The Effects of Stocking Density and Food Deprivation on Mucous Cells and Lysozyme Activity in the Skin and Gills of Silver Catfish
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. The Experiment and Animals
2.2. Water Quality Parameters
2.3. Collection of Biological Material
2.4. Biological Material Analysis
2.5. Statistical Analysis
3. Results
3.1. Skin Analysis in the Basal Group
3.2. Cutaneous Response to Stocking Density and Food Deprivation
3.3. Gill Response to Stocking Density and Food Deprivation
| HSD | LSD | |
|---|---|---|
| Gill filaments | 77.59 ± 6.41 | 59.16 ± 7.27 * |
| Gill lamellae | 76.24 ± 6.43 | 59.22 ± 7.29 * |
| HSD-F | HSD-FS | LSD-F | LSD-FS | |
|---|---|---|---|---|
| Lysozyme activity | 75.0 ± 12.2 c | 133.0 ± 22.7 a | 94.5 ± 13.3 b | 72.0 ± 10.2 c |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Esteban, M.A. An overview of the immunological defenses in fish skin. Int. Sch. Res. Not. 2012, 2012, 853470. [Google Scholar] [CrossRef]
- Van der Salm, A.L.; Nolan, D.T. In vitro evidence that cortisol directly modulates stress-related responses in the skin epidermis of the rainbow trout (Oncorhynchus mykiss Walbaum). Fish Physiol. Biochem. 2004, 27, 9–18. [Google Scholar] [CrossRef]
- Alverez-Pellitero, P. Fish immunity and parasite infections: From innate immunity to prophylactic prospects. Vet. Immunol. Immunopathol. 2008, 126, 171–198. [Google Scholar] [CrossRef] [PubMed]
- Guardiola, F.A.; Cuesta, A.; Abellán, E.; Meseguer, J.; Esteban, M.A. Comparative analysis of the humoral immunity of skin mucus from several marine teleost fish. Fish Shellfish Immunol. 2014, 40, 24–31. [Google Scholar] [CrossRef]
- Portz, D.E.; Woodley, C.M.; Cech, J.J. Stress-associated impacts of short-term holding on fishes. Rev. Fish Biol. Fish. 2006, 16, 125–170. [Google Scholar] [CrossRef]
- Wu, S.M.; Liu, J.-H.; Shu, L.-H.; Chen, C.H. Anti-oxidative responses of zebrafish (Danio rerio) gill, liver and brain tissues upon acute cold shock. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2015, 187, 202–213. [Google Scholar] [CrossRef]
- Fernández-Montero, A.; Torrecillas, S.; Tort, L.; Ginés, R.; Acosta, F.; Izquierdo, M.; Montero, D. Stress response and skin mucus production of greater amberjack (Seriola dumerili) under different rearing conditions. Aquaculture 2020, 520, 735005. [Google Scholar] [CrossRef]
- Gisbert, E.; Luz, R.K.; Fernández, I.; Pradhan, P.K.; Salhi, M.; Mozanzadeh, M.T.; Kumar, A.; Kotzamanis, Y.; Castro-Ruiz, D.; Bessonart, M.; et al. Development, nutrition, and rearing practices of relevant catfish species (Siluriformes) at early stages. Rev. Aquac. 2022, 14, 73–105. [Google Scholar] [CrossRef]
