4.1. Milk Yield and Components
In the present research, milk production was lower than that of other breeds of dairy goats but close to that reported in the literature for goats from the local Carpathian breed [
7,
12,
28]. The milk fat, protein, and lactose contents were similar to those reported by Anghel et al. [
28] for goats of the same breed but with very different diets. Thus, neither mixed shrubs–grass rangeland nor Hs dietary supplementation negatively affect milk production and composition in Carpathian goats.
Even though the nutritional quality of the pasture (mixed shrubs–grass rangeland) was slightly better than that of hay (
Table 1 and
Table 2), milk production was lower in SG than in H (
Table 3). Thus, the most important factor driving differences in milk production between SG and H would be energy expenditure for locomotion on pasture. Based on walking distances, Steinshamn et al. [
18] calculated that goats grazing on woodland rangeland expended an average of 5.8 to 9.0 MJ NEL daily for locomotion. In addition, woody pastures often contain high amounts of tannins, which can reduce feed digestibility and utilization [
18].
The higher fat content of SG milk than H may be due to lower milk production in SG goats, which lead to an increase in the level of components in milk [
7]. On the other hand, it may be due to the mobilization of body fat in SG goats due to a lower energy balance in the diet. In this regard, relevant studies have shown that there is a correlation between the lower energy balance of the diet and the high fat content of goat milk [
18,
29].
The study previously carried out by Min et al. [
30] also found a similar milk protein content but lower milk urea content in grazing goats than hay-fed goats. In the present study, the higher milk urea content in SG than in H indicates that the balance between energy and protein for milk protein synthesis was probably more optimal in goats fed with hay than those fed on pasture. Contrary to the results obtained in this study, Bodnár et al. [
31] concluded that grazing goats on a native-grass-only pasture had a beneficial effect on milk protein content and milk-to-cheese efficiency compared to goats fed indoors with canned forage.
In the present study, milk production and milk fat and total solids content increased when goats were fed Hs-supplemented diets (
Table 3), in agreement with the results previously obtained by Mierlita [
11,
13] in dairy sheep. Cremonesi et al. [
32] reported that the introduction of hemp seeds into the diets of goats (9.4% of DM) did not change milk production; instead, the milk fat content increased significantly (from 2.84 to 3.55%). Similarly, Emami et al. [
33] found that supplementing goat diets with 2.5% pomegranate or linseed oil increased milk fat content.
The FA profile of milk fat is majorly influenced by the degradability rate of dietary fat in the rumen. In this regard, oilseeds with a high ruminal degradability (e.g., rapeseed) negatively affect milk fat content, unlike oilseeds that have a low ruminal degradability (e.g., flaxseed) [
34]. Therefore, it can be assumed that hemp seeds have a low ruminal degradability and slowly release unsaturated FAs in the rumen, reducing the amount of FAs transformed in the rumen via biohydrogenation, favoring the increase in milk fat [
35].
Dietary supplementation with Hs did not affect milk protein content (
Table 3), which is in agreement with other reports [
36,
37] but different from the results reported by Cozma et al. [
12], where supplementing the diet with hemp oil had a positive effect.
In the present study, dietary supplementation with Hs led to a significant decrease in milk cholesterol levels (
Table 3), contrary to Cozma et al. [
12], who reported that dietary supplementation with hemp oil had no influence on the concentration of cholesterol in goat milk. Results similar to those obtained in this study were reported in Holstein cows, when dietary supplementation with vegetable oil (soybean oil, rapeseed oil) decreased the milk cholesterol concentration [
38].
4.2. Fatty Acid Composition of Milk
Although many studies in the literature have shown that grazing increases milk MUFAs and PUFAs and decreases SFAs compared to hay feeding [
11,
13,
18,
19,
39,
40], in the present study, the proportion of MUFA in milk was not affected by the type of basic forage in the goats’ diets (
Table 4 and
Table 5). The difference could be explained by the good quality of the hay diet, which included concentrates and induced a healthier fatty acid profile in the milk fat [
41]. Similar to the results obtained in this study, De Lucena et al. [
42] found a higher proportion of UFA and lower SFA in the milk of goats fed tannin-rich diets, because tannins from woody pastures reduced FA biohydrogenation in the rumen. In addition, it is known that increasing the flow of unsaturated FAs into the mammary gland reduces the proportion of short- and medium-chain saturated FAs in milk fat by reducing acetyl-CoA carboxylase activity [
43]. The presence of tannins in woody pastures probably affected nutrient absorption and minimized de novo FA synthesis in the mammary gland, accentuating the mobilization of FA from adipose tissue in the goats’ bodies [
10]. Thus, the milk of SG goats had lower concentrations of short- and medium-chain FAs (mainly C16:0) and increased proportions of long-chain FAs, mainly C18:0 and C18:1, which originate from adipose tissue mobilized from the body [
10].
