Sand-Related Factors Influencing Nest Burrowing Potential of the Sand Martins
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Measurements of Nesting Colonies and Nest Morphology
2.3. Soil Sampling and Analyses
2.4. Statistical Analyses
3. Results
3.1. General Characteristics of Nesting Colonies
3.2. Factors Influencing on Nest Characteristics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garrison, B.A. Bank Swallow Riparia riparia. In The Birds of North America; American Ornithologists’ Union, Cornell Laboratory of Ornithology, Academy of Natural Sciences: Washington, DC, USA, 1999; Volume 414, pp. 1–20. Available online: http://bna.birds.cornell.edu.proxy.lib.umich.edu/bna/species/414 (accessed on 30 March 2009).
- Etxezarreta, J.; Arizaga, J. Characteristics of Sand Martin Riparia riparia colonies in artificial river walls. Ardeola 2014, 6, 127–134. [Google Scholar] [CrossRef]
- Lind, B.B.; Stigh, J.; Larsson, L. Sediment type and breeding strategy of the Bank Swallow Riparia riparia in western Sweden. Ornis Svec. 2002, 12, 157–163. [Google Scholar] [CrossRef]
- Mainwaring, M.C.; Hartley, I.R.; Lambrechts, M.M.; Deeming, D.C. The design and function of birds’ nests. Ecol. Evol. 2014, 4, 3909–3928. [Google Scholar] [CrossRef]
- Hansell, M. Bird Nests and Construction Behaviour; Cambridge University Press: Cambridge, UK, 2000. [Google Scholar]
- Szabó, Z.D.; Szép, T. Breeding dispersal patterns within a large Sand Martin (Riparia riparia) colony. J. Ornithol. 2010, 151, 185–191. [Google Scholar] [CrossRef]
- Gryaznova, A.N.; Savchenko, A.P. Distribution peculiarities of the sand (Riparia riparia Linnaeus 1758) martin and the pale sand (Riparia diluta Sharpe et Wyatt 1893) martin (Passeriformes, Hirundinidae) in the sympatry zone in Southern Central Siberia. Biol. Bull. 2017, 44, 991–997. [Google Scholar] [CrossRef]
- Benmazouz, I.; Jokimäki, J.; Lengyel, S.; Juhász, L.; Kaisanlahti-Jokimäki, M.L.; Kardos, G.; Kövér, L. Corvids in urban environments: A systematic global literature review. Animals 2021, 11, 3226. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.P.; Siefferman, L.; Wang, Y.J.; Ding, T.S.; Chiou, C.R.; Shieh, B.S.; Yuan, H.W. Nest site restoration increases the breeding density of blue-tailed bee-eaters. Biol. Conserv. 2009, 142, 1748–1753. [Google Scholar] [CrossRef]
- Fry, C.H.; Fry, K. Madagascar Bee-eater and Blue-tailed Bee-eater. In Kingfishers, Bee-Eaters and Rollers: A Handbook, 1st ed.; Fry, H.C., Fry, K., Harris, A., Eds.; Christopher Helm: London, UK, 1992; pp. 273–275. [Google Scholar]
- Karaardiç, H.; Kizilkaya, E. Sand banks in pseudo-steppe areas provide suitable nesting sites: High breeding numbers of the European Roller (Coracias garrulus L. 1758) in Southwest Turkey. Acta Biol. Turc. 2021, 34, 205–210. [Google Scholar]
- Haug, E.; Millsap, B.; Martell, M. Burrowing owl: Athene cunicularia. The Birds of North America Online, 061: 1. Available online: http://bna.birds.cornell.edu/bna/species/061/articles/introduction (accessed on 3 September 2014).
