Vessels Disturb Bottlenose Dolphin Behavior and Movement in an Active Ship Channel
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
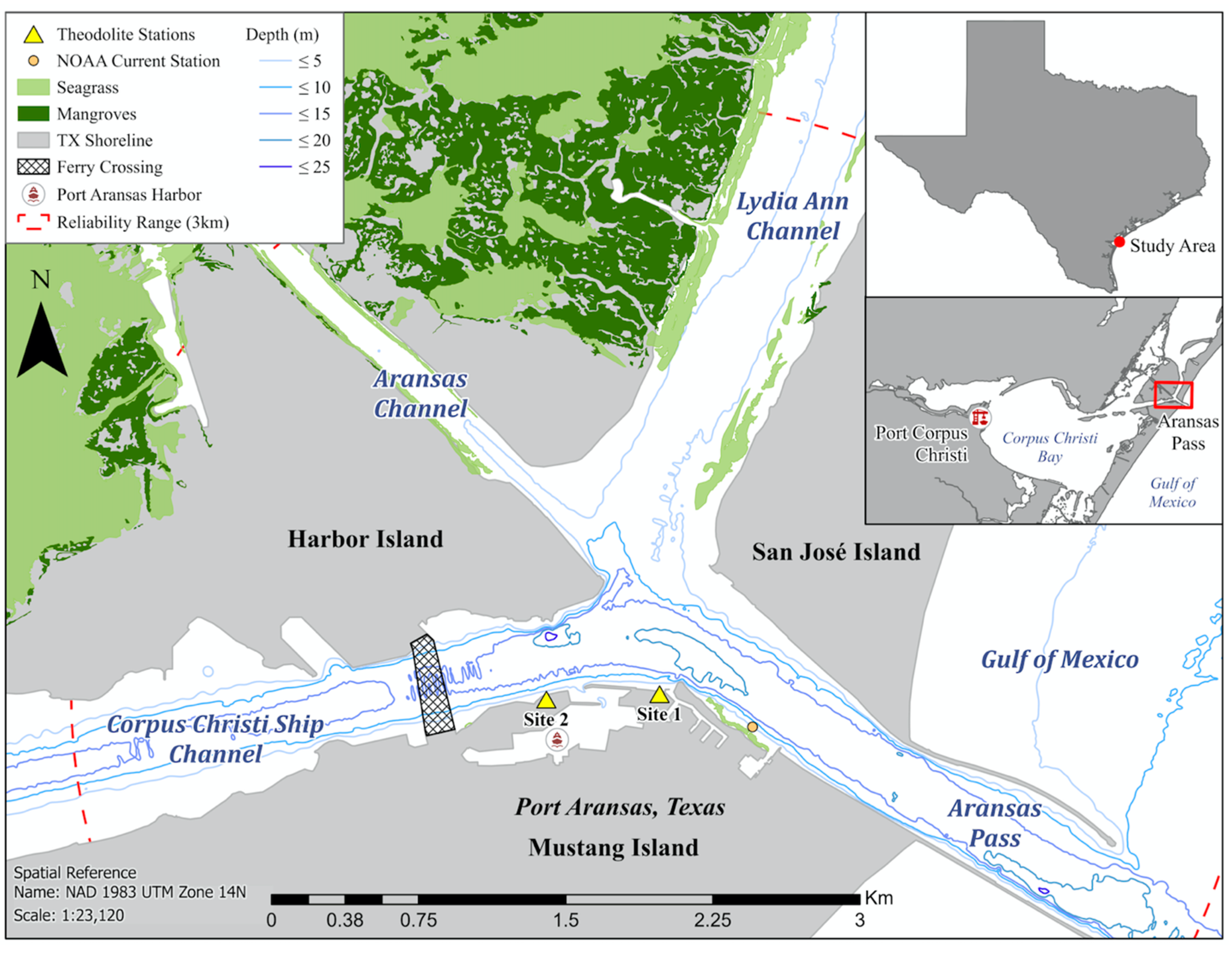
2.1. Study Area
2.2. Sampling Method
2.3. Analyses
3. Results
3.1. Behavioral Patterns
3.2. Swimming Speed and Bearing-Change Patterns
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Culloch, R.M.; Anderwald, P.; Brandecker, A.; Haberlin, D.; McGovern, B.; Pinfield, R.; Visser, F.; Jessopp, M.; Cronin, M. Effect of construction-related activities and vessel traffic on marine mammals. Mar. Ecol. Prog. Ser. 2016, 549, 231–242. [Google Scholar] [CrossRef]
- Marley, S.A.; Kent, C.P.S.; Erbe, C. Occupancy of bottlenose dolphins (Tursiops aduncus) in relation to vessel traffic, dredging, and environmental variables within a highly urbanized estuary. Hydrobiologia 2017, 792, 243–263. [Google Scholar] [CrossRef]
- Piwetz, S. Common bottlenose dolphin (Tursiops truncatus) behavior in an active narrow seaport. PLoS ONE 2019, 14, e0211971. [Google Scholar] [CrossRef] [PubMed]
- Lusseau, D. Effects of tour boats on the behavior of bottlenose dolphins: Using Markov chains to model anthropogenic impacts. Conserv. Biol. 2003, 17, 1785–1793. [Google Scholar] [CrossRef]
- Schoeman, R.P.; Patterson-Abrolat, C.; Plön, S. A global review of vessel collisions with marine animals. Front. Mar. Sci. 2020, 7, 292. [Google Scholar] [CrossRef]
- Koroza, A.; Evans, P.G. Bottlenose dolphin responses to boat traffic affected by boat characteristics and degree of compliance to code of conduct. Sustainability 2022, 14, 5185. [Google Scholar] [CrossRef]
- Pirotta, E.; Laesser, B.E.; Hardaker, A.; Riddoch, N.; Marcoux, M.; Lusseau, D. Dredging displaces bottlenose dolphins from an urbanized foraging patch. Mar. Pollut. Bull. 2013, 74, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Todd, V.L.G.; Todd, I.B.; Gardiner, J.C.; Morrin, E.C.N.; MacPherson, N.A.; DiMarzio, N.A.; Thomsen, F. A review of impacts of marine dredging activities on marine mammals. ICES J. Mar. Sci. 2015, 72, 328–340. [Google Scholar] [CrossRef]
- van Ginkel, C.; Becker, D.M.; Gowans, S.; Simard, P. Whistling in a noisy ocean: Bottlenose dolphins adjust whistle frequencies in response to real-time ambient noise levels. Bioacoustics 2018, 27, 391–405. [Google Scholar] [CrossRef]
- Creel, L. Ripple Effects: Population and Coastal Regions; Population Reference Bureau: Washington, DC, USA, 2003; pp. 1–8. Available online: https://www.prb.org/wp-content/uploads/2020/12/RippleEffects_Eng.pdf (accessed on 1 June 2022).