- Barcellos, L.J.G.; Marqueze, A.; Trapp, M.; Quevedo, R.M.; Ferreira, D. The effects of fasting on cortisol, blood glucose and liver and muscle glycogen in adult jundiá Rhamdia quelen. Aquaculture 2010, 300, 231–236. [Google Scholar] [CrossRef]
- Barcellos, L.J.G.; Kreutz, L.C. Nursery rearing of jundiá, Rhamdia quelen (Quoy & Gaimard) in cases: Cage type, stocking density and stress response to confinement. Aquaculture 2004, 232, 383–394. [Google Scholar]
- Piaia, R.; Baldisserotto, B. Densidade de estocagem e crescimento de alevinos de jundiá Rhamdia quelen (Quoy & Gaimard, 1824). Ciência Rural 2000, 30, 509–513. [Google Scholar]
- Cordero, H.; Ceballos-Francisco, D.; Cuesta, A.; Esteban, M.A.Â. Dorso-ventral skin characterization of the farmed fish gilthead seabream (Sparus aurata). PLoS ONE 2017, 12, e0180438. [Google Scholar] [CrossRef] [PubMed]
- Pickering, A.D. The distribution of mucous cells in the epidermis of the brown trout Salmo trutta (L.) and the char Salvelinus alpinus (L.). J. Fish Biol. 1974, 6, 111–118. [Google Scholar] [CrossRef]
- Vastos, I.N.; Kotzamanis, M. Monitoring stress in fish by applying image analysis to their skin mucous cells. Eur. J. Histochem. 2010, 54, e22. [Google Scholar]
- Da Cunha, M.A.; Zeppenfeld, C.C.; Garcia, L.d.O.; Loro, V.L.; da Fonseca, M.B.; Emanuelli, T.; Veeck, A.P.d.L.; Copatti, C.E.; Baldisserotto, B. Anesthesia of silver catfish with eugenol: Time of induction, cortisol response and sensory analysis of fillet. Ciência Rural 2010, 40, 2107–2114. [Google Scholar] [CrossRef]
- Menezes, C.; Ruiz-Jarabo, I.; Martos-Sitcha, J.A.; Toni, C.; Salbego, J.; Becker, A.; Loro, V.L.; Martínez-Rodríguez, G.; Mancera, J.M.; Baldisserotto, B. The influence of stocking density and food deprivation in silver catfish (Rhamdia quelen): A metabolic and endocrine approach. Aquaculture 2015, 435, 257–264. [Google Scholar] [CrossRef]
- Da Silva, H.N.P.; Almeida, A.P.G.; Souza, C.d.F.; Mancera, J.M.; Martos-Sitcha, J.A.; Martínez-Rodríguez, G.; Baldisserotto, B. Stress response of Rhamdia quelen to the interaction stocking density—Feeding regimen. Gen. Comp. Endocrinol. 2023, 335, 114228. [Google Scholar] [CrossRef]
- Verdouw, H.; Van Echteld, C.; Dekkers, E. Ammonia determination based on indophenol formation with sodium salicylate. Water Res. 1978, 12, 399–402. [Google Scholar] [CrossRef]
- Eaton, A.D.; Franson, M.A.H.; Clesceri, L.S.; Rice, E.W.; Greenberg, A.E. Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association: Washington, DC, USA, 2005; 1325p. [Google Scholar]
- Nigam, A.K.; Kumari, U.; Mittal, S.; Mittal, A.K. Comparative analysis of innate immune parameters of the skin mucous secretions from certain freshwater teleosts; inhabiting different ecological niches. Fish. Physiol. Biochem. 2012, 38, 1245–1256. [Google Scholar] [CrossRef]