- Stevenson, C.; Woehler, E.J. Population decreases in Little Penguins Eudyptula minorin southeastern Tasmania, Australia, over the past 45 years. Mar. Ornithol. 2007, 35, 71–76. [Google Scholar]
- Fischer, J.B.; Griffin, C.R. Can burrow-nesting seabirds be identified from their burrow dimensions? Wildl. Soc. Bull. 2000, 28, 586–591. [Google Scholar]
- Rodway, M.S.; Lemon, M.J. Use of permanent plots to monitor trends in burrow-nesting seabird populations in British Columbia. Mar. Ornithol. 2011, 39, 243–253. [Google Scholar]
- Rosenberg, D.; Haley, K. The ecology of burrowing owls in the agroecosystem of the Imperial Valley, California. Stud. Avian Biol. 2004, 27, 120–135. [Google Scholar]
- Deeming, D.C.; Reynolds, S.J. (Eds.) Nests, Eggs, & Incubation: New Ideas about Avian Reproduction; Oxford University Press: Oxford, UK, 2015. [Google Scholar]
- Mainwaring, M.C.; Reynolds, S.J.; Weidinger, K. The infuence of predation on the location and design of nests. In Nests, Eggs, & Incubation: New Ideas about Avian Reproduction; Deeming, D.C., Reynolds, S.J., Eds.; Oxford University Press: Oxford, UK, 2015; pp. 50–64. [Google Scholar]
- Burke, T.R.; Cadman, M.D.; Nol, E. Reproductive success and health of breeding Bank Swallows (Riparia riparia) in aggregate (sand and gravel) pit and natural lakeshore habitats. Condor 2019, 121, 1–10. [Google Scholar] [CrossRef]
- Windsor, D.; Emlen, S.T. Predator-prey interactions of adult and prefledgling Bank Swallows and American Kestrels. Condor 1975, 77, 359–361. [Google Scholar] [CrossRef]
- McGowan, P.C.; Reinstma, K.; Sullivan, J.D.; DeVoss, K.P.; Wall, J.L.; Zimnik, M.D.; Prosser, D.J. Use of Bank Swallow (Riparia riparia) burrows as shelter by Common Tern (Sterna hirundo) chicks. Waterbirds 2018, 41, 179–182. [Google Scholar] [CrossRef]
- Burke, T. Predation of Bank Swallow nestlings by Ring-billed Gull and Common Grackle. Ont. Birds 2020, 38, 159–164. [Google Scholar]
- Dybala, K.E.; Seavy, N.E.; Dettling, M.D.; Gilbert, M.; Melcer, R.; Gardali, T. Does restored riparian habitat create ecological traps for riparian birds through increased Brown-headed Cowbird nest parasitism? Ecol. Restor. 2014, 32, 239–248. [Google Scholar] [CrossRef]
- Jara, R.F.; Crego, R.D.; Samuel, M.D.; Rozzi, R.; Jiménez, J.E. Nest-site selection and breeding success of passerines in the world’s southernmost forests. PeerJ 2020, 8, e9892. [Google Scholar] [CrossRef]
- Heneberg, P. Soil penetrability as a key factor affecting the nesting of burrowing birds. Ecol. Res. 2009, 24, 453–459. [Google Scholar] [CrossRef]
- Zhang, L.; Ma, X.; Chen, Z.; Wang, C.; Liu, Z.; Li, X.; Xing, X. Negative effects of artificial nest boxes on birds: A review. Avian Res. 2023, 14, 100101. [Google Scholar] [CrossRef]
- Castaño-Vázquez, F.; Schumm, Y.R.; Bentele, A.; Quillfeldt, P.; Merino, S. Experimental manipulation of cavity temperature produces differential effects on parasite abundances in blue tit nests at two different latitudes. Int. J. Parasitol. Parasites Wildl. 2021, 14, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Mead, C.J.; Pepler, G.R.M. Birds and other animals at Sand Martin colonies. Br. Birds 1975, 68, 89–99. [Google Scholar]
- Heneberg, P. Size of sand grains as a significant factor affecting the nesting of bank swallows (Riparia riparia). Biol. Bratisl. 2001, 56, 205–210. [Google Scholar]
- Smalley, I.; Blake-Smalley, R.; O’Hara-Dhand, K.; Jary, Z.; Svircev, Z. Sand martins favour loess: How the properties of loess ground facilitate the nesting of sand martins/bank swallows/uferschwalben (Riparia riparia Linnaeus 1758). Quat. Int. 2013, 296, 216–219. [Google Scholar] [CrossRef]
- Sieber, O. Bestand und Verbreitung der Uferschwalbe (Riparia riparia) 1980 in der Schweiz. Z. Tierpsychol. 1980, 52, 19–56. (In German) [Google Scholar] [CrossRef]
- Petersen, A.J. The breeding cycle in the Bank Swallow. Wilson Bull. 1955, 67, 235–286. [Google Scholar]
- González, S.; Villarino, A. Nidotópica y situación actual del Avión Zapador, Riparia riparia (L. 1758), en la provincia de Ourense (NO España). Ardeola 1997, 44, 41–49. [Google Scholar]
- Svensson, S. Studies on the breeding biology of the sand martin Riparia riparia in a colony in southern Lapland 1968. Vår Fågelvärld 1969, 28, 236–240, (In Swedish with an English Summary). [Google Scholar]
- Bancroft, W.J. Environmental Response to Burrowing Seabird Colonies: A Study in Ecosystem Engineering. Ph.D. Thesis, School of Animal Biology, The University of Western Australia, Perth, Australia, 2004. [Google Scholar]
