Mapping Subchondral Bone Density Distribution in the Canine C6-C7 Vertebral Endplates: A CT-OAM Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
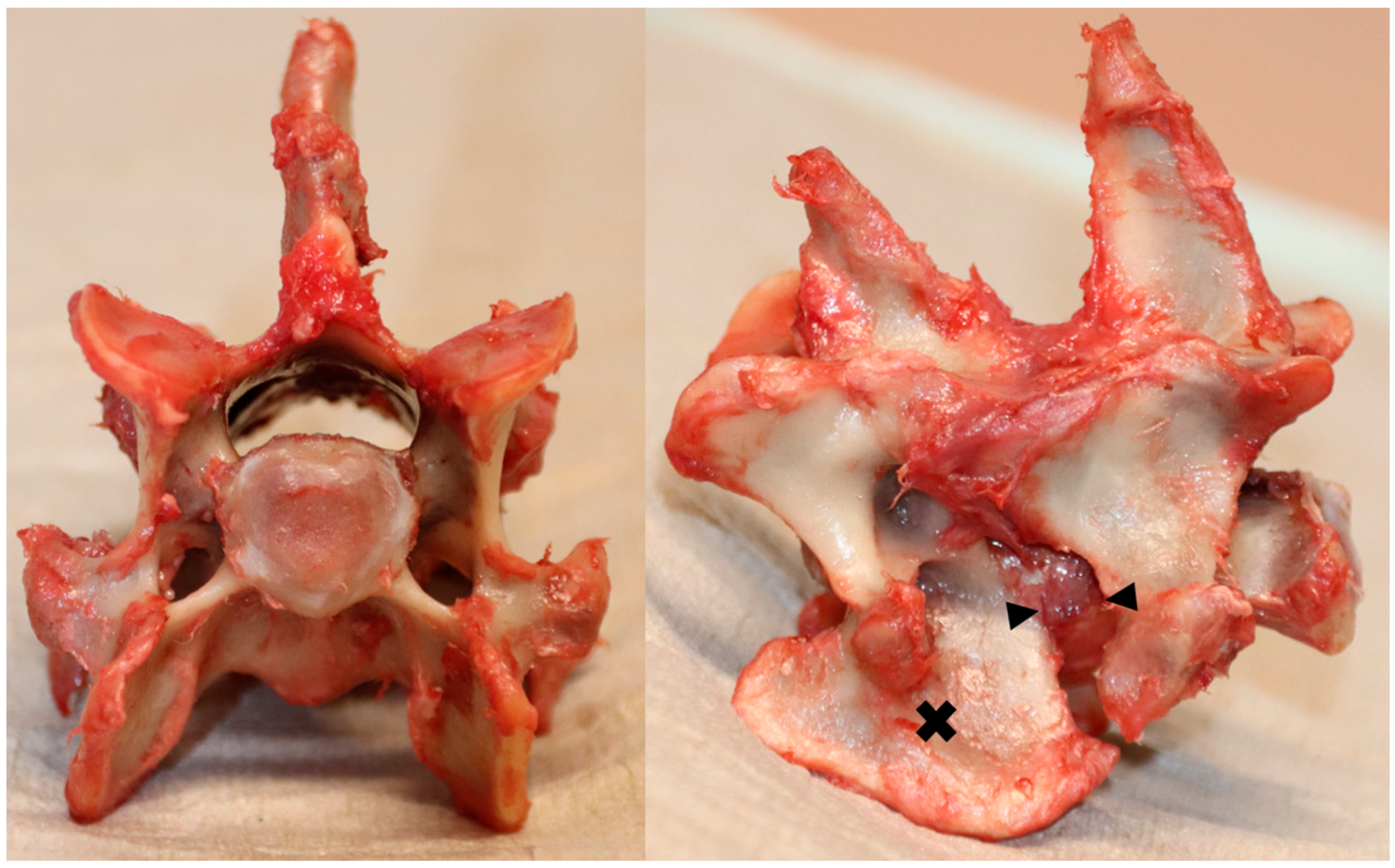
2.1. Specimen Preparations
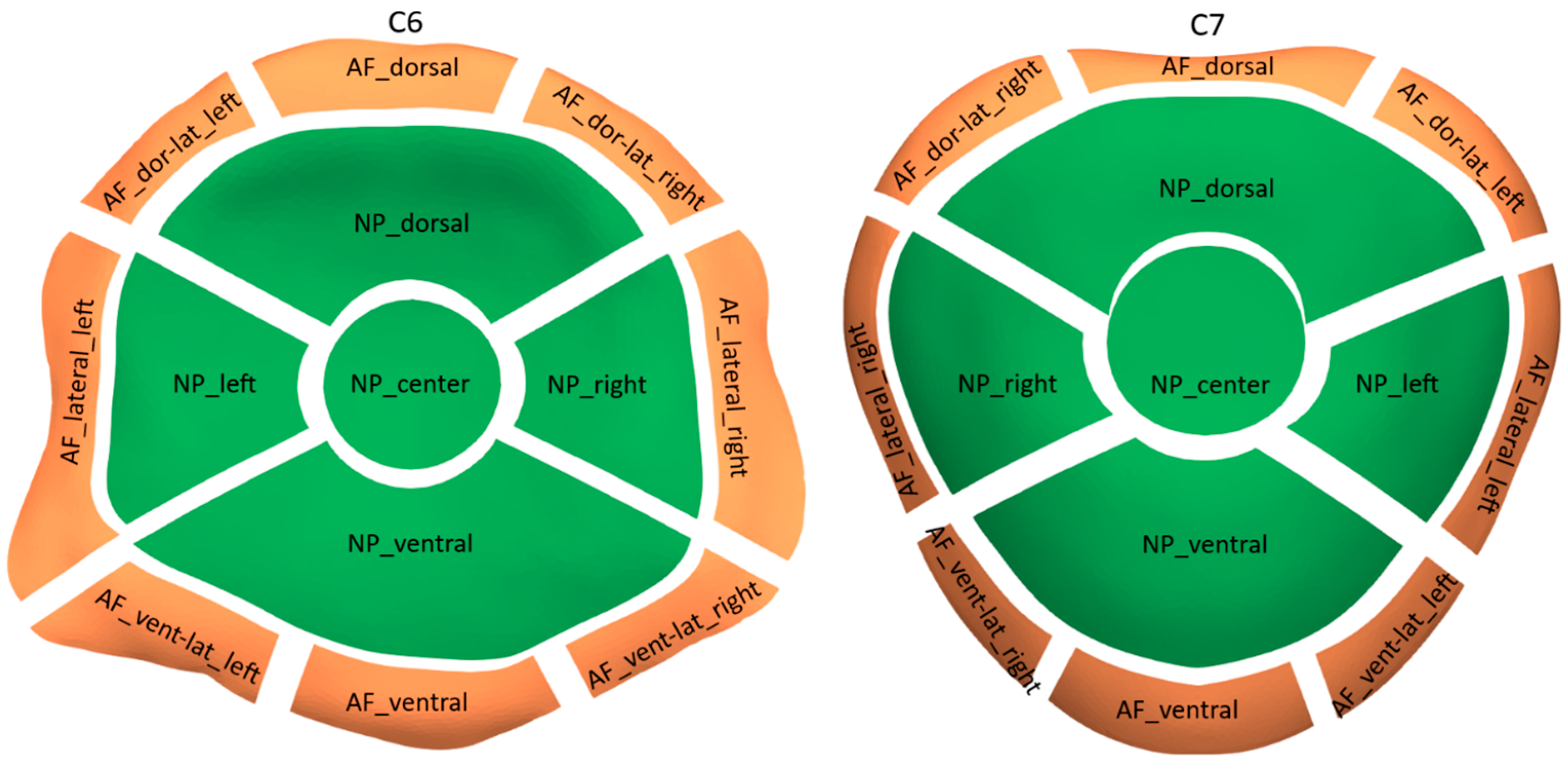
2.2. CT-OAM Mapping
2.3. Statistical Analysis
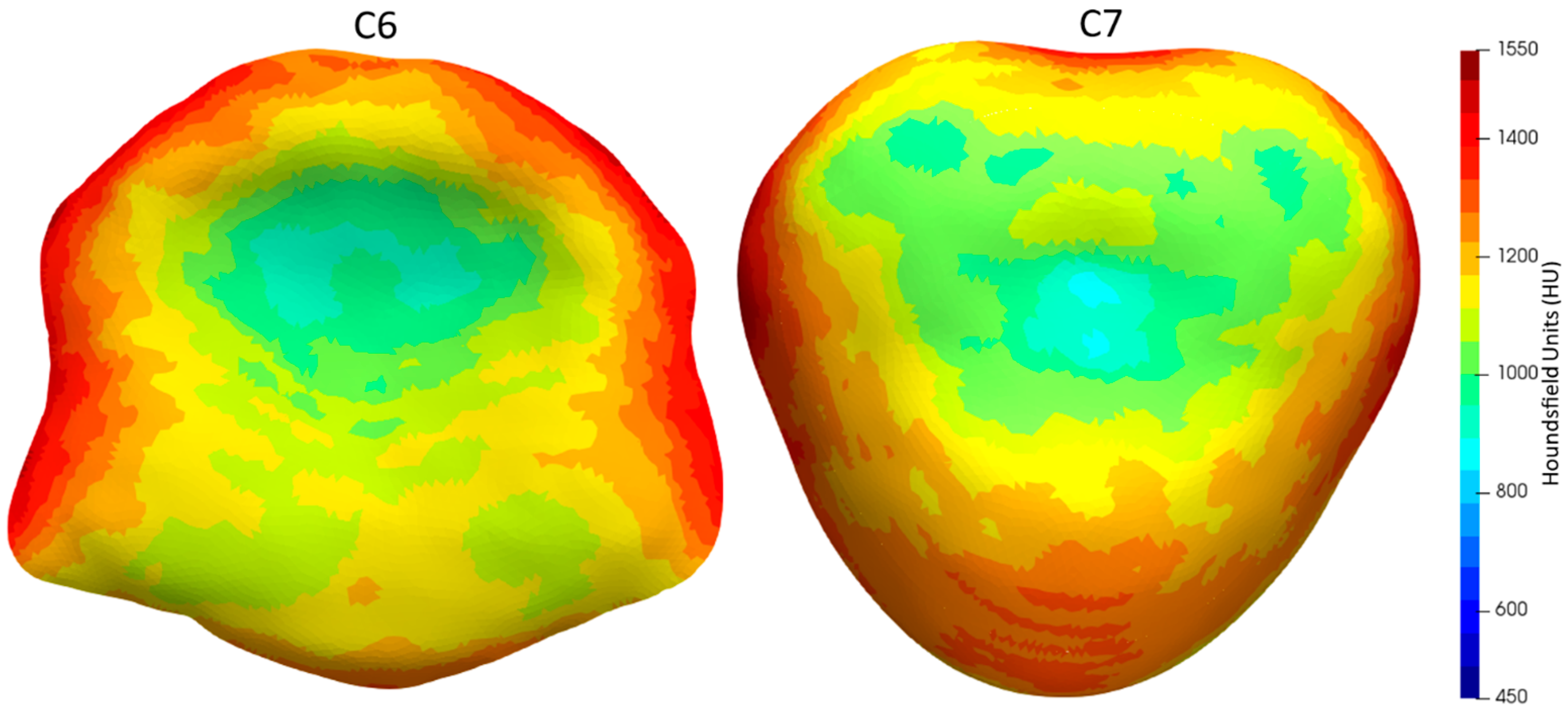
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- da Costa, R.C.; Parent, J.M.; Partlow, G.; Dobson, H.; Holmberg, D.L.; Lamarre, J. Morphologic and morphometric magnetic resonance imaging features of Doberman Pinschers with and without clinical signs of cervical spondylomyelopathy. Am. J. Vet. Res. 2006, 67, 1601–1612. [Google Scholar] [CrossRef] [PubMed]
- De Decker, S.; Gielen, I.M.V.L.; Duchateau, L.; Oevermann, A.; Polis, I.; Van Soens, I.; van Bree, H.J.J.; Van Ham, L.M.L. Evolution of clinical signs and predictors of outcome after conservative medical treatment for disk-associated cervical spondylomyelopathy in dogs. J. Am. Vet. Med. Assoc. 2012, 240, 848–857. [Google Scholar] [CrossRef] [PubMed]
- Rohner, D.; Kowaleski, M.P.; Schwarz, G.; Forterre, F. Short-Term Clinical and Radiographical Outcome after Application of Anchored Intervertebral Spacers in Dogs with Disc-Associated Cervical Spondylomyelopathy. Vet. Comp. Orthop. Traumatol. 2019, 32, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Farrokhi, M.R.; Torabinezhad, S.; Ghajar, K.A. Pilot Study of a New Acrylic Cage in a Dog Cervical Spine Fusion Model. Clin. Spine Surg. 2010, 23, 272–277. [Google Scholar] [CrossRef] [PubMed]