- Chilvers, B.L.; Lawler, I.R.; Macknight, F.; Marsh, H.; Noad, M.; Paterson, R. Moreton Bay, Queensland, Australia: An example of the co-existence of significant marine mammal populations and large-scale coastal development. Biol. Conserv. 2005, 122, 559–571. [Google Scholar] [CrossRef]
- Wells, R.S.; Scott, M.D. Bottlenose dolphin, Tursiops truncatus, common bottlenose dolphin. In Encyclopedia of Marine Mammals, 3rd ed.; Würsig, B., Thewissen, J.G.M., Kovacs, K.M., Eds.; American Press: San Diego, CA, USA, 2018; pp. 118–124. [Google Scholar]
- Toth, J.L.; Hohn, A.A.; Able, K.W.; Gorgone, A.M. Patterns of seasonal occurrence, distribution, and site fidelity of coastal bottlenose dolphins (Tursiops truncatus) in southern New Jersey, U.S.A. Mar. Mammal Sci. 2011, 27, 94–110. [Google Scholar] [CrossRef]
- Lusseau, D. The hidden cost of tourism: Detecting long-term effects of tourism using behavioral information. Ecol. Soc. 2004, 9, 2. Available online: http://www.ecologyandsociety.org/vol9/iss1/art2/ (accessed on 1 October 2021). [CrossRef]
- Papale, E.; Azzolin, M.; Giacoma, C. Vessel traffic affects bottlenose dolphin (Tursiops truncatus) behavior in waters surrounding Lampedusa Island, South Italy. J. Mar. Biol. Assoc. UK 2011, 92, 1877–1885. [Google Scholar] [CrossRef]
- Rivard, A.E.; Gelwick, F.P.; von Zharen, W. Behavioral patterns of common bottlenose dolphins (Tursiops truncatus) within the Galveston-Port Bolivar ferry lane. Southeast. Nat. 2016, 15, 742–759. [Google Scholar] [CrossRef]
- Arcangeli, A.; Crosti, R. The short-term impact of dolphin-watching on the behavior of bottlenose dolphins (Tursiops truncatus) in western Australia. JMATE 2009, 2, 3–9. [Google Scholar]
- Bejder, L.; Samuels, A.; Whitehead, H.; Gales, N.; Mann, J.; Connor, R.; Heithaus, M.; Watson-Capps, J.; Flaherty, C.; Krützen, M. Decline in relative abundance of bottlenose dolphins exposed to long-term disturbance. Conserv. Biol. 2006, 20, 1791–1798. [Google Scholar] [CrossRef]
- Mattson, M.C.; Thomas, J.A.; St. Aubin, D. Effects of boat activity on the behavior of bottlenose dolphins (Tursiops truncatus) in waters surrounding Hilton Head Island, South Carolina. Aquat. Mamm. 2005, 31, 133–140. [Google Scholar] [CrossRef]
- Vergara-Peña, A. Effects of Marine Recreation on Bottlenose Dolphins in Cardigan Bay. Ph.D. Thesis, Bangor University, Bangor, UK, 2020. [Google Scholar]
- Würsig, B.; Lynn, S.K.; Jefferson, T.A.; Mullin, K.E. Behavior of cetaceans in the northern Gulf of Mexico relative to survey ships and aircraft. Aquat. Mamm. 1998, 24, 41–50. [Google Scholar]