- Palaksha, K.J.; Shin, G.W.; Kim, Y.R.; Jung, T.S. Evaluation of non-specific immune components from the skin mucus of olive flounder (Paralichthys olivaceus). Fish Shellfish Immunol. 2008, 24, 479–488. [Google Scholar] [CrossRef]
- Mang, P.V.S.L.; Jiraungkoorskul, W. Comparative Analysis of Morphometric Characteristics and Mucous Cell Distribution between Pangasius hypophthalmus and Clarias batrachus. Egypt. J. Aquat. Biol. Fish. 2020, 24, 351–364. [Google Scholar] [CrossRef]
- Jørgensen, J.B.; Sharp, G.J.; Secombes, C.J.; Robertsen, B. Effect of a cell wall glucan yeast on the bactericidal activity of rainbow trout macrophages. Fish Shellfish Immunol. 1993, 3, 267–277. [Google Scholar] [CrossRef]
- Kreutz, L.C.; Barcellos, L.J.G. Altered hematological and immunological parameters in silver catfish (Rhamdia quelen) following short term exposure to sublethal concentration of glyphosate. Fish Shellfish Immunol. 2011, 30, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Mistri, A.; Kumari, U.; Mittal, S.; Mittal, A.K. Morphological specializations of epidermis of na angler catfish Chaca chaca (Silurifomes, Chacidae) in relations ti its ecological niche: A scanning eléctron microscopic investigation. Microsc. Res. Tech. 2018, 81, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Sveen, L.; Timmerhaus, G. Deep neural network analysis—A paradigm shift for histological examination of health and welfare of farmed fish. Aquaculture 2021, 532, 736024. [Google Scholar] [CrossRef]
- Schulz, U.H.; Leuchtenberger, C. Activity patterns of South American silver catfish (Rhamdia quelen). Braz. J. Biol. 2006, 66, 565–574. [Google Scholar] [CrossRef]
- Cone, R.A. Barrier properties of mucus. Adv. Drug Deliv. Rev. 2009, 61, 75–85. [Google Scholar] [CrossRef]
- Phrompanya, P.; Panase, P.; Saenphet, S.; Saenphet, K. Histopathology and oxidative stress responses of Nile tilapia Oreochromis niloticus exposed to temperature shocks. Fish Sci. 2021, 87, 491–502. [Google Scholar] [CrossRef]
- Furtado, F.; Breiland, M.W.; Strand, D.; Timmerhaus, G.; Carletto, D.; Pedersen, L.F.; Afonso, F.; Lazado, C.C. Regulation of the molecular repertoires of oxidative stress response in the gills and olfactory organ of Atlantic salmon following infection and treatment of the parasite Neoparameoba perurans. Fish Shellfish Immunol. 2022, 130, 612–623. [Google Scholar] [CrossRef] [PubMed]
- Moron, S.E.; Andrade, C.A.; Fernandes, M.N. Response of mucous cells of the gills of traíra (Hoplias malabaricus) and jeju (Hoplerythrinus unitaeniatus) (Teleostei: Erythrinidae) to hypo-and hyper-osmotic ion stress. Neotrop. Ichthyol. 2009, 7, 491–498. [Google Scholar] [CrossRef]