Grazing is known to increase the concentration of FAs that have positive effects on human health (in particular, C18:1 t-11, CLA c-9,t-11, and n-3 FA) in milk fat [
11,
13,
18,
19]. In the present study, the higher proportion of healthy FAs (C18:1 t-11, CLA c-9,t-11, C18:3 n-3, C20:5 n-3, and C22:5 n-3) in milk produced in SG than in H can be explained by a higher supply of precursors (C18:2 n-6 and C18:3 n-3) present in the pasture (shrubs–grass mixed pasture) than in hay. In addition, the high content of tannins in woody pastures affects the biohydrogenation processes of dietary FA in the rumen. For example, the high content of tannins from woody pastures caused an increase in the proportion of LA and ALA in sheep milk [
44]. The alteration of the ruminal biohydrogenation process due to tannins from woody pastures could also explain the higher concentration of long-chain n-3 FA (EPA and DPA) resulting from the conversion of dietary ALA (C18:3 n-3) [
45].
Although grazing increases the intake of UFA and mainly PUFA [
39,
40], in our study, SG goats had a higher content of C18:0 and similar MUFA content in milk fat with H goats, particularly as a result of higher C18:1 in the milk of SG goats (
Table 4 and
Table 5). Previous studies reported a higher rate of FA biohydrogenation (C18:1 → C18:0) for fresh feed [
46], which negates the effect of higher PUFA consumption in SG goats. A higher amount of substrate (C18:0) determined a higher proportion of C18:1 in the milk of SG goats due to the fact that a part of C18:0 through ∆9-desaturase activity is converted to C18:1 [
39]. The higher proportion of C18:0 in SG milk than in H milk could also be explained by the lower desaturation of C18:0 into C18:1 in the mammary gland, as shown by ∆9C18 activity (
Table 5).
The content of RA (rumenic acid) was higher in the milk of pasture-fed goats, which agreed with the results reported by Decandia et al. [
47], who found a higher RA in the milk of goats grazing on a shrub-dominated pasture than in the milk of goats fed indoors with canned forage. Grazing has been shown to significantly improve the development of rumen bacteria (such as
Butyrivibrio fibrisolvens), with a positive effect on the production of VA and CLA isomers [
