Partially Alternative Feeding with Fermented Distillers’ Grains Modulates Gastrointestinal Flora and Metabolic Profile in Guanling Cattle
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Ethics
2.2. Preparation of FDG
2.3. Animals, Experimental Design, and Sample Collection
2.4. 16S rDNA Sequencing and Data Analysis
2.5. LC-MS-Based Metabolomics Detection and Data Analysis
2.6. Correlation Analysis
2.7. Statistical Analysis
3. Results
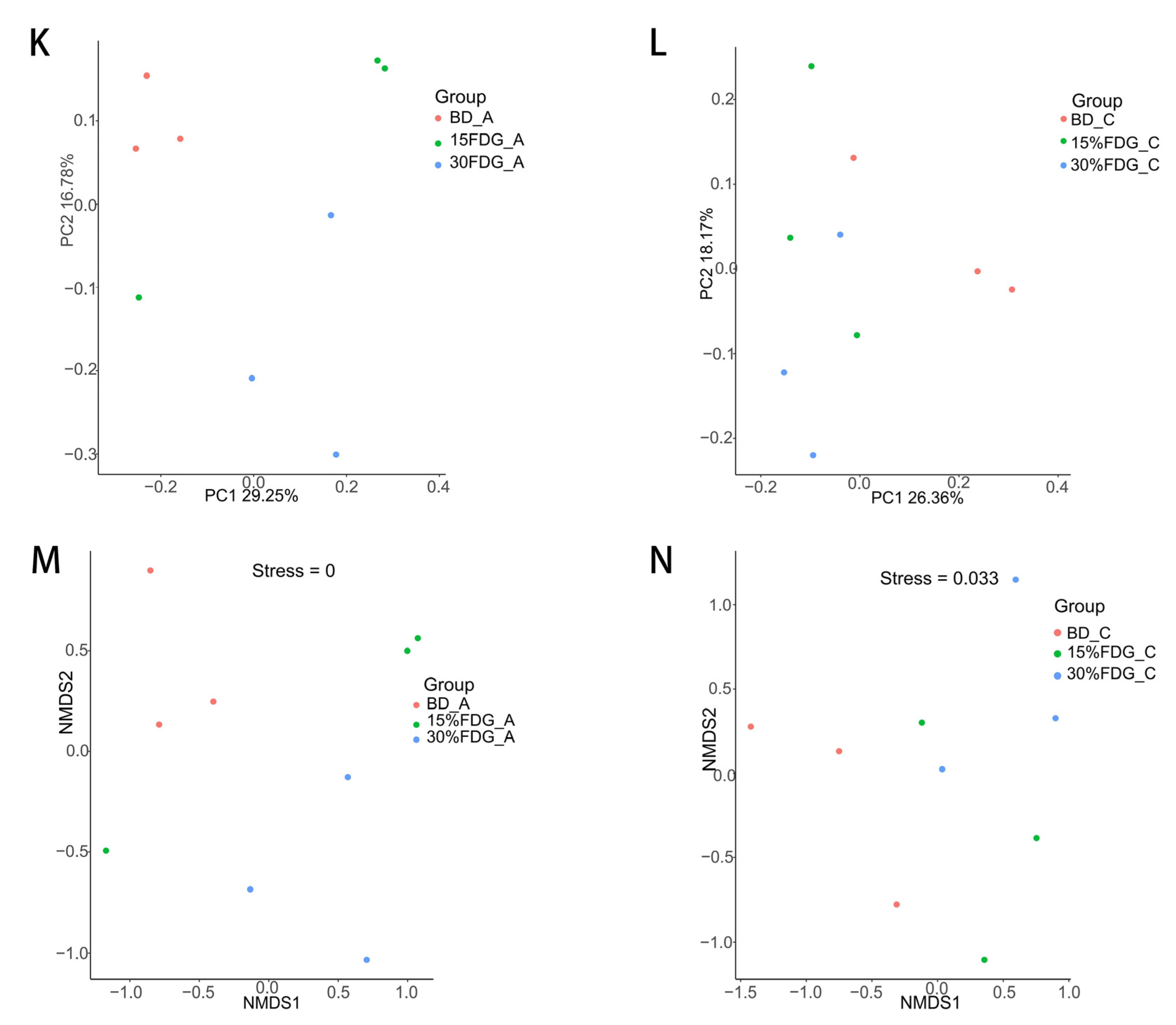
3.1. 16S rDNA Sequencing Results and Microbial Diversity Characteristics
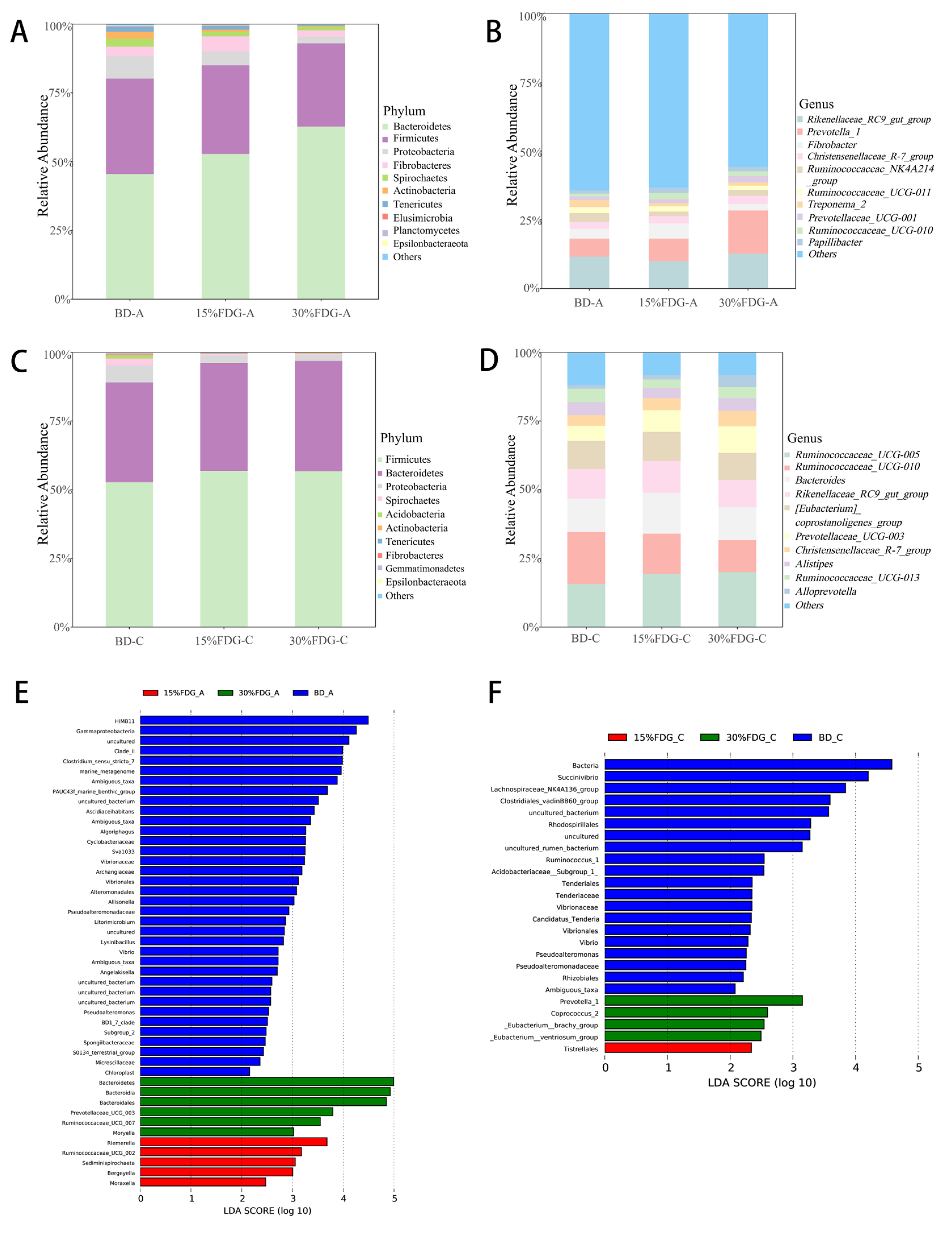
3.2. Analysis of the Structural Composition of Microbial Communities in Abomasal and Cecal Contents
3.3. Metabolomics Analysis of Abomasal and Caecal Contents
3.4. Correlation Analysis between Microbiome and Metabolome in Abomasal Content and Caecal Content
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kilama, J.; Yakir, Y.; Shaani, Y.; Adin, G.; Kaadan, S.; Wagali, P.; Sabastian, C.; Ngomuo, G.; Mabjeesh, S.J. Chemical composition, in vitro digestibility, and storability of selected agro-industrial by-products: Alternative ruminant feed ingredients in Israel. Heliyon 2023, 9, e14581. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, Y.; Xu, L.; Xin, C.; Tan, Z.; Zhang, X.; Ma, C.; Chen, S.; Li, H. Changes of the main components, physicochemical properties of distiller’s grains after extrusion processing with focus on modification mechanism. Food Chem. 2022, 390, 133187. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.P.; Wang, L.; Sun, Z.Y.; Wang, S.T.; Shen, C.H.; Tang, Y.Q.; Kida, K. Biochar addition reduces nitrogen loss and accelerates composting process by affecting the core microbial community during distilled grain waste composting. Bioresour. Technol. 2021, 337, 125492. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, N.A.; Serao, N.V.; Kerr, B.J.; Zijlstra, R.T.; Patience, J.F. Relationships among dietary fiber components and the digestibility of energy, dietary fiber, and amino acids and energy content of nine corn coproducts fed to growing pigs. J. Anim. Sci. 2014, 92, 4505–4517. [Google Scholar] [CrossRef]