- Nowacek, S.M.; Wells, R.S.; Solow, A.R. Short-term effects of boat traffic on bottlenose dolphins, Tursiops truncatus, in Sarasota Bay, Florida. Mar. Mammal Sci. 2001, 17, 673–688. [Google Scholar] [CrossRef]
- Steckenreuter, A.; Harcourt, R.; Möller, L. Are speed restriction zones an effective management tool for minimizing impacts of boats on dolphins in an Australian marine park? Mar. Policy 2012, 36, 258–264. [Google Scholar] [CrossRef]
- Swim with and Approach Regulation for Hawaiian Spinner Dolphins under the Marine Mammal Protection Act, Volume 86 50 C.F.R. § Part 216. Available online: https://www.fisheries.noaa.gov/action/final-rule-prohibit-swimming-and-approaching-hawaiian-spinner-dolphins (accessed on 1 April 2023).
- PCCA Press Release. Port of Corpus Christi Finishes Fiscal Year 2022 with Record Tonnage. Port of Corpus Christi, 23 January 2023. Available online: https://portofcc.com/port-of-corpus-christi-finishes-fiscal-year-2022-with-record-tonnage/ (accessed on 15 February 2023).
- PCCA Press Release. Port of Corpus Christi Closes 2019 with Record Tonnage. Port of Corpus Christi, 14 January 2020. Available online: https://portofcc.com/port-of-corpus-christi-closes-2019-with-record-tonnage/ (accessed on 1 August 2021).
- U.S. Coast Guard. Ports and Waterways Safety Assessment: Workshop Report Corpus Christi, Texas. United States Coast Guard Navigation Center, September 2019. Available online: https://www.navcen.uscg.gov/pdf/pawsa/WorkshopReports/Corpus_Christi_Sep_2019.pdf (accessed on 1 August 2021).
- Hrvacevic, Z. Funding Secured for Phase 4 of the Corpus Christi Dredging Project. Dredging Today. 27 December 2022. Available online: https://www.dredgingtoday.com/2022/12/27/funding-secured-for-phase-4-of-the-corpus-christi-dredging-project/ (accessed on 20 February 2023).
- Shane, S.H. The Population Biology of the Atlantic Bottlenose Dolphin, Tursiops truncatus, in the Aransas Pass Area of Texas. Master’s Thesis, Texas A&M University-Galveston, Galveston, TX, USA, 1977. [Google Scholar]
- Shane, S.H. Occurrence, movements, and distribution of bottlenose dolphin, Tursiops truncatus, in southern Texas. Fish. Bull. 1980, 78, 593–601. [Google Scholar]
- Leatherwood, S.; Reeves, R.R. Abundance of bottlenose dolphins in Corpus Christi Bay and coastal Southern Texas. Contrib. Mar. Sci. 1983, 26, 179–199. [Google Scholar]
- NOAA National Ocean Service. Tides and Currents [Data Set]. Center for Operational Oceanographic Products and Services. Available online: https://tidesandcurrents.noaa.gov/map/index.html (accessed on 1 June 2022).