39]. VA is converted to RA (CLA c-9,t-11) by Δ9-desaturase in the mammary gland and some human tissues [
48].
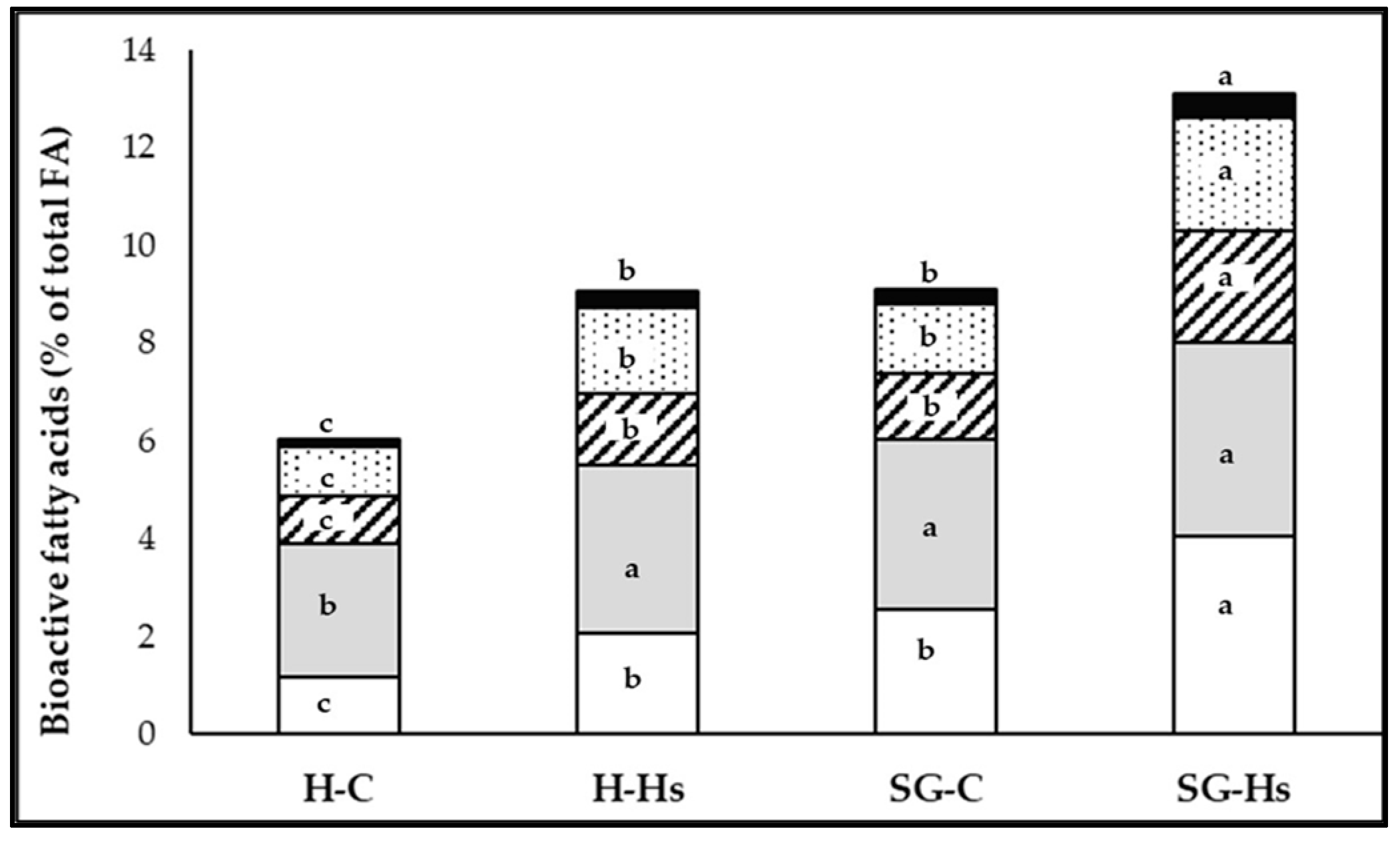
The milk of goats supplemented with Hs (H-Hs and SG-Hs) had lower proportions of SFA (mainly FAs with atherogenic effects: 12:0, 14:0, and 16:0) and higher concentrations of MUFAs (mainly 18:1 c-9 and 18:1 t-11) and PUFAs (mainly RA and ALA) than the milk of the groups that received a standard concentrate (
Table 4 and
Table 5), as reported by previous studies, when the goats’ diet was supplemented with hemp oil [
11] or linseed [
37,
49].
Supplementation of the diet with Hs decreased the proportion of de novo synthesized FA (C10:0, C12:0, C14:0, and C16:0) in milk fat, as the increased amount of long-chain FAs reduced the activity of acetyl -CoA carboxylase in the mammary gland [
50].
The high proportion of MUFA found in the milk fat of goats supplemented with Hs can be related to the higher intake of LA from Hs, which, through rumen biohydrogenation, led to a significant increase in the proportion of oleic acid (C18:1 c-9, p < 0.001). On the other hand, the high biohydrogenation of dietary C18:2 n-6 in the rumen in goats supplemented with Hs is also supported by the higher proportion of C18:0 compared to milk from goats that did not receive Hs feed (H-C and SG-C groups) (p < 0.001).
The higher level of PUFA in the milk of goats supplemented with Hs was due to the higher levels of LA and ALA in Hs (
Table 2). The high proportion of ALA in Hs ensured higher concentrations of n-3 FAs (ALA, EPA, and DHA) in milk fat. The concentration of ALA found in goat milk in our research was considerably higher (1.42% of total FA) than the value reported by Zan et al. [
51] (0.88%) in goats grazing on alpine pasture, but the total number of FAs identified in their study was higher. However, Cozma et al. [
12] reported that dietary supplementation of goats with 4.7% hemp oil did not affect the concentration of ALA in milk fat. The lack of an increase in the concentration of ALA in milk fat compared to the present study could be attributed to the fact that ALA was provided in the form of oil (a free form) that was vulnerable to rumen biohydrogenation.