- Iram, A.; Cekmecelioglu, D.; Demirci, A. Distillers’ dried grains with solubles (DDGS) and its potential as fermentation feedstock. Appl. Microbiol. Biotechnol. 2020, 104, 6115–6128. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, C.; Su, W.; Jiang, Z.; He, H.; Gong, T.; Kai, L.; Xu, H.; Wang, Y.; Lu, Z. Co-fermented yellow wine lees by Bacillus subtilis and Enterococcus faecium regulates growth performance and gut microbiota in finishing pigs. Front. Microbiol. 2022, 13, 1003498. [Google Scholar] [CrossRef]
- Zhai, S.S.; Tian, L.; Zhang, X.F.; Wang, H.; Li, M.M.; Li, X.C.; Liu, J.L.; Ye, H.; Wang, W.C.; Zhu, Y.W.; et al. Effects of sources and levels of liquor distiller’s grains with solubles on the growth performance, carcass characteristics, and serum parameters of Cherry Valley ducks. Poult. Sci. 2020, 99, 6258–6266. [Google Scholar] [CrossRef]
- Zengin, M.; Sur, A.; Ilhan, Z.; Azman, M.A.; Tavsanli, H.; Esen, S.; Bacaksiz, O.K.; Demir, E. Effects of fermented distillers grains with solubles, partially replaced with soybean meal, on performance, blood parameters, meat quality, intestinal flora, and immune response in broiler. Res. Vet. Sci. 2022, 150, 58–64. [Google Scholar] [CrossRef]
- Yao, K.; Jiang, L.; Liu, J.; Wang, D.; Liu, H.; Ren, D. Effect of Yellow Wine Lees Supplementation on Milk Antioxidant Capacity and Hematological Parameters in Lactating Cows under Heat Stress. Animals 2021, 11, 2643. [Google Scholar] [CrossRef]
- Barko, P.C.; McMichael, M.A.; Swanson, K.S.; Williams, D.A. The Gastrointestinal Microbiome: A Review. J. Vet. Intern. Med. 2018, 32, 9–25. [Google Scholar] [CrossRef]
- Mukherjee, A.G.; Wanjari, U.R.; Bradu, P.; Murali, R.; Kannampuzha, S.; Loganathan, T.; Doss C., G.P.; Prakash, B.P.A.; Renu, K.; Dey, A.; et al. The crosstalk of the human microbiome in breast and colon cancer: A metabolomics analysis. Crit. Rev. Oncol. Hemat. 2022, 176, 103757. [Google Scholar] [CrossRef] [PubMed]
- Zhi, T.; Ma, A.; Liu, X.; Chen, Z.; Li, S.; Jia, Y. Dietary Supplementation of Brevibacillus laterosporus S62-9 Improves Broiler Growth and Immunity by Regulating Cecal Microbiota and Metabolites. Probiotics Antimicrob. Proteins, 2023; ahead of print. [Google Scholar] [CrossRef]