- De Decker, S.; Caemaert, J.; Tshamala, M.C.; Gielen, I.M.V.L.; Van Bree, H.J.J.; Bosmans, T.; Wegge, B.; Van Ham, L.M.L. Surgical Treatment of Disk-Associated Wobbler Syndrome by a Distractable Vertebral Titanium Cage in Seven Dogs. Vet. Surg. 2011, 40, 544–554. [Google Scholar] [CrossRef]
- Dixon, B.C.; Tomlinson, J.L.; Kraus, K.H. Modified distraction-stabilization technique using an interbody polymethyl methacrylate plug in dogs with caudal cervical spondylomyelopathy. J. Am. Vet. Med. Assoc. 1996, 208, 61–68. [Google Scholar]
- Bernard, F.; Bardet, J.-F.; da Silva, A. Caudal cervical arthrodesis using a distractable fusion cage in a dog. Vet. Comp. Orthop. Traumatol. 2010, 23, 209–213. [Google Scholar] [CrossRef]
- Steffen, F.; Voss, K.; Morgan, J.P. Distraction–Fusion for Caudal Cervical Spondylomyelopathy Using an Intervertebral Cage and Locking Plates in 14 Dogs. Vet. Surg. 2011, 40, 743–752. [Google Scholar] [CrossRef]
- Solano, M.A.; Fitzpatrick, N.; Bertran, J. Cervical Distraction-Stabilization Using an Intervertebral Spacer Screw and String-of Pearl (SOP™) Plates in 16 Dogs With Disc-Associated Wobbler Syndrome. Vet. Surg. 2015, 44, 627–641. [Google Scholar] [CrossRef]
- King, J.; Corfield, G.; Mouatt, J.; Kan, C.; Moses, P. Surgical management and long-term outcome of dogs with cervical spondylomyelopathy with an anchored intervertebral titanium device. Aust. Vet. J. 2020, 98, 156–163. [Google Scholar] [CrossRef]
- Bok, T.E.R.; Willemsen, K.; van Rijen, M.H.P.; Grinwis, G.C.M.; Tryfonidou, M.A.; Meij, B.P. Instrumented cervical fusion in nine dogs with caudal cervical spondylomyelopathy. Vet. Surg. 2019, 48, 1287–1298. [Google Scholar] [CrossRef]
