The miR-214-5p/Lactoferrin/miR-224-5p/ADAM17 Axis Is Involved in Goat Mammary Epithelial Cells’ Immune Regulation
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Mammary Tissue Collection and Cell Culture
2.3. MiRNA Sequencing
2.4. Vector Construction
2.5. GMECs Cell Transfection
2.6. RNA Extraction and Quantitative Real-Time PCR (qRT-PCR)
2.7. Western Blot
2.8. Luciferase Assays
2.9. Statistical Analysis
3. Results
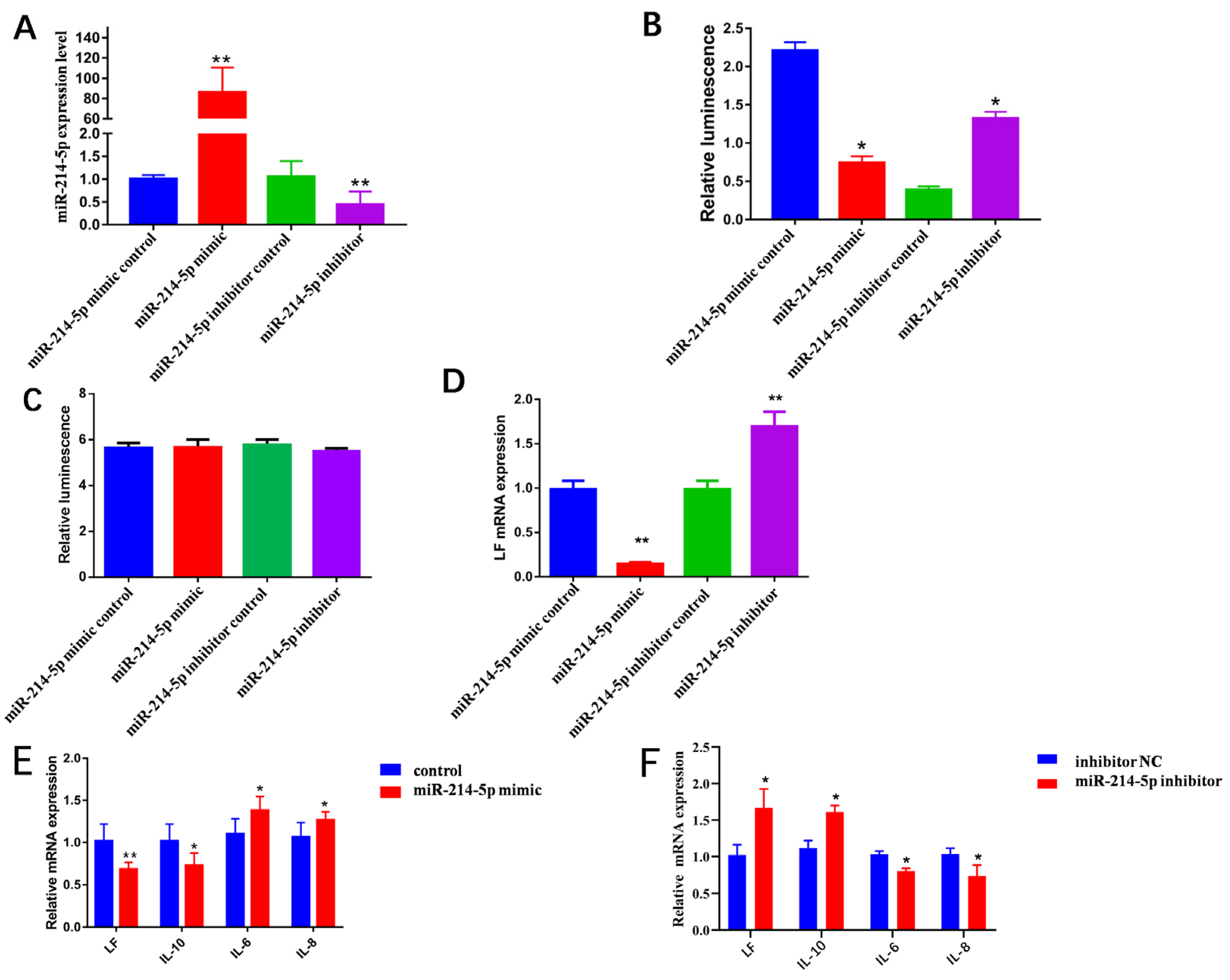
3.1. miR-214-5p Target 3′UTR of LF mRNA
3.2. miR-214-5p Decreases the Expression of Inflammatory Factors
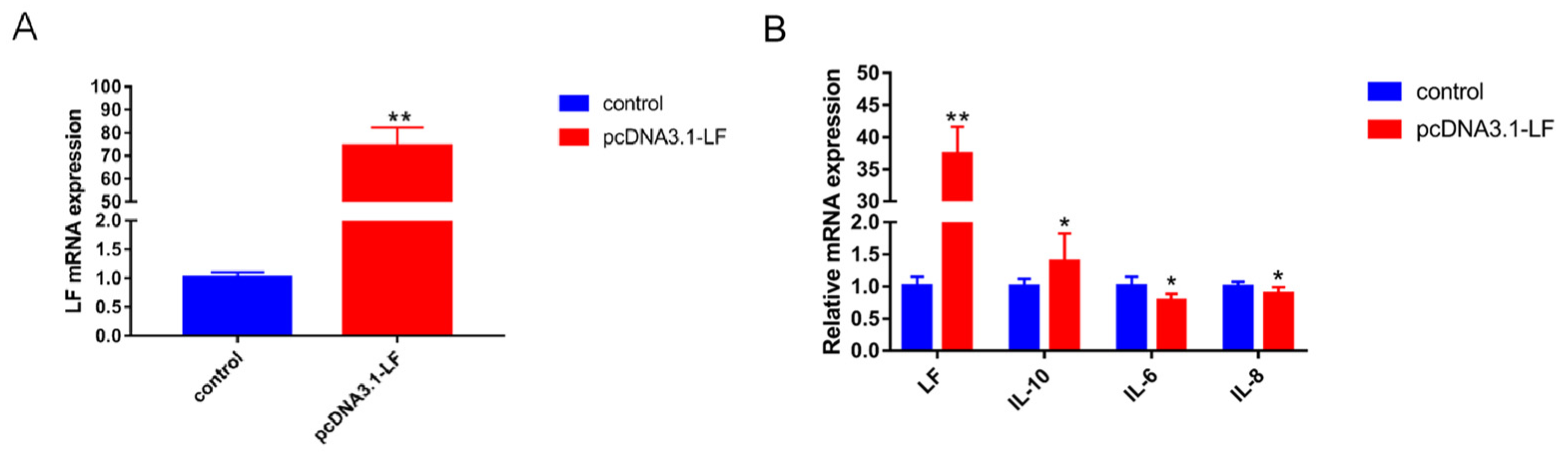
3.3. LF Decreases the Expression of Inflammatory Factors
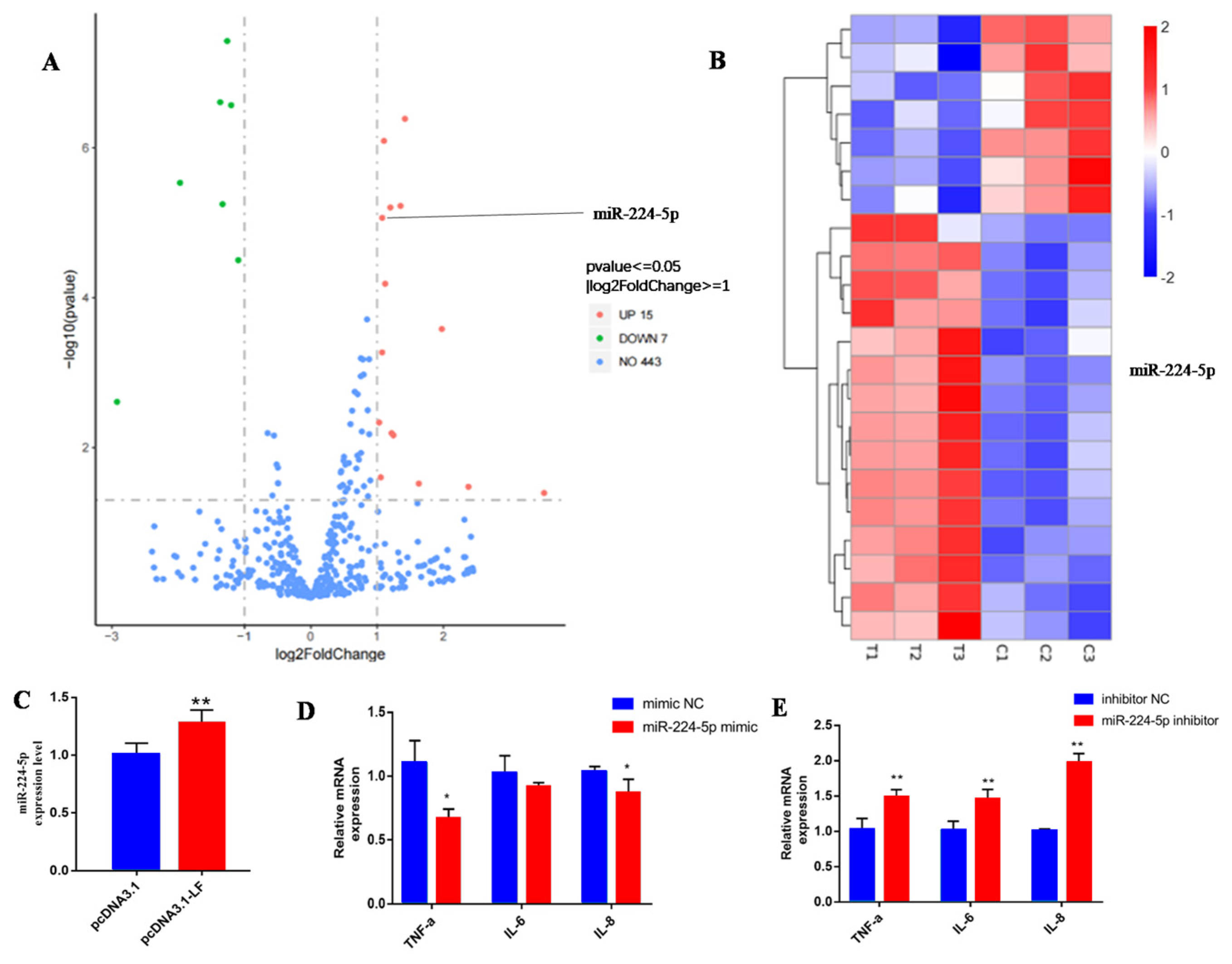
3.4. LF Attenuates the Inflammatory Response via Targeting miR-224-5p
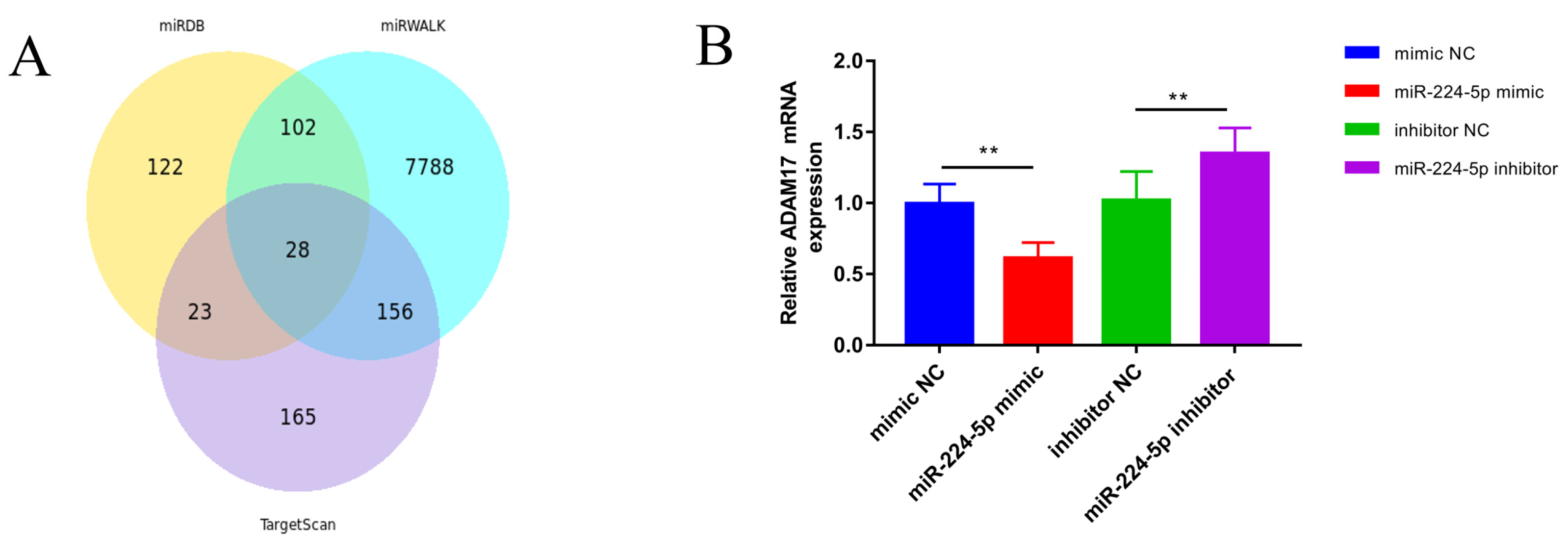
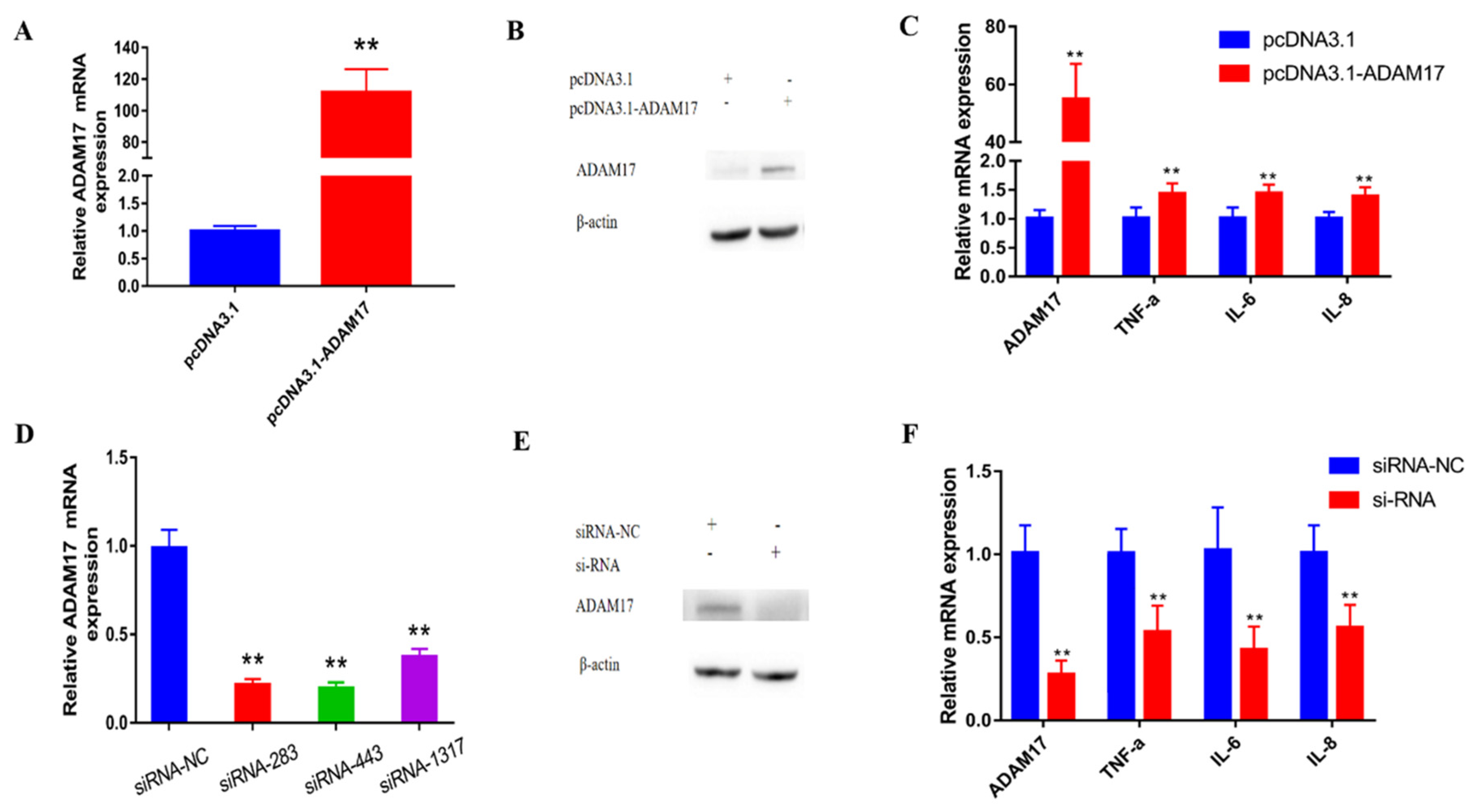
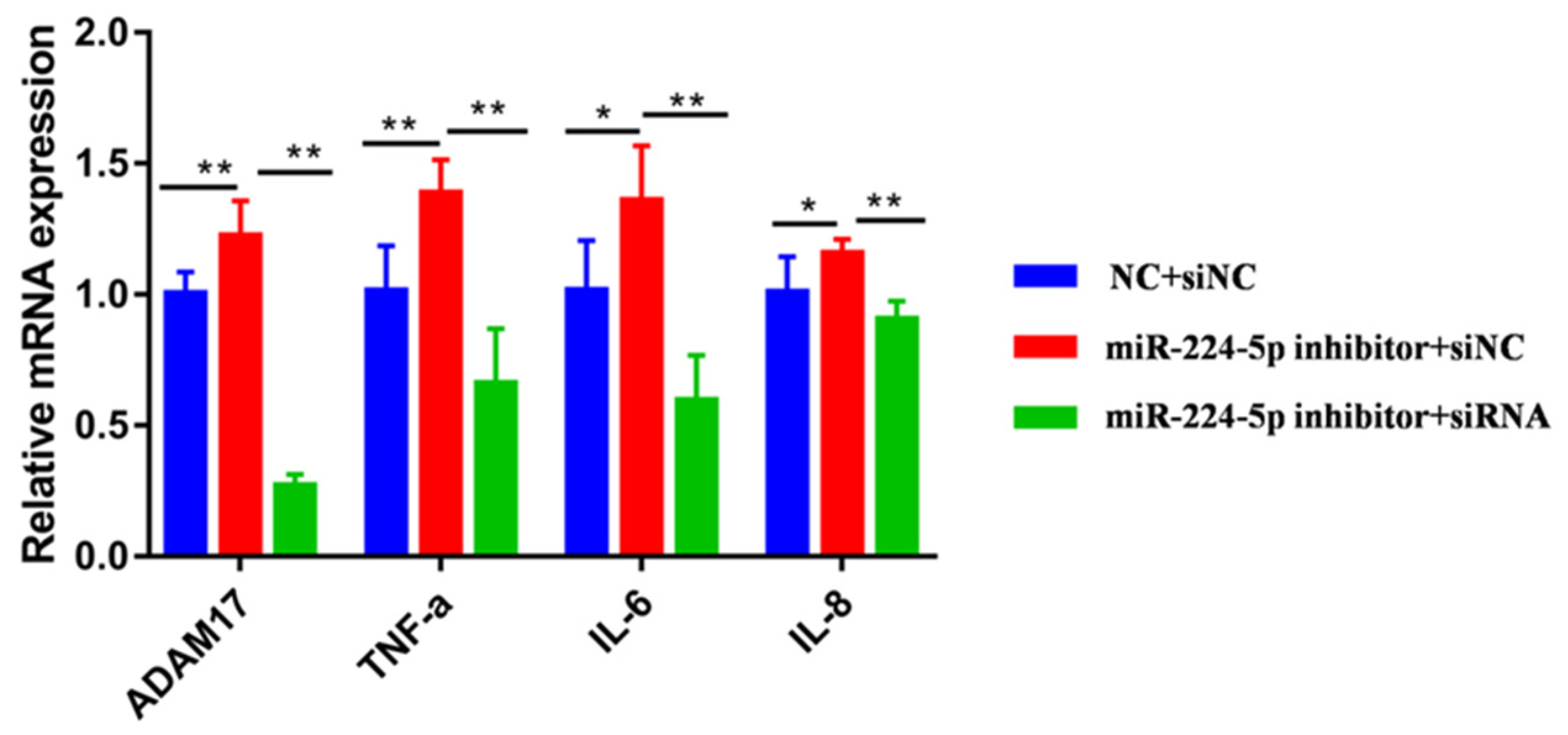
3.5. miR-224-5p Attenuates the Inflammatory Response via Targeting of ADAM17
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gao, Y.; Tong, J.Z.; Gu, K.; Gao, F.; Ma, W.T.; Chen, D.K. Effects of IL-17A on the secretion of immune defense factors in Goat mammary epithelial cells. Chin. J. Vet. Med. 2020, 40, 2205–2213. [Google Scholar]