Hemp seeds, due to their high content of ALA (18.63% of total FA) compared to other vegetable oils (<9%, except for linseed [
6]), ensured a high content of n-3 FAs (ALA, EPA, and DPA) in goat’s milk, which have beneficial effects on human health. ALA in ruminant feed, mainly in the mammary gland, undergoes a series of elongation and desaturation reactions, leading to the formation of long-chain PUFAs (EPA, DPA, and DHA) [
2]. The high LA and ALA content of Hs (
Table 2) probably contributed to the higher proportion of VA and CLA c-9,t-11 (RA, rumenic acid) in milk fat. In fact, RA comes from the ruminal biohydrogenation of dietary LA and ALA but also from the endogenous synthesis carried out in the mammary gland via ∆9-desaturase, starting from VA. However, ∆9-desaturase activity (calculated as product/product + substrate) did not change significantly in the Hs-supplemented groups (
Table 5), probably due to the increased availability of PUFA in the mammary gland [
29]. Thus, we can speculate that the higher concentration of CLA c-9,t-11 in the milk of goats supplemented with Hs is due to the higher intake of the two precursors (C18:2 n-6 and C18:3 n-3) that were biohydrogenated in the rumen. In addition, fats containing large amounts of C18:2 n-6 and C18:3 n-3, as is also the case with Hs, have an inhibitory effect on cellulolytic bacteria in the rumen [
52], reducing the process of biohydrogenation of the two unsaturated FAs, and thus could positively influence the levels of rumen biohydrogenation intermediates in milk.
The ∆9-desaturase activity (∆9C14 and ∆9C16) decreased significantly when goats were fed Hs-supplemented diets, while the desaturase index (DI) increased compared to unsupplemented diets (
Table 5). A reduction in Δ9-desaturase and an increase in DI were observed in goats supplemented with linseed or soybean oil [
53] and in sheep supplemented with hemp seed or hempseed cake [
11].
In this study, the positive correlation of ALA with total C18:1, C18:1 t-11, and CLA c-9,t-11 confirms its biohydrogenation to C18:1 trans isomers. In addition, the correlation of ALA with C18:1 c-9 and CLA c-9,t-11 suggests their formation via the desaturation of C18:0 in the mammary gland.
4.3. Health-Related Lipid Indices
The sanogenic lipid indices of milk fats found in the current study fell within the values presented in the specialized literature for goat milk [
45]. The quality of milk fat is frequently evaluated based on the PUFA/SFA ratio, which should have a value above 0.45 for the prevention of cardiovascular disease and other chronic diseases [
54]. In the present study, using mixed shrubs–grass rangeland but also supplementing the diet with Hs led to a significant increase (
p < 0.001) in the PUFA/SFA ratio in milk. However, the values of the PUFA/SFA ratio were much lower than those recommended for the human diet (0.07–0.15) (
Table 6).
Nutritionists recommend foods with a low n-6/n-3 FA ratio to reduce the risk of hypertension, cardiovascular disease, diabetes, and cancer in humans [
55]. In the current study, the n-6/n-3 FA ratio of goat milk was within the recommended values (<4) for human health in all treatment groups, but the best values were obtained in goats supplemented with Hs.
The atherogenic index (AI) describes the relationship between SFAs, which are considered pro-atherogenic, and UFAs, which are considered anti-atherogenic. The thrombogenicity index (TI) assesses the propensity of FA to form clots in blood vessels. AI and TI values lower than three are considered beneficial for human health [
55]. In the present study, all milk samples fell within the recommended value; the lowest values for AI and TI were found in SG-Hs milk (goats that grazed and whose diets were supplemented with Hs), and the highest values were found in conventionally produced milk (H-C: goats were fed hay and standard concentrates). In agreement with the current study, De Lucena et al. [
52] reported a decrease in AI and TI in goat milk when the diet was high in tannins (28 g tannins per kg DM); woody pastures are known for their high tannin content. The health-promoting index (HPI) is considered the inverse of TI, so milk with a high HPI value is healthier for humans [
54]. In the present study, higher HPI values were found in SG milk, and these were within the range reported by Claps et al. [
56] (0.34–0.45) for pasture-raised goat milk. The supplementation of diets with Hs significantly increased (
p < 0.01) the HPI value (0.49–0.54).