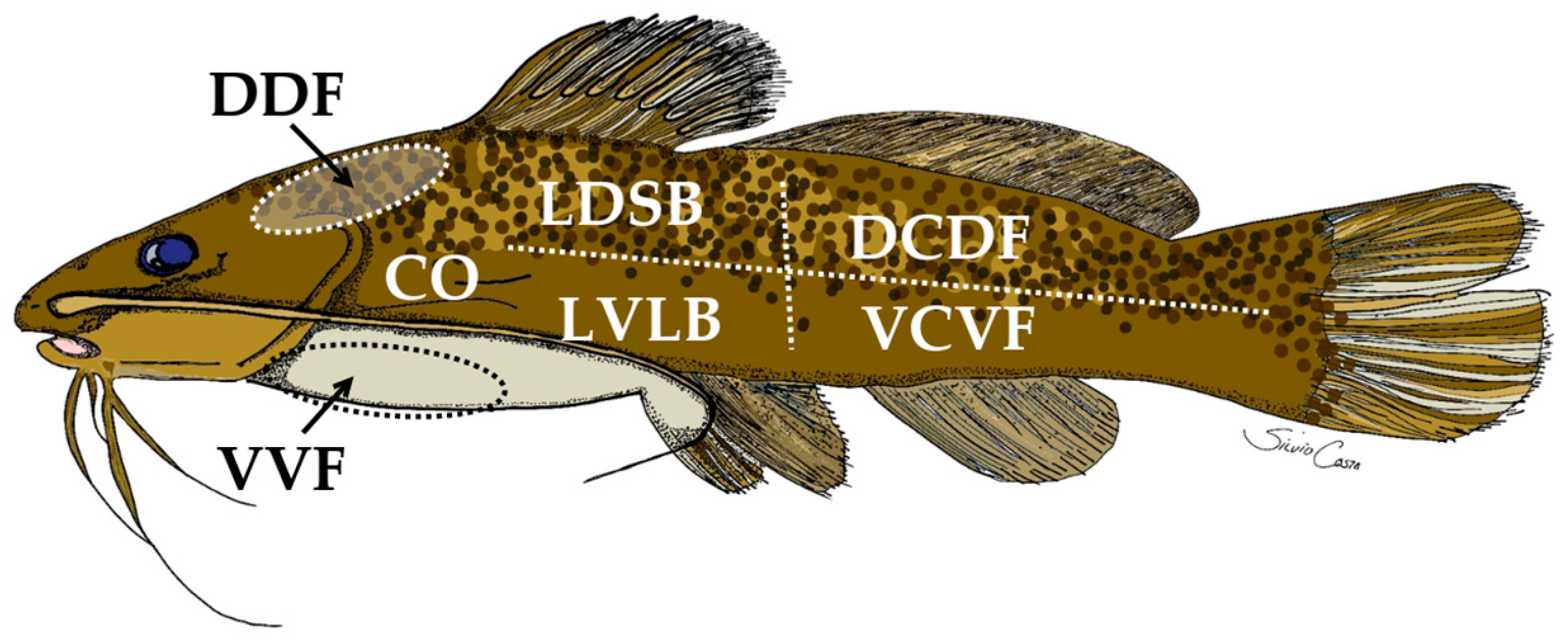


| Collection Site | Fed | Fasted | |||
|---|---|---|---|---|---|
| LSD-F | HSD-F | LSD-FS | HSD-FS | ||
| CO | median | 126.235 a | 102.423 b | 77.096 c | 56.687 d |
| 25% | 100.129 | 76.899 | 62.454 | 35.399 | |
| 75% | 155.660 | 128.913 | 102.069 | 79.290 | |
| DCDF | median | 148.339 a | 120.769 b | 88.611 c | 88.326 c |
| 25% | 98.722 | 100.862 | 69.422 | 64.780 | |
| 75% | 179.823 | 154.366 | 121.049 | 120.124 | |
| DDF | median | 111.610 a | 76.647 c | 79.654 c | 83.775 b |
| 25% | 88.865 | 59.114 | 65.486 | 57.406 | |
| 75% | 132.596 | 102.554 | 98.327 | 99.057 | |
| LDSB | median | 129.411 a | 86.423 c | 94.477 b | 70.143 d |
| 25% | 110.458 | 68.668 | 65.286 | 45.093 | |
| 75% | 153.985 | 101.965 | 124.256 | 96.803 | |
| VCVF | median | 137.414 a | 100.690 c | 105.819 b | 91.398 d |
| 25% | 117.477 | 87.176 | 81.955 | 68.303 | |
| 75% | 156.255 | 121.582 | 126.348 | 117.334 | |
| VVF | median | 161.006 a | 126.023 b | 115.069 c | 72.141 d |
| 25% | 137.196 | 96.673 | 94.864 | 53.418 | |
| 75% | 188.434 | 157.191 | 162.466 | 105.813 | |
| LVLB | median | 111.644 a | 91.869 b | 88.724 c | 76.797 c |
| 25% | 88.502 | 78.320 | 42.892 | 40.609 | |
| 75% | 154.810 | 112.583 | 111.305 | 121.051 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scherer, G.P.; Zavaglia, I.M.O.; Sutili, F.J.; Silva, H.N.P.; Furquim, M.D.; da Veiga, M.L.; Baldisserotto, B.; da Costa, S.T. The Effects of Stocking Density and Food Deprivation on Mucous Cells and Lysozyme Activity in the Skin and Gills of Silver Catfish. Animals 2023, 13, 3438. https://doi.org/10.3390/ani13223438
Scherer GP, Zavaglia IMO, Sutili FJ, Silva HNP, Furquim MD, da Veiga ML, Baldisserotto B, da Costa ST. The Effects of Stocking Density and Food Deprivation on Mucous Cells and Lysozyme Activity in the Skin and Gills of Silver Catfish. Animals. 2023; 13(22):3438. https://doi.org/10.3390/ani13223438
Chicago/Turabian StyleScherer, Gabriela Pires, Isadora Maria Oliveira Zavaglia, Fernando Jonas Sutili, Hugo Napoleão Pereira Silva, Magale Dallaporta Furquim, Marcelo Leite da Veiga, Bernardo Baldisserotto, and Sílvio Teixeira da Costa. 2023. "The Effects of Stocking Density and Food Deprivation on Mucous Cells and Lysozyme Activity in the Skin and Gills of Silver Catfish" Animals 13, no. 22: 3438. https://doi.org/10.3390/ani13223438
APA StyleScherer, G. P., Zavaglia, I. M. O., Sutili, F. J., Silva, H. N. P., Furquim, M. D., da Veiga, M. L., Baldisserotto, B., & da Costa, S. T. (2023). The Effects of Stocking Density and Food Deprivation on Mucous Cells and Lysozyme Activity in the Skin and Gills of Silver Catfish. Animals, 13(22), 3438. https://doi.org/10.3390/ani13223438