- Yan, Y.T. Study on the Relationship between Antibacterial Peptide S100A7 and Mastitis in Goat Milk Cell; Northwest Agriculture and Forestry University of Science and Technology: Xianyang, China, 2021. [Google Scholar]
- Rachman, A.B.; Maheswari, R.R.A.; Bachroem, M.S. Composition and isolation of lactoferrin from colostrum and milk of various goat breeds. Procedia Food Sci. 2015, 3, 200–210. [Google Scholar] [CrossRef]
- Legrand, D.; Elass, E.; Carpentier, M.; Mazurier, J. Lactoferrin: A modulator of immune and inflammatory responses. Cell. Mol. Life. Sci. 2005, 62, 2549–2559. [Google Scholar] [CrossRef] [PubMed]
- Teng, C.T. Lactoferrin: The path from protein to gene. Biometals 2010, 23, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Sessa, R.; Di Pietro, M.; Filardo, S.; Bressan, A.; Rosa, L.; Cutone, A.; Frioni, A.; Berlutti, F.; Paesano, R.; Valenti, P. Effect of bovine lactoferrin on Chlamydia trachomatis infection and inflammation. Biochem. Cell Biol. 2016, 95, 34–40. [Google Scholar] [CrossRef]
- Puddu, P.; Valenti, P.; Gessani, S. Immunomodulatory Effects of Lactoferrin On Antigen Presenting Cells. Biochimie 2009, 91, 11–18. [Google Scholar] [CrossRef]