- Dhar, U.K.; Menzer, E.L.; Lin, M.; Hagerty, V.; O’connor, T.; Tsai, C.-T.; Vrionis, F.D. Factors influencing cage subsidence in anterior cervical corpectomy and discectomy: A systematic review. Eur. Spine J. 2023, 32, 957–968. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Shan, Z.; Zhao, F.; Cheung, J.P.Y. Poor Bone Quality, Multilevel Surgery, and Narrow and Tall Cages Are Associated with Intraoperative Endplate Injuries and Late-onset Cage Subsidence in Lateral Lumbar Interbody Fusion: A Systematic Review. Clin. Orthop. Relat. Res. 2021, 480, 163–188. [Google Scholar] [CrossRef] [PubMed]
- Noordhoek, I.; Koning, M.T.; Jacobs, W.C.H.; Vleggeert-Lankamp, C.L.A. Incidence and clinical relevance of cage subsidence in anterior cervical discectomy and fusion: A systematic review. Acta Neurochir. 2018, 160, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Toth, J.M.; Wang, M.; Estes, B.T.; Scifert, J.L.; Seim, H.B.; Turner, A.S. Polyetheretherketone as a biomaterial for spinal applications. Biomaterials 2006, 27, 324–334. [Google Scholar] [CrossRef]
- Kersten, R.F.M.R.; van Gaalen, S.M.; de Gast, A.; Öner, F.C. Polyetheretherketone (PEEK) cages in cervical applications: A systematic review. Spine J. 2015, 15, 1446–1460. [Google Scholar] [CrossRef]
- Shimizu, T.; Fujibayashi, S.; Yamaguchi, S.; Otsuki, B.; Okuzu, Y.; Matsushita, T.; Kokubo, T.; Matsuda, S. In vivo experimental study of anterior cervical fusion using bioactive polyetheretherketone in a canine model. PLoS ONE 2017, 12, e0184495. [Google Scholar] [CrossRef]