- Guo, X.; Huang, T.; Yin, X.; Yang, J.; Zhang, Z. Research on the development of breeding cooperatives in Guizhou Province under the background of rural revitalization—A case study of Guanling yellow cattle. Rural. Econ. Technol. 2021, 32, 32–34. [Google Scholar]
- Cheng, Q.; Xu, D.; Chen, Y.; Zhu, M.; Fan, X.; Li, M.; Tang, X.; Liao, C.; Li, P.; Chen, C. Influence of Fermented-Moutai Distillers’ Grain on Growth Performance, Meat Quality, and Blood Metabolites of Finishing Cattle. Front. Vet. Sci. 2022, 9, 874453. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Guo, C.; Zhou, L.; Zhong, Z.; Zhu, W.; Huang, Y.; Zhang, Z.; Gorgels, T.G.; Berendschot, T.T. Effects of dietary supplementation with epidermal growth factor-expressing Saccharomyces cerevisiae on duodenal development in weaned piglets. Br. J. Nutr. 2016, 115, 1509–1520. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhu, Q.; Azad, M.; Li, H.; Huang, P.; Kong, X. The Impacts of Dietary Fermented Mao-tai Lees on Growth Performance, Plasma Metabolites, and Intestinal Microbiota and Metabolites of Weaned Piglets. Front. Microbiol. 2021, 12, 778555. [Google Scholar] [CrossRef]
- O’Hara, E.; Neves, A.; Song, Y.; Guan, L.L. The Role of the Gut Microbiome in Cattle Production and Health: Driver or Passenger? Annu. Rev. Anim. Biosci. 2020, 8, 199–220. [Google Scholar] [CrossRef]
- Li, H.; Li, H.; Xie, P.; Li, Z.; Yin, Y.; Blachier, F.; Kong, X. Dietary supplementation with fermented Mao-tai lees beneficially affects gut microbiota structure and function in pigs. AMB Express 2019, 9, 26. [Google Scholar] [CrossRef]
- Torres, M.M.; Gizzi, F.O.; Martin, M.; Espariz, M.; Magni, C.; Blancato, V.S. Metagenomic approach to infer rumen microbiome derived traits of cattle. World J. Microbiol. Biotechnol. 2023, 39, 250. [Google Scholar] [CrossRef]
- Mariat, D.; Firmesse, O.; Levenez, F.; Guimaraes, V.; Sokol, H.; Dore, J.; Corthier, G.; Furet, J.P. The Firmicutes/Bacteroidetes ratio of the human microbiota changes with age. BMC Microbiol. 2009, 9, 123. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef]
- Jiang, X.; Xu, H.J.; Ma, G.M.; Sun, Y.K.; Li, Y.; Zhang, Y.G. Digestibility, lactation performance, plasma metabolites, ruminal fermentation, and bacterial communities in Holstein cows fed a fermented corn gluten-wheat bran mixture as a substitute for soybean meal. J. Dairy Sci. 2021, 104, 2866–2880. [Google Scholar] [CrossRef] [PubMed]