- Puddu, P.; Latorre, D.; Carollo, M.; Catizone, A.; Ricci, G.; Valenti, P.; Gessani, S. Bovine Lactoferrin Counteracts Toll-Like Receptor Mediated Activation Signals in Antigen Presenting Cells. PLoS ONE 2011, 6, 22504. [Google Scholar] [CrossRef] [PubMed]
- Berlutti, F.; Schippa, S.; Morea, C.; Sarli, S.; Perfetto, B.; Donnarumma, G.; Valenti, P. Lactoferrin downregulates pro-inflammatory cytokines upexpressed in intestinal epithelial cells infected with invasive or noninvasive Escherichia coli strains. Biochem. Cell Biol. 2006, 84, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Kruzel, M.L.; Harari, Y.; Mailman, D.; Actor, J.K.; Zimecki, M. Differential effects of prophylactic, concurrent and therapeutic lactoferrin treatment on LPS-induced inflammatory responses in mice. Clin. Exp. Immunol. 2002, 130, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Friedman, P.C.; Farh, K.K.; Burge, C.B.; Bartel, D.P. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2008, 19, 92–105. [Google Scholar] [CrossRef] [PubMed]
- Siqueiros-Cendón, T.; Arévalo-Gallegos, S.; Iglesias-Figueroa, B.F.; García-Montoya, I.A.; Salazar-Martínez, J.; Rascón-Cruz, Q. Immunomodulatory effects of lactoferrin. Acta Pharmacol. Sin. 2014, 35, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and functions. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Yin, Y.; Li, X.; He, T.; Wang, P.; Song, M.; Gao, J. Effect of miR-26a-5p targeting ADAM17 gene on apoptosis, inflammatory factors and oxidative stress response of myocardial cells in hypoxic model. J. Bioenerg. Biomembr. 2020, 52, 83–92. [Google Scholar] [CrossRef]
- Qu, Y.; Zhang, J.; Zhang, J.; Xiao, W. MiR-708-3p Alleviates Inflammation and Myocardial Injury After Myocardial Infarction by Suppressing ADAM17 Expression. Inflammation 2021, 44, 1083–1095. [Google Scholar] [CrossRef] [PubMed]
- Hasmall, S. Downregulation of Lactoferrin by PPARalpha Ligands: Role in Perturbation of Hepatocyte Proliferation and Apoptosis. Toxicol. Sci. 2002, 68, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.J.; Li, J.; Meng, S. Research progress on the molecular mechanism of lactoferrin regulating the expression of NF-κB in inflammatory response. Shandong Pharm. 2016, 56, 106–109. [Google Scholar]
- Mcmaster, M.T.; Dey, S.K.; Andrews, G.K. Association of monocytes and neutrophils with early events of blastocyst implantation in mice. Reproduction 1993, 99, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Shigeta, H.; Newbold, R.R.; McLachlan, J.A.; Teng, C. Estrogenic effect on the expression of estrogen receptor, COUP-TF, and lactoferrin mRNA in developing mouse tissues. Mol. Reprod. Dev. 2015, 45, 21–30. [Google Scholar] [CrossRef]