- Driver, C.J.; Lopez, V.; Walton, B.; Jones, D.; Fentem, R.; Tomlinson, A.; Rose, J. Instrumented cervical fusion using patient specific end-plate conforming interbody devices with a micro-porous structure in nine dogs with disk-associated cervical spondylomyelopathy. Front. Vet. Sci. 2023, 10, 1208593. [Google Scholar] [CrossRef]
- Parr, W.C.H.; Tan, C.; Walsh, W.R.; Brunel, L.; Joffe, M.R. Development of a Customized Interbody Fusion Device for Treatment of Canine Disc-Associated Cervical Spondylomyelopathy. Vet. Comp. Orthop. Traumatol. 2019, 32, 079–086. [Google Scholar] [CrossRef]
- Muller-Gerbl, M. The subchondral bone plate. Adv. Anat. Embryol. Cell Biol. 1998, 141, 1–134. [Google Scholar]
- Orías, A.A.E.; Sheha, E.; Zavras, A.; John, P.; Fitch, A.A.; An, H.S.; Inoue, N.; Colman, M. CT Osteoabsorptiometry Assessment of Subchondral Bone Density Predicts Intervertebral Implant Subsidence in a Human ACDF Cadaver Model. Glob. Spine J. 2021, 13, 1374–1383. [Google Scholar] [CrossRef] [PubMed]
- Poilliot, A.; Li, K.C.; Müller-Gerbl, M.; Toranelli, M.; Zhang, M.; Zwirner, J.; Hammer, N. Subchondral bone strength of the sacroiliac joint-a combined approach using computed tomography osteoabsorptiometry (CT-OAM) imaging and biomechanical validation. J. Mech. Behav. Biomed. Mater. 2020, 111, 103978. [Google Scholar] [CrossRef] [PubMed]
- Hoechel, S.; Wirz, D.; Müller-Gerbl, M. Density and strength distribution in the human subchondral bone plate of the patella. Int. Orthop. 2012, 36, 1827–1834. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kraljević, M.; Zumstein, V.; Wirz, D.; Hügli, R.; Müller-Gerbl, M. Mineralisation and mechanical strength of the glenoid cavity subchondral bone plate. Int. Orthop. 