- Harzen, S.E. Use of an electronic theodolite in the study of movements of the bottlenose dolphin (Tursiops truncatus) in the Sado Estuary, Portugal. Aquat. Mamm. 2002, 28, 251–260. [Google Scholar]
- Azzellino, A.; Gaspari, S.; Airoldi, S.; Nani, B. Habitat use and preferences of cetaceans along the continental slope and the adjacent pelagic waters in the western Ligurian Sea. Deep Sea Res. Part I Oceanogr. Res. Pap. 2008, 55, 296–323. [Google Scholar] [CrossRef]
- Shane, S.H. Behavior and ecology of the bottlenose dolphin at Sanibel Island, Florida. In The Bottlenose Dolphin; Leatherwood, S., Reeves, R.R., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 245–265. [Google Scholar]
- Sagnol, O.; Reitsma, F.; Richter, C.; Field, L.H. Correcting positional error in shore-based theodolite measurements of animals at sea. J. Mar. Biol. 2014, 2014, 267917. [Google Scholar] [CrossRef]
- Henderson, E.E.; Würsig, B. Behavior patterns of bottlenose dolphins in San Luis Pass, Texas. Gulf Mex. Sci. 2007, 25, 153–161. [Google Scholar] [CrossRef]
- Torres, L.G.; Read, A.J. Where to catch a fish? The influence of foraging tactics on the ecology of bottlenose dolphins (Tursiops truncatus) in Florida Bay, Florida. Mar. Mammal Sci. 2009, 25, 797–815. [Google Scholar] [CrossRef]
- Shane, S.H.; Wells, R.S.; Würsig, B. Ecology, behavior, and social organization of the bottlenose dolphin: A review. Mar. Mammal Sci. 1986, 2, 34–63. [Google Scholar] [CrossRef]
- Baker, I.; O’Brien, J.; McHugh, K.; Berrow, S. An ethogram for bottlenose dolphins (Tursiops truncatus) in the Shannon Estuary, Ireland. Aquat. Mamm. 2017, 43, 594–613. [Google Scholar] [CrossRef]
- Upadhyay, A. Formula to Find Bearing or Heading Angle between Two Points: Latitude Longitude. iGISMAP. Available online: https://www.igismap.com/formula-to-find-bearing-or-heading-angle-between-two-points-latitude-longitude/ (accessed on 11 May 2023).
- Marine Traffic. Vessels database. AIS Marine Traffic: Global Ship Tracking Intelligence. Available online: https://www.marinetraffic.com (accessed on 1 June 2021).
- Hua, C.; Choi, Y.; Shi, Q. Companion to BER 643: Advanced Regression Methods; Bookdown; GitHub: San Francisco, CA, USA, 2021; Available online: https://bookdown.org/chua/ber642_advanced_regression/multinomial-logistic-regression.html (accessed on 1 August 2022).
- Félix, F.; Fernández, J.E.; Paladines, A.; Centeno, R.; Romero, J.; Burneo, S.F. Habitat use of the common bottlenose dolphin (Tursiops truncatus) in the Gulf of Guayaquil, Ecuador: Management needs for a threatened population. Ocean Coast. Manag. 2022, 223, 106174. [Google Scholar] [CrossRef]
- McNulty, K. Handbook of Regression Modeling in People Analytics; Bookdown; GitHub: San Francisco, CA, USA, 2021; Available online: https://peopleanalytics-regression-book.org/multinomial-logistic-regression-for-nominal-category-outcomes.html (accessed on 1 August 2022).
- Shukla, A. Multinomial Logistic Regression in R [Data Set]. RPubs-RStudio. Available online: https://rpubs.com/Anupam/588952 (accessed on 1 August 2022).
- Pipis, G. Contingency Tables in R. R-Bloggers. 19 December 2020. Available online: https://www.r-bloggers.com/2020/12/contingency-tables-in-r/ (accessed on 1 August 2022).
- Wood, S.N. Mgcv: GAMs and generalized ridge regression for R. R News 2001, 1, 20–25. [Google Scholar]
- Wood, S.N. Generalized Additive Models: An Introduction with R, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2006; pp. 61–245. [Google Scholar]
- Zuur, A.F.; Ieno, E.N.; Walker, N.J.; Saveliev, A.A.; Smith, G.M. Mixed Effects Models and Extensions in Ecology with R, 1st ed.; Springer Science and Business Media: New York, NY, USA, 2009; pp. 1–574. [Google Scholar]
- Jackson, S. Machine Learning: Generalized Additive Models [Lecture Notes]; Bookdown; GitHub: San Francisco, CA, USA, 2023; Available online: https://bookdown.org/ssjackson300/Machine-Learning-Lecture-Notes/generalised-additive-models.html#generalised-additive-models (accessed on 1 April 2023).