- Hertaviani, R.F. Nutritional Ingredients and Lactoferrin Concentration in Dairy Goat Milk from Etawah Grades and Jawarandu Breed. Ipb 2009. Available online: https://repository.ipb.ac.id/handle/123456789/59954 (accessed on 9 July 2023).
- Paesano, R.; Natalizi, T.; Berlutti, F.; Valenti, P. Body iron delocalization: The serious drawback in iron disorders in both developing and developed countries. Pathog. Glob. Health 2012, 106, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Frioni, A.; Conte, M.P.; Cutone, A.; Longhi, C.; Musci, G.; di Patti, M.C.; Natalizi, T.; Marazzato, M.; Lepanto, M.S.; Puddu, P.; et al. Lactoferrin differently modulates the inflammatory response in epithelial models mimicking human inflammatory and infectious diseases. Biometals 2014, 27, 843–856. [Google Scholar] [CrossRef] [PubMed]
- Valenti, P.; Catizone, A.; Pantanella, F.; Frioni, A.; Natalizi, T.; Tendini, M.; Berlutti, F. Lactoferrin decreases inflammatory response by cystic fibrosis bronchial cells invaded with Burkholderia cenocepacia iron-modulated biofilm. Int. J. Immunopathol. Pharmacol. 2011, 24, 1057–1068. [Google Scholar] [CrossRef] [PubMed]