2011, 35, 1813–1819. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hara, T.; Ohara, Y.; Abe, E.; Takami, K.; Orías, A.A.E.; Arai, H.; Inoue, N. Cervical endplate bone density distribution measured by CT osteoabsorptiometry and direct comparison with mechanical properties of the endplate. Eur. Spine J. 2021, 30, 2557–2564. [Google Scholar] [CrossRef] [PubMed]
- Müller-Gerbl, M.; Weißer, S.; Linsenmeier, U. The distribution of mineral density in the cervical vertebral endplates. Eur. Spine J. 2008, 17, 432–438. [Google Scholar] [CrossRef]
- Cheon, B.; Park, S.; Lee, S.-K.; Park, J.-G.; Cho, K.-O.; Choi, J. Variation of canine vertebral bone architecture in computed tomography. J. Vet. Sci. 2018, 19, 145–150. [Google Scholar] [CrossRef]
- Fedorov, A.; Beichel, R.; Kalpathy-Cramer, J.; Finet, J.; Fillion-Robin, J.-C.; Pujol, S.; Bauer, C.; Jennings, D.; Fennessy, F.; Sonka, M.; et al. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn. Reson. Imaging 2012, 30, 1323–1341. [Google Scholar] [CrossRef]
- Shamonin, D.P.; Bron, E.E.; Lelieveldt, B.P.F.; Smits, M.; Klein, S.; Staring, M. Fast parallel image registration on CPU and GPU for diagnostic classification of Alzheimer’s disease. Front. Neuroinform. 2013, 7, 50. [Google Scholar] [CrossRef]
- Klein, S.; Staring, M.; Murphy, K.; Viergever, M.A.; Pluim, J.P.W. elastix: A Toolbox for Intensity-Based Medical Image Registration. IEEE Trans. Med. Imaging 2009, 29, 196–205. [Google Scholar] [CrossRef]