- Wedderburn, R.W.M. Quasi-likelihood functions, generalized linear models, and Gauss-Newton method. Biometrika 1974, 61, 439–447. [Google Scholar] [CrossRef]
- Stokes, G.M. Life history studies of Southern flounder (Paralichthys lethostigma) and Gulf flounder (P. albigutta) in the Aransas Bay area of Texas. TPWD 1977, 25, 1–36. [Google Scholar]
- Bushon, A. Recruitment, Spatial Distribution, and Fine-Scale Movement Patterns of Estuarine Dependent Species through Tidal Inlets in Texas. Ph.D. Thesis, Texas A&M University-Corpus Christi, Corpus Christi, TX, USA, 2006. [Google Scholar]
- Payne, L.M. Evaluation of Large-Scale Movement Patterns of Spotted Seatrout (Cynoscion nebulosus) Using Acoustic Telemetry. Master’s Thesis, Texas A&M University-Corpus Christi, Corpus Christi, TX, USA, 2011. [Google Scholar]
- Rossbach, K.A. Cooperative feeding among bottlenose dolphins (Tursiops truncatus) near Grand Bahamas Island, Bahamas. Aquat. Mamm. 1999, 25, 163–167. [Google Scholar]
- Nañez-James, S.E.; Stunz, G.W.; Holt, S.A. Habitat use patterns of newly settled southern flounder (Paralichthys lethostigma) in Aransas-Copano Bay, Texas. Estuaries Coast 2009, 32, 350–359. [Google Scholar] [CrossRef]
- Mattos, P.H.; Rosa, L.D.; Fruet, P.F. Activity budgets and distribution of bottlenose dolphins (Tursiops truncatus) in the Patos Lagoon estuary, southern Brazil. Lat. Am. J. Aquat. Mamm. 2007, 6, 161–169. [Google Scholar] [CrossRef]
- Mate, B.R.; Rossbach, K.A.; Nieukirk, S.L.; Wells, R.S.; Irvine, A.B.; Scott, M.D.; Read, A.J. Satellite-monitored movements and dive behavior of a bottlenose dolphin (Tursiops truncatus) in Tampa Bay, Florida. Mar. Mammal Sci. 1995, 11, 452–463. [Google Scholar] [CrossRef]
- Bejder, L.; Samuels, A.; Whitehead, H.; Finn, H.; Allen, S. Impact assessment research: Use and misuse of habituation, sensitization, and tolerance in describing wildlife responses to anthropogenic stimuli. Mar. Ecol. Prog. Ser. 2009, 395, 177–185. [Google Scholar] [CrossRef]
- Higham, J.E.S.; Shelton, E.J. Tourism and wildlife habituation: Reduced population fitness or cessation of impact? Tour. Manag. 2011, 32, 1290–1298. [Google Scholar] [CrossRef]
- Constantine, R.; Brunton, D.H.; Dennis, T. Dolphin-watching tour boats change bottlenose dolphin (Tursiops truncatus) behavior. Biol. Conserv. 2004, 117, 299–307. [Google Scholar] [CrossRef]



| Behavioral State | Description | Source |
|---|---|---|
| Forage | Variable movement directions, high arching dives (tail flukes out of water), interacting with fish (trapping fish against hard structures) | [37,38] |
| Mill | Nondirectional movement, absence of physical contact, frequent changes in heading | [37,39,40] |
| Orient against current (OAC) | Frequent surfacing, no position change, oriented against a visible current | [29], termed “rest” [39], “forage” |
| Rest | Slow movement, drifting in one direction at the surface | [39] |
| Socialize | Individuals in close proximity, body contact, sexual behavior, leaps, playing with objects | [29,40] |
| Travel | Steady or rapid movement in one direction | [37] |
| Vessel Type | Vessel Size (m) | Mean Speed (m/s) |
|---|---|---|