- Black, R.A.; Rauch, C.T.; Kozlosky, C.J.; Peschon, J.J.; Slack, J.L.; WoLFson, M.F.; Castner, B.J.; Stocking, K.L.; Reddy, P.; Srinivasan, S.; et al. A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature 1997, 385, 729–733. [Google Scholar] [CrossRef]
- Liao, Y.; Du, X.; Lonnerdal, B. miR-214 Regulates Lactoferrin Expression and Pro-Apoptotic Function in Mammary Epithelial Cells. J. Nutr. 2010, 140, 1552–1556. [Google Scholar] [CrossRef] [PubMed]
- Song, N.; Wang, X.; Gui, L.; Raza, S.H.A.; Luoreng, Z.; Zan, L. MicroRNA-214 regulates immunity-related genes in bovine mammary epithelial cells by targeting NFATc3 and TRAF3. Mol. Cell. Probes 2017, 35, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Peng, J.Q.; Qin, P.; Luo, X.R.; Zhang, M. miR-214-5p inhibits high glucose-induced inflammation and fibrosis in glomerular mesangial cells by regulating HOXA13. Chin. J. Gerontol. 2021, 41, 1719–1723. [Google Scholar]
- Yang, X.Y.; Guan, X.N.; Zhao, W.S.; Liu, J.; Zhao, L.; Yang, D. The regulatory effect of miR-214-5p targeting PAK4 on myocardial injury and immune response in rats with ischemia-reperfusion. Chin. J. Physiol. 2020, 36, 394–400. [Google Scholar]