The h/H FA ratio describes the relationship between FAs with a hypocholesterolemic effect and those with a hypercholesterolemic effect, so high values for this ratio are desirable. A higher h/H ratio was found in the milk of goats supplemented with Hs. In our study, the h/H FA ratio was in accordance with the results previously published by Chen and Liu [
54] and Bodnár et al. [
31], who found higher values for milk obtained on pastures consisting of different herbaceous species compared to milk obtained indoors with diets based on canned forages. The desirable FA (DFA) represents the sum of anti-atherogenic fatty acids (UFAs + C18:0) that lower plasma cholesterol and triacylglycerols [
54]. Thus, higher values are preferred for DFA. Higher values for DFA were found in the milk produced by SG goats (
p < 0.01) but also when the goats’ diet was supplemented with Hs (
p < 0.001) (
Table 6).
4.4. Fat-Soluble Vitamins and Antioxidant Capacity
Natural antioxidants in milk are important for human health because they can neutralize and eliminate free radicals and their harmful effects [
57]. Uncontrolled free radicals can lead to atherosclerosis, cardiovascular disease, diabetes, cancer, and the breakdown of some biochemical compounds in the diet. The results of this study showed that SG milk has a higher content (
p < 0.01) of natural antioxidants (α-tocopherol and retinol) than H milk (
Table 7). These results agree with those previously reported by Delgado-Pertínez et al. [
46] regarding the α-tocopherol content of milk obtained from goats grazing on Mediterranean shrublands, although in our study, the values were higher, probably due to the different botanical composition of the mixed shrubs–grass rangeland, the vegetation stage of the plants, and probably differences in the leaf:stem ratio or environmental factors. The lower content of α-tocopherol and retinol in H milk is due to the wilting and drying of plants, which causes oxidative degradation of these nutrients [
58].
The supplementation of the diet with Hs did not change the retinol content of milk, although Hs are a β-carotene form [
11]. The results obtained in this study are in agreement with Cozma et al. [
12], who supplemented goats’ diets with hemp oil, but in disagreement with Puppel et al. [
59], who showed that supplementing the diet with flaxseed increased milk retinol concentration in cows. The differences between these studies could be due to a species effect (goat vs. cow) or the nature of the fat supplement (hemp vs. flax). Unlike cows, goats convert all the β-carotene in their feed into retinol, so their milk has more vitamin A, which is comparable from this point of view to the milk of humans [
60].
The results of this study demonstrate that the total antioxidant capacity (TAC) of goat milk is positively correlated with the content of α-tocopherol and retinol, since these vitamins intervene in the capture of free radicals and the inhibition of lipid peroxidation [
61]. In addition, high-fat milk has been shown to have a higher antioxidant capacity, as measured using the ABTS assay, than low-fat milk due to its higher content of lipophilic vitamins; positive correlations have been established between the fat content and the TAC value of milk [
54].
Significant decreases in α-tocopherol and retinol content were observed during milk storage in all experimental variants, probably because these vitamins acted as a hydrogen donor, which led to the accumulation of lipid hydroperoxides in milk. This mechanism could be responsible for the significant decrease in the TAC of stored milk in all types of milk. The results of this study agree with those previously reported by Havemose et al. [
62] for cow’s milk.
In contrast to the results of this study, Yilmaz-Ersan et al. [
63] reported an increase in TAC in pasteurized milk (90 °C for 10 min), as measured using the ABTS test, compared to raw milk. Similarly, other studies have demonstrated that heating milk to over 100 °C increases the antioxidant capacity of milk because thiol groups formed by protein unfolding act as hydrogen donors [
61]. In the present study, milk pasteurization was performed at a lower temperature, namely 63 °C, for 30 min, which may explain why pasteurization did not change the TAC value, in agreement with other recent studies [
19,
23,
61].
After 4 days of refrigerated storage of milk, the TAC value decreased for all types of milk, suggesting that higher concentrations of α-tocopherol and retinol in milk do not prevent the oxidation of unsaturated FAs from milk fat but delay this process, increasing the oxidative stability of milk [
16].