| Ecotour Ferry Fishing (charter) Personal recreational Enforcement (law enforce) Tug | Small (<10) | 4.02 ± (5.02) |
| Coastal cargo (barge) Ecotour Ferry Fishing (charter, trawler) Personal recreational Enforcement (law enforce, port tender) Supply (inshore, offshore) Tug | Medium (10–30) | 1.96 ± (1.32) |
| Coastal cargo (barge, tank-barge) Ferry Fishing (trawler) Supply (offshore) Tug | Large (31–70) | 1.42 ± (0.98) |
| Coastal cargo (barge) Offshore commercial (carrier, tanker) Tug | Extra-Large (>70) | 2.47 ± (0.69) |
| Independent Variables | Behavioral States | |||
|---|---|---|---|---|
| Forage | Mill | Social | ||
| Season | Fall | 1.27 (0.10) | 0.45 (0.14) | 1.68 (0.14) |
| Spring | 1.95 (0.11) | 0.81 (0.15) | 3.34 (0.14) | |
| Winter | 4.07 (0.14) | 0.75 (0.20) | 1.38 (0.22) | |
| Time of day | Morning | 1.76 (0.12) | 1.08 (0.15) | 1.05 (0.16) |
| Early PM | 0.73 (0.09) | 0.67 (0.12) | 1.12 (0.12) | |
| Late PM | 3.66 (0.17) | 1.42 (0.22) | 2.47 (0.22) | |
| Group size | –– | 1.10 (0.01) | 1.17 (0.02) | 1.20 (0.02) |
| Vessel Type | Coastal cargo | 0.43 (0.31) | 1.58 (0.34) | 1.14 (0.41) |
| Ecotour | 0.98 (0.25) | 1.42 (0.30) | 1.87 (0.31) | |
| Ferry | 2.15 (0.26) | 0.55 (0.45) | 0.25 (0.51) | |
| Fishing | 1.51 (0.24) | 1.05 (0.34) | 0.84 (0.35) | |
| Mixed | 1.36 (0.21) | 1.83 (0.27) | 1.23 (0.29) | |
| Offshore commercial | 0.75 (0.57) | 0.93 (0.65) | 0.60 (0.83) | |
| Personal | 1.07 (0.22) | 1.21 (0.28) | 0.85 (0.30) | |
| Enforcement | 2.05 (0.63) | 1.35 (0.81) | 4.45 (0.71) | |
| Supply vessels | 0.34 (0.51) | 0.95 (0.62) | 1.27 (0.51) | |
| Tug | 0.55 (0.53) | 0.34 (1.07) | 0.28 (0.86) | |
| Vessel size | Small | 0.68 (0.21) | 1.22 (0.28) | 1.15 (0.29) |
| Medium | 1.16 (0.21) | 1.34 (0.27) | 0.70 (0.29) | |
| Large | 1.19 (0.35) | 0.92 (0.56) | 1.19 (0.53) | |
| Extra large | 1.13 (0.49) | 1.32 (0.52) | 0.72 (0.69) | |
| Mixed | 0.54 (0.22) | 0.60 (0.30) | 0.64 (0.31) | |
| Number of vessels | –– | 1.12 (0.03) | 1.17 (0.04) | 1.10 (0.04) |
| Predictor | Log-Likelihood | X2 | df | Pr (>X2) |
|---|---|---|---|---|
| Season | −5305.80 | 325.48 | −9 | <2.2 × 10−16 |
| Time of day | −5228.80 | 171.46 | −9 | <2.2 × 10−16 |
| Group size | −5224.40 | 162.68 | −3 | <2.2 × 10−16 |
| Vessel type | −5198.80 | 111.52 | −27 | 0.0000 |
| Vessel size | −5155.10 | 24.07 | −12 | 0.0199 |
| Number of vessels | −5153.10 | 20.12 | −3 | 0.0002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mills, E.M.M.; Piwetz, S.; Orbach, D.N. Vessels Disturb Bottlenose Dolphin Behavior and Movement in an Active Ship Channel. Animals 2023, 13, 3441. https://doi.org/10.3390/ani13223441
Mills EMM, Piwetz S, Orbach DN. Vessels Disturb Bottlenose Dolphin Behavior and Movement in an Active Ship Channel. Animals. 2023; 13(22):3441. https://doi.org/10.3390/ani13223441
Chicago/Turabian StyleMills, Eliza M. M., Sarah Piwetz, and Dara N. Orbach. 2023. "Vessels Disturb Bottlenose Dolphin Behavior and Movement in an Active Ship Channel" Animals 13, no. 22: 3441. https://doi.org/10.3390/ani13223441
APA StyleMills, E. M. M., Piwetz, S., & Orbach, D. N. (2023). Vessels Disturb Bottlenose Dolphin Behavior and Movement in an Active Ship Channel. Animals, 13(22), 3441. https://doi.org/10.3390/ani13223441