- Li, P.; Wang, J.; Guo, F.; Zheng, B.; Zhang, X. A novel inhibitory role of microRNA-224 in particulate matter 2.5-induced asthmatic mice by inhibiting TLR2. J. Cell. Mol. Med. 2020, 24, 3040–3052. [Google Scholar] [CrossRef] [PubMed]
- Hiss, S.; Meyer, T.; Sauerwein, H. Lactoferrin concentrations in goat milk throughout lactation. Small Rumin. Res. 2008, 79, 87–90. [Google Scholar] [CrossRef]
- Palau, V.; Jarrín, J.; Villanueva, S.; Benito, D.; Márquez, E.; Rodríguez, E.; Soler, M.J.; Oliveras, A.; Gimeno, J.; Sans, L.; et al. Endothelial ADAM17 Expression in the Progression of Kidney Injury in an Obese Mouse Model of Pre-Diabetes. Int. J. Mol. Sci. 2021, 23, 221. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Huang, W.; Chen, H.; Wei, H.; Luo, A.; Xia, G.; Deng, X.; Zhang, G. MicroRNA-224, negatively regulated by c-jun, inhibits growth and epithelial-to-mesenchymal transition phenotype via targeting ADAM17 in oral squamous cell carcinoma. J. Cell. Mol. Med. 2019, 23, 4913–4920. [Google Scholar] [CrossRef] [PubMed]
| Gene | Primer Sequences (5′~3′) |
|---|---|
| LF | F: TCTGGCTGCCCCGAGGAAAAACGTT |
| R: TCCTGCAGGCACTTGAAGGCACCAG | |
| miR-214-5p | F: ACAGCAGGCACAGACAGGCAGT |
| R: TCTCAGAAGCTAAACAGGGTCG | |
| ADAM17 | F: GGCGCGGGAGGAATAAGAAG |
| R: TGTCCAGGAAATCAGAGAGCC | |
| IL6 | F: AGATATACCTGGACTTCCT |
| R: TGTTCTGATACTGCTCTG | |