- Petri, R.M.; Munnich, M.; Zebeli, Q.; Klevenhusen, F. Graded replacement of corn grain with molassed sugar beet pulp modulates the fecal microbial community and hindgut fermentation profile in lactating dairy cows. J. Dairy Sci. 2019, 102, 5019–5030. [Google Scholar] [CrossRef] [PubMed]
- La Reau, A.J.; Suen, G. The Ruminococci: Key symbionts of the gut ecosystem. J. Microbiol. 2018, 56, 199–208. [Google Scholar] [CrossRef]
- Ning, K.; Lu, K.; Chen, Q.; Guo, Z.; Du, X.; Riaz, F.; Feng, L.; Fu, Y.; Yin, C.; Zhang, F.; et al. Epigallocatechin Gallate Protects Mice against Methionine-Choline-Deficient-Diet-Induced Nonalcoholic Steatohepatitis by Improving Gut Microbiota to Attenuate Hepatic Injury and Regulate Metabolism. ACS Omega 2020, 5, 20800–20809. [Google Scholar] [CrossRef]
- De Wit, N.; Derrien, M.; Bosch-Vermeulen, H.; Oosterink, E.; Keshtkar, S.; Duval, C.; de Vogel-van den Bosch, J.; Kleerebezem, M.; Muller, M.; van der Meer, R. Saturated fat stimulates obesity and hepatic steatosis and affects gut microbiota composition by an enhanced overflow of dietary fat to the distal intestine. Am. J. Physiol.-Gastrointest. Liver Physiol. 2012, 303, G589–G599. [Google Scholar] [CrossRef]
- Devillard, E.; McIntosh, F.M.; Duncan, S.H.; Wallace, R.J. Metabolism of linoleic acid by human gut bacteria: Different routes for biosynthesis of conjugated linoleic acid. J. Bacteriol. 2007, 189, 2566–2570. [Google Scholar] [CrossRef] [PubMed]
- Baddini, F.A.; Fernandes, P.A.; Ferreira, D.C.N.; Goncalves, R.B. Conjugated linoleic acid (CLA): Effect modulation of body composition and lipid profile. Nutr. Hosp. 2009, 24, 422–428. [Google Scholar]
- Devillard, E.; McIntosh, F.M.; Paillard, D.; Thomas, N.A.; Shingfield, K.J.; Wallace, R.J. Differences between human subjects in the composition of the faecal bacterial community and faecal metabolism of linoleic acid. Microbiology-SGM 2009, 155, 513–520. [Google Scholar] [CrossRef]
- Chen, H.; Jia, Z.; He, M.; Chen, A.; Zhang, X.; Xu, J.; Wang, C. Arula-7 powder improves diarrhea and intestinal epithelial tight junction function associated with its regulation of intestinal flora in calves infected with pathogenic Escherichia coli O(1). Microbiome 2023, 11, 172. [Google Scholar] [CrossRef]