- Adamo, P.F. Cervical arthroplasty in two dogs with disk-associated cervical spondylomyelopathy. J. Am. Vet. Med. Assoc. 2011, 239, 808–817. [Google Scholar] [CrossRef] [PubMed]
- Falzone, C.; Tranquillo, V.; Gasparinetti, N. Comparison of Two Surgical Techniques for the Treatment of Canine Disc Associated-Cervical Spondylomyelopathy. Front. Vet. Sci. 2022, 9, 880018. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Mei, J.; Feng, X.; Deng, C.; Tian, X.; Lv, J.; Sun, L. Low cervical vertebral CT value increased early subsidence of titanium mesh cage after anterior cervical corpectomy and fusion. J. Orthop. Surg. Res. 2022, 17, 355. [Google Scholar] [CrossRef] [PubMed]
- Zavras, A.G.; Dandu, N.; Espinoza-Orias, A.A.; Singh, K.; An, H.S.; Inoue, N.; Colman, M.W. Computed Tomography Osteoabsorptiometry Evaluation of Cervical Endplate Subchondral Bone Mineral Density. Glob. Spine J. 2021, 13, 1803–1811. [Google Scholar] [CrossRef]
- Agnello, K.A.; Kapatkin, A.S.; Garcia, T.C.; Hayashi, K.; Welihozkiy, A.T.; Stover, S.M. Intervertebral Biomechanics of Locking Compression Plate Monocortical Fixation of the Canine Cervical Spine. Vet. Surg. 2010, 39, 991–1000. [Google Scholar] [CrossRef]
- Beishuizen, R.; Bok, T.E.R.; Teunissen, M.; van der Veen, A.J.; Emanuel, K.S.; Tryfonidou, M.A.; Meij, B.P. Biomechanical effects of a titanium intervertebral cage as a stand-alone device, and in combination with locking plates in the canine caudal cervical spine. Vet. Surg. 2021, 50, 1087–1097. [Google Scholar] [CrossRef]