| IL8 | F: AAGCTGGCTGTTGCTCTCTTG |
| R: TGTTCTGATACTGCTCTG | |
| IL10 | F: CATGGGCCTGACATCAAGGA |
| R: CTCTTGTTTTCGCAGGGCAG | |
| TNF-α | F: TGGTTCAGACACTCAGGT |
| R: CGCTGATGTTGGCTACAA | |
| siADAM17 | F: CCGCUUUGGAGACUAAUUATT |
| R: UAAUUAGUCUCCAAAGCGGTT | |
| MRPL39 | F: AGGTTCTCTTTTGTTGGCATCC |
| R: TTGGTCAGAGCCCCAGAAGT | |
| UXT | F: TGTGGCCCTTGGATATGGTT |
| R: GTGTCTGGGACCACTGTGTCAA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pang, S.; Shao, Y.; Yu, Y.; Sha, K.; Jiang, Y.; Zhang, X.; Zhong, Y.; Shi, H.; Li, W. The miR-214-5p/Lactoferrin/miR-224-5p/ADAM17 Axis Is Involved in Goat Mammary Epithelial Cells’ Immune Regulation. Animals 2023, 13, 2835. https://doi.org/10.3390/ani13182835
Pang S, Shao Y, Yu Y, Sha K, Jiang Y, Zhang X, Zhong Y, Shi H, Li W. The miR-214-5p/Lactoferrin/miR-224-5p/ADAM17 Axis Is Involved in Goat Mammary Epithelial Cells’ Immune Regulation. Animals. 2023; 13(18):2835. https://doi.org/10.3390/ani13182835
Chicago/Turabian StylePang, Shilong, Yuexin Shao, Yan Yu, Kela Sha, Yanting Jiang, Xian Zhang, Yuling Zhong, Huaiping Shi, and Weijuan Li. 2023. "The miR-214-5p/Lactoferrin/miR-224-5p/ADAM17 Axis Is Involved in Goat Mammary Epithelial Cells’ Immune Regulation" Animals 13, no. 18: 2835. https://doi.org/10.3390/ani13182835