- Gupta, R.S.; Mahmood, S.; Adeolu, M. A phylogenomic and molecular signature based approach for characterization of the phylum Spirochaetes and its major clades: Proposal for a taxonomic revision of the phylum. Front. Microbiol. 2013, 4, 217. [Google Scholar] [CrossRef]
- Gotley, D.C.; Morgan, A.P.; Ball, D.; Owen, R.W.; Cooper, M.J. Composition of gastro-oesophageal refluxate. Gut 1991, 32, 1093–1099. [Google Scholar] [CrossRef]
- Liu, R.; Li, X.; Hylemon, P.B.; Zhou, H. Conjugated Bile Acids Promote Invasive Growth of Esophageal Adenocarcinoma Cells and Cancer Stem Cell Expansion via Sphingosine 1-Phosphate Receptor 2-Mediated Yes-Associated Protein Activation. Am. J. Pathol. 2018, 188, 2042–2058. [Google Scholar] [CrossRef] [PubMed]
- Rezen, T.; Rozman, D.; Kovacs, T.; Kovacs, P.; Sipos, A.; Bai, P.; Miko, E. The role of bile acids in carcinogenesis. Cell. Mol. Life Sci. 2022, 79, 243. [Google Scholar] [CrossRef] [PubMed]
- Jiao, N.; Baker, S.S.; Chapa-Rodriguez, A.; Liu, W.; Nugent, C.A.; Tsompana, M.; Mastrandrea, L.; Buck, M.J.; Baker, R.D.; Genco, R.J.; et al. Suppressed hepatic bile acid signalling despite elevated production of primary and secondary bile acids in NAFLD. Gut 2018, 67, 1881–1891. [Google Scholar] [CrossRef]
- Motohashi, H.; Tahara, Y.; Whittaker, D.S.; Wang, H.B.; Yamaji, T.; Wakui, H.; Haraguchi, A.; Yamazaki, M.; Miyakawa, H.; Hama, K.; et al. The circadian clock is disrupted in mice with adenine-induced tubulointerstitial nephropathy. Kidney Int. 2020, 97, 728–740. [Google Scholar] [CrossRef] [PubMed]
- Kuang, H.; Sun, X.; Liu, Y.; Tang, M.; Wei, Y.; Shi, Y.; Li, R.; Xiao, G.; Kang, J.; Wang, F.; et al. Palmitic acid-induced ferroptosis via CD36 activates ER stress to break calcium-iron balance in colon cancer cells. FEBS J. 2023, 290, 3664–3687. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yang, X.; Wu, J.; Ye, S.; Gong, J.; Cheng, W.M.; Luo, Z.; Yu, J.; Liu, Y.; Zeng, W.; et al. Reprogramming of palmitic acid induced by dephosphorylation of ACOX1 promotes beta-catenin palmitoylation to drive colorectal cancer progression. Cell Discov. 2023, 9, 26. [Google Scholar] [CrossRef]