- McAfee, P.C.; Cunningham, B.; Dmitriev, A.; Hu, N.; Kim, S.W.; Cappuccino, A.; Pimenta, L. Cervical Disc Replacement—Porous Coated Motion Prosthesis: A Comparative Biomechanical Analysis Showing the Key Role of the Posterior Longitudinal Ligament. Spine 2003, 28, S176–S185. [Google Scholar] [CrossRef]
- Hartmann, K.; Düver, P.; Kaiser, S.; Fischer, C.; Forterre, F. CT-Scan Based Evaluation of Dorsal-to-Ventral Ratios of Paraspinal Musculature in Chondrodystrophic and Non-chondrodystrophic Dogs. Front. Vet. Sci. 2020, 7, 577394. [Google Scholar] [CrossRef]
- Yamaguchi, F.; Nishi, H.; Misumi, K.; Fujiki, M. Serum cross-linked N-telopeptide of type I collagen across the canine life span: An investigation in intact and neutered male and female dogs. Res. Vet. Sci. 2021, 136, 609–615. [Google Scholar] [CrossRef]
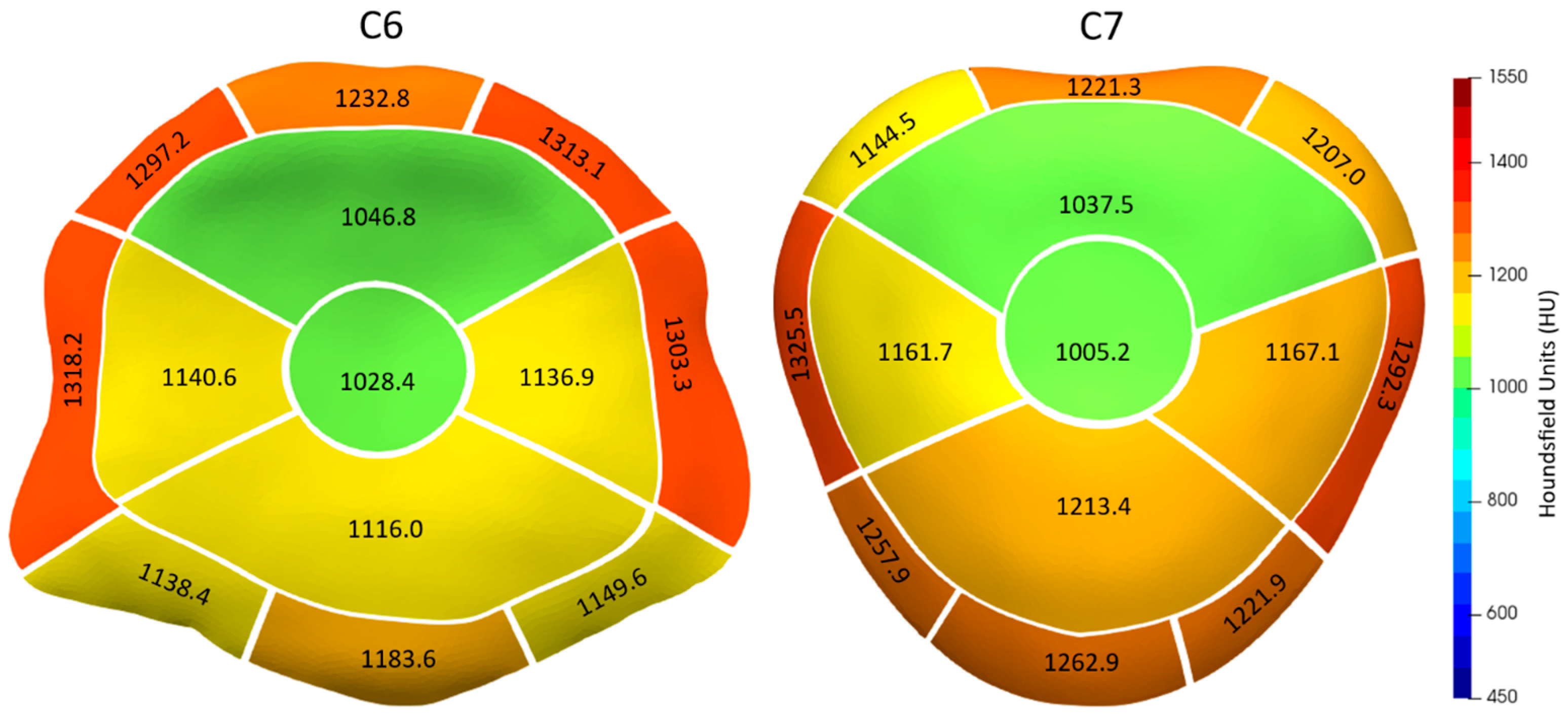
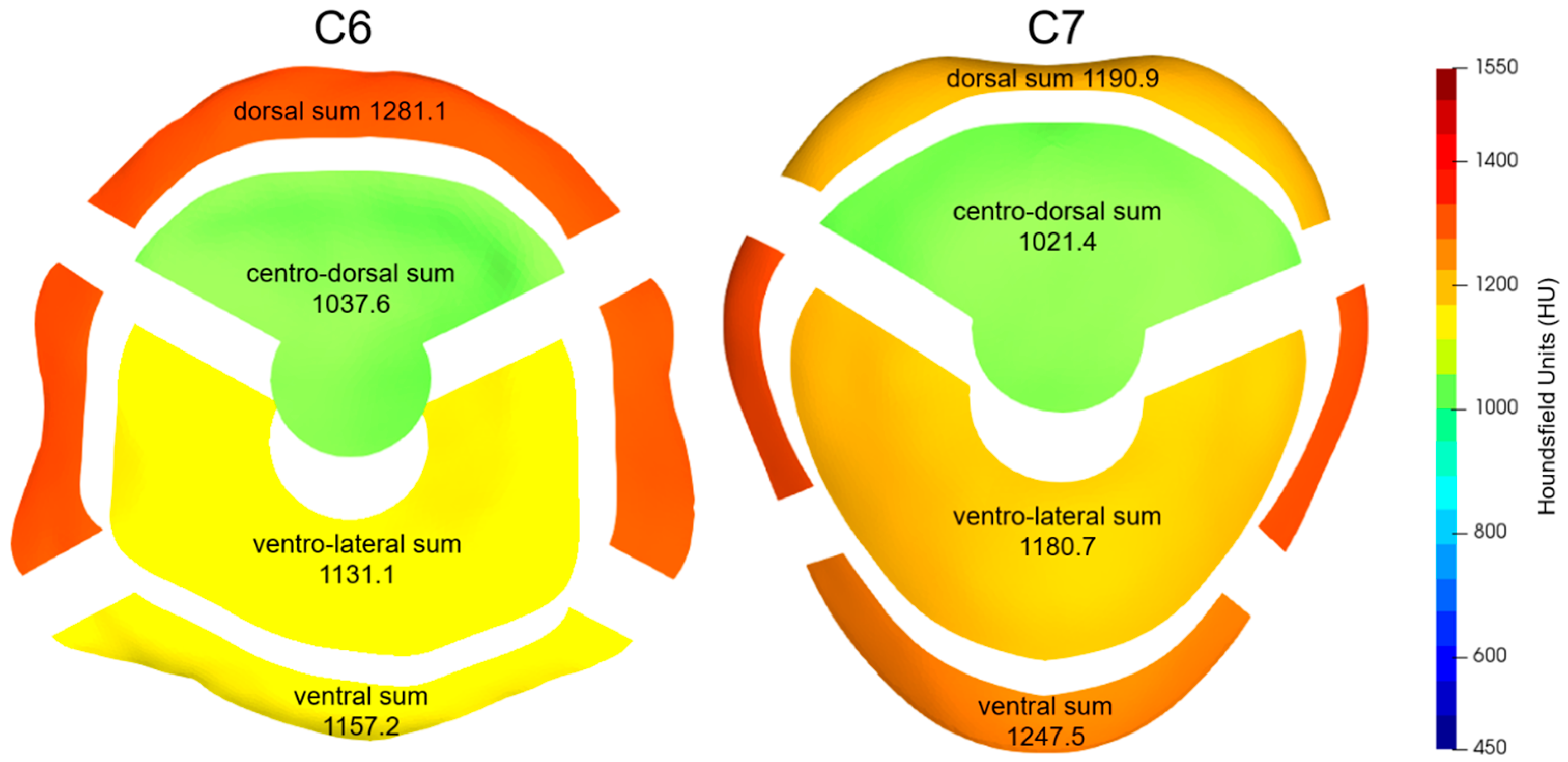
| C6 | |||||||||
| 1169.9 | |||||||||
| 77.6 | AF | ||||||||
| 1248.5 | |||||||||
| 81.5 | AF-Dorsal-Sum | AF-Vental-Sum | AF-Lateral-Left | AF-Lateral-Right | |||||
| 1281.1 | 1157.2 | 1318.2 | 1303.3 | ||||||
| 73.9 | 99.9 | 89.8 | 88.2 | ||||||
| AF-Dorsal-Left | AF-Dorsal-Center | AF-Dorsal-Right | AF-Ventral-Left | AF-Ventral-Center | AF-Ventral-Right | ||||
| 1297.2 | 1232.8 | 1313.1 | 1138.4 | 1183.6 | 1149.6 | ||||
| 78.2 | 79.5 | 81.8 | 105.1 | 104.8 | 111.4 | ||||
| NP | |||||||||
| 1092.6 | |||||||||
| 81.5 | NP-Centrodorsal-Sum | NP-Ventrolateral-Sum | |||||||
| 1037.6 | 1131.1 | ||||||||
| 89.9 | 85.3 | ||||||||
| NP-Dorsal | NP-Central | NP-Left | NP-Ventral | NP-Right | |||||
| 1046.8 | 1028.4 | 1140.6 | 1116.0 | 1136.9 | |||||
| 76.1 | 109.3 | 92.6 | 85.3 | 84.9 | |||||
| C7 | |||||||||
| 1172.3 | |||||||||