- Nava, L.C.; Tiberti, S.; Corsetto, P.A.; Conte, F.; Tyagi, P.; Machwirth, M.; Ebert, S.; Loffreda, A.; Scheller, L.; Sheta, D.; et al. Linoleic acid potentiates CD8(+) T cell metabolic fitness and antitumor immunity. Cell Metab. 2023, 35, 633–650. [Google Scholar] [CrossRef]
- Fuchs, D.; Hamberg, M.; Skold, C.M.; Wheelock, A.M.; Wheelock, C.E. An LC-MS/MS workflow to characterize 16 regio- and stereoisomeric trihydroxyoctadecenoic acids. J. Lipid Res. 2018, 59, 2025–2033. [Google Scholar] [CrossRef]
- Shirahata, T.; Sunazuka, T.; Yoshida, K.; Yamamoto, D.; Harigaya, Y.; Nagai, T.; Kiyohara, H.; Yamada, H.; Kuwajima, I.; Omura, S. Total synthesis and adjuvant activity of all stereoisomers of pinellic acid. Bioorg. Med. Chem. Lett. 2003, 13, 937–941. [Google Scholar] [CrossRef]
- D’Mello, S.A.; Finlay, G.J.; Baguley, B.C.; Askarian-Amiri, M.E. Signaling Pathways in Melanogenesis. Int. J. Mol. Sci. 2016, 17, 1144. [Google Scholar] [CrossRef]
- Wu, S.; Han, J.; Laden, F.; Qureshi, A.A. Long-term ultraviolet flux, other potential risk factors, and skin cancer risk: A cohort study. Cancer Epidemiol. Biomark. Prev. 2014, 23, 1080–1089. [Google Scholar] [CrossRef]
- Pitchot, W.; Scantamburlo, G.; Pinto, E.; Karila, L. [Cocaine addiction]. Rev. Med. Liège 2013, 68, 294–297. [Google Scholar] [PubMed]
- Bramness, J.G.; Rognli, E.B. Psychosis induced by amphetamines. Curr. Opin. Psychiatry 2016, 29, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Martin-Gonzalez, C.; Ribot-Hernandez, I.; Fernandez-Rodriguez, C.; Gonzalez-Navarrete, L.; Vera-Delgado, V.; Rodriguez-Gaspar, M.; Martinez-Riera, A.; Gonzalez-Reimers, E. Incidence of cancer and mortality among heavy alcoholics. Clin. Nutr. ESPEN 2020, 37, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Lv, R.; Du, L.; Zhou, F.; Yuan, X.; Liu, X.; Zhang, L. Rosmarinic Acid Alleviates Inflammation, Apoptosis, and Oxidative Stress through Regulating miR-155-5p in a Mice Model of Parkinson’s Disease. ACS Chem. Neurosci. 2020, 11, 3259–3266. [Google Scholar] [CrossRef]
- Hitl, M.; Kladar, N.; Gavaric, N.; Bozin, B. Rosmarinic Acid-Human Pharmacokinetics and Health Benefits. Planta Med. 2021, 87, 273–282. [Google Scholar] [CrossRef]
- Correia, A.S.; Vale, N. Tryptophan Metabolism in Depression: A Narrative Review with a Focus on Serotonin and Kynurenine Pathways. Int. J. Mol. Sci. 2022, 23, 8493. [Google Scholar] [CrossRef]
- Davidson, M.; Rashidi, N.; Nurgali, K.; Apostolopoulos, V. The Role of Tryptophan Metabolites in Neuropsychiatric Disorders. Int. J. Mol. Sci. 2022, 23, 9968. [Google Scholar] [CrossRef]
- Shimizu, S. Pantothenic acid. Nihon Rinsho 1999, 57, 2218–2222. [Google Scholar]




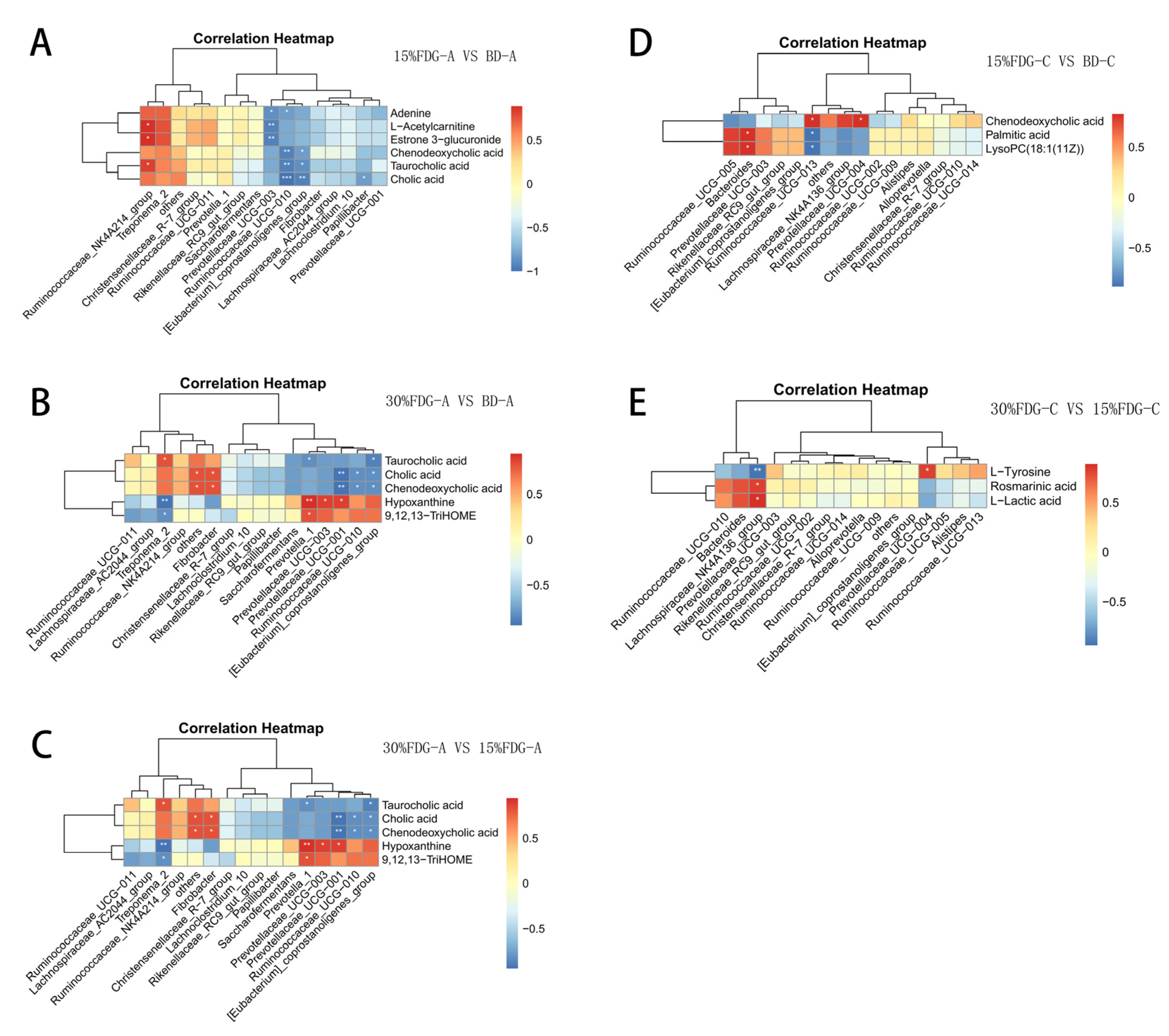
| Nos. | Metabolites | VIP | p-Value | FC | Annotation |
|---|---|---|---|---|---|
| 15%FDG-A vs. BD-A | |||||
| 1 | Adenine | 1.023 | <0.001 | 0.025 | Purine metabolism |
| 2 | Chenodeoxycholic acid | 2.119 | 0.037 | 0.002 | Primary bile acid biosynthesis |
| 3 | Cholic acid | 13.380 | 0.005 | 0.031 | Primary bile acid biosynthesis|Bile secretion |
| 4 | Estrone 3-glucuronide | 1.037 | 0.026 | 0.072 | Steroid hormone biosynthesis |
| 5 | L-Acetylcarnitine | 1.637 | 0.008 | 0.094 | Insulin resistance |
| 6 | Taurocholic acid | 41.737 | 0.028 | 0.230 | Primary bile acid biosynthesis| Bile secretion|Cholesterol metabolism| Taurine and hypotaurine metabolism |
| 30%FDG-A vs. BD-A | |||||
| 1 | 9, 12, 13-TriHOME | 1.104 | 0.003 | 4.171 | Linoleic acid metabolism |
| 2 | Chenodeoxycholic acid | 1.779 | 0.041 | 0.033 | Primary bile acid biosynthesis |
| 3 | Cholic acid | 9.674 | 0.023 | 0.252 | Primary bile acid biosynthesis|Bile secretion |
| 4 | Hypoxanthine | 3.987 | <0.001 | 7.063 | Purine metabolism |
| 5 | Taurocholic acid | 40.776 | 0.001 | 0.119 | Primary bile acid biosynthesis| Bile secretion|Cholestero lmetabolism| Taurine and hypotaurine metabolism |
| 30%FDG-A vs. 15%FDG-A | |||||
| 1 | 3-(3-Indolyl)-2-oxopropanoic acid | 1.220 | 0.046 | 2.822 | Tryptophan metabolism |
| 2 | Guanine | 1.417 | 0.006 | 3.135 | Purine metabolism |
| 3 | Hypoxanthine | 10.526 | 0.001 | 5.143 | Purine metabolism |
| 4 | L-Tyrosine | 1.740 | 0.029 | 2.901 | Melanogenesis|Cocaine addiction|Amphetamine addiction|Alcoholism|Prolactin signaling pathway|Dopaminergic synapse|Parkinson disease|Thiamine metabolism|Phenylalanine, tyrosine and tryptophan biosynthesis|Aminoacyl-tRNA biosynthesis|Phenylalanine metabolism|Ubiquinone and other terpenoid-quinone biosynthesis|Tyrosine metabolism |