| 72.2 | AF | ||||||||
| 1245.5 | |||||||||
| 65.3 | AF-Dorsal-Sum | AF-Vental-Sum | AF-Lateral-Left | AF-Lateral-Right | |||||
| 1190.9 | 1247.5 | 1292.3 | 1325.5 | ||||||
| 60.7 | 84.2 | 75.4 | 73.8 | ||||||
| AF-Dorsal-Left | AF-Dorsal-Center | AF-Dorsal-Right | AF-Ventral-Left | AF-Ventral-Center | AF-Ventral-Right | ||||
| 1207.0 | 1221.3 | 1144.6 | 1221.9 | 1262.9 | 1257.9 | ||||
| 65.1 | 75.6 | 67.6 | 86.6 | 98.4 | 86.8 | ||||
| NP | |||||||||
| 1121.0 | |||||||||
| 86.8 | NP-Centrodorsal-Sum | NP-Ventrolateral-Sum | |||||||
| 1021.4 | 1180.7 | ||||||||
| 93.8 | 85.8 | ||||||||
| NP-Dorsal | NP-Central | NP-Left | NP-Ventral | NP-Right | |||||
| 1037.5 | 1005.2 | 1167.1 | 1213.4 | 1161.7 | |||||
| 95.3 | 96.7 | 87.4 | 93.4 | 84.4 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kramer, V.; Böttcher, P. Mapping Subchondral Bone Density Distribution in the Canine C6-C7 Vertebral Endplates: A CT-OAM Study. Animals 2023, 13, 3432. https://doi.org/10.3390/ani13223432
Kramer V, Böttcher P. Mapping Subchondral Bone Density Distribution in the Canine C6-C7 Vertebral Endplates: A CT-OAM Study. Animals. 2023; 13(22):3432. https://doi.org/10.3390/ani13223432
Chicago/Turabian StyleKramer, Vincenz, and Peter Böttcher. 2023. "Mapping Subchondral Bone Density Distribution in the Canine C6-C7 Vertebral Endplates: A CT-OAM Study" Animals 13, no. 22: 3432. https://doi.org/10.3390/ani13223432
APA StyleKramer, V., & Böttcher, P. (2023). Mapping Subchondral Bone Density Distribution in the Canine C6-C7 Vertebral Endplates: A CT-OAM Study. Animals, 13(22), 3432. https://doi.org/10.3390/ani13223432