| 5 | Pantothenic acid | 1.385 | 0.027 | 6.518 | Pantothenate and CoA biosynthesis|beta-Alanine metabolism|Vitamin digestion and absorption |
| 15% FDG-C vs. BD-C | |||||
| 1 | Chenodeoxycholic acid | 13.983 | 0.002 | 0.448 | Primary bile acid biosynthesis |
| 2 | LysoPC(18:1(11Z)) | 1.126 | 0.011 | 4.981 | Choline metabolism in cancer| Glycerophospholipid metabolism |
| 3 | Palmitic acid | 3.628 | 0.021 | 3.297 | Fatty acid elongation|Fatty acid degradation| Fatty acid biosynthesis| Biosynthesis of unsaturated fatty acids |
| 30% FDG-C vs. 15% FDG-C | |||||
| 1 | L-Lactic acid | 1.057 | 0.046 | 0.310 | HIF-1 signaling pathway|cAMP signaling pathway|Glucagon signaling pathway|Central carbon metabolism in cancer|Glycolysis/Gluconeogenesis|Pyruvate metabolism|Propanoate metabolism|Fructose and mannose metabolism |
| 2 | L-Tyrosine | 1.343 | 0.031 | 1.697 | Tyrosine metabolism|Melanogenesis|Cocaine addiction|Amphetamine addiction|Alcoholism|Prolactin signaling pathway|Dopaminergic synapse|Parkinson disease|Thiamine metabolism|Phenylalanine, tyrosine and tryptophan biosynthesis|Aminoacyl-tRNA biosynthesis|Phenylalanine metabolism|Ubiquinone and other terpenoid-quinone biosynthesis |
| 3 | Rosmarinic acid | 1.981 | 0.028 | 0.309 | Tyrosine metabolism |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, G.; Chen, C.; Mei, S.; Chen, Z.; Zhang, R.; Zhang, T.; Xu, D.; Zhu, M.; Luo, X.; Zeng, C.; et al. Partially Alternative Feeding with Fermented Distillers’ Grains Modulates Gastrointestinal Flora and Metabolic Profile in Guanling Cattle. Animals 2023, 13, 3437. https://doi.org/10.3390/ani13223437
He G, Chen C, Mei S, Chen Z, Zhang R, Zhang T, Xu D, Zhu M, Luo X, Zeng C, et al. Partially Alternative Feeding with Fermented Distillers’ Grains Modulates Gastrointestinal Flora and Metabolic Profile in Guanling Cattle. Animals. 2023; 13(22):3437. https://doi.org/10.3390/ani13223437
Chicago/Turabian StyleHe, Guangxia, Chao Chen, Shihui Mei, Ze Chen, Rong Zhang, Tiantian Zhang, Duhan Xu, Mingming Zhu, Xiaofen Luo, Chengrong Zeng, and et al. 2023. "Partially Alternative Feeding with Fermented Distillers’ Grains Modulates Gastrointestinal Flora and Metabolic Profile in Guanling Cattle" Animals 13, no. 22: 3437. https://doi.org/10.3390/ani13223437