- Brevik, E.C.; Fenton, T.E.; Lazari, A. Soil electrical conductivity as a function of soil water content and implications for soil mapping. Precis. Agric. 2006, 7, 393–404. [Google Scholar] [CrossRef]
- Alam, S.I.; Hammoda, H.; Khan, F.; Al Enazi, R.; Goktepe, I. Electrical Conductivity, pH, Organic Matter and Texture of Selected Soils Around the Qatar University Campus. Res. Agric. Livest. Fish 2020, 7, 403–409. [Google Scholar] [CrossRef]
- Evans, R. Soils at risk of accelerated erosion in England and Wales. Soil Use Manag. 1990, 6, 125–131. [Google Scholar] [CrossRef]
- Turner, A. Collared Sand Martin (Riparia riparia). In Handbook of the Birds of the World Alive; del Hoyo, J., Elliott, A., Sargatal, J., Christie, D.A., de Juana, E., Eds.; Lynx Edicions: Barcelona, Spain, 2004; Volume 9, pp. 647–648. [Google Scholar]
- Ben-Hur, M.; Yolcu, G.; Uysal, H.; Lado, M.; Paz, A. Soil structure changes: Aggregate size and soil texture effects on hydraulic conductivity under different saline and sodic solutions. Australian. J. Soil Res. 2009, 47, 688–696. [Google Scholar] [CrossRef]
- Hickling, R.A.O. The burrow-excavation phase in the breeding cycle of the sand martin Riparia riparia. Ibis 1959, 101, 497–502. [Google Scholar] [CrossRef]
- COSEWIC. COSEWIC Assessment and Status Report on the Bank Swallow Riparia riparia in Canada; Committee on the Status of Endangered Wildlife in Canada: Ottawa, ON, Canada, 2013; p. ix,48. Available online: www.registrelep-sararegistry.gc.ca/default_e.cfm (accessed on 21 September 2023).
- Turner, A.K. Time and energy constraints on the brood size of swallows, Hirundo rustica, and sand martins, Riparia riparia. Oecologia 1983, 59, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Morgan, R.A. Sand Martin nest record cards. Bird Study 1979, 26, 129–132. [Google Scholar] [CrossRef]
- Pelletier, N.; Arndt, J.E.; Darvill, R.; Cyr, M.A. Natural and human-made nesting habitat use by Bank Swallow (Riparia riparia) in Canada. Can. Field-Nat. 2022, 136, 228–236. [Google Scholar] [CrossRef]
- Tucker, G.M.; Heath, M.F. Birds in Europe, Their Conservation Status; BirdLife International Conservation Series 3; BirdLife International: Cambridge, UK, 1994. [Google Scholar]
- Ahlman, S. Sand Martin in Finland in 2009. Linnut-Vuosikirja 2010, 2011, 130–135, (In Finnish with an English Summary). [Google Scholar]
- BirdLife International. European Birds of Conservation Concern: Populations, Trends and National Responsibilities; BirdLife International: Cambridge, UK, 2017. [Google Scholar]
- IUCN. 2023. Available online: https://www.iucnredlist.org/species/103815961/155536007 (accessed on 25 June 2023).
- BirdLife International. 2015. Available online: http://datazone.birdlife.org/userfiles/file/Species/erlob/summarypdfs/22712176_riparia_riparia (accessed on 9 February 2023).
- Per, E.; Kiraz Erciyas, Y.; Yavuz, N. Hirundinidae familyası türlerinin Türkiye dağılımı ve göç fenolojisi ile bölgesel ve zamansal farklılıkları. Trak. Univ. J. Nat. Sci. 2016, 17, 7–15. [Google Scholar]
- Nergiz, H.; Durmuş, A. Effects of road construction works on some bird communities in Van (Turkey). Bitlis Eren Univ. J. Sci. Technol. 2016, 6, 73–75. [Google Scholar] [CrossRef][Green Version]
- Ditzler, C.; Scheffe, K.; Monger, H.C. Soil Survey Manual: Soil Science Division Staff; Goverment Printing Office: Washington, DC, USA, 2017.
- Siqueira, G.M.; Dafonte, J.D.; Bueno Lema, J.; Valcárcel Armesto, M. Using soil apparent electrical conductivity to optimize sampling of soil penetration resistance and to improve the estimations of spatial patterns of soil compaction. Sci. World J. 2014, 2014, 269480. [Google Scholar] [CrossRef]
- EUNIS (European Nature Information System). ArcGIS Online Database. ArcMap 10.2. 2018. Available online: https://eunis.eea.europa.eu/ (accessed on 9 February 2023).
- Gilbert, G.; Gibbons, D.; Evans, J. Bird Monitoring Methods. A Manual of Techniques for Key UK Species; Royal Society for the Protection of Birds: Edinburgh, UK, 1998. [Google Scholar]
- Heneberg, P. Sand Martin (Riparia riparia) in the Czech Republic at the turn of the millennium. Linz. Biol. Beitr. 2007, 39, 293–312. [Google Scholar]
- Szép, T.; Szabó, D.Z.; Vallner, J. Integrated population monitoring of sand martin Riparia riparia—An opportunity to monitor the effects of environmental disasters along the river Tisza. Ornis Hung. 2003, 12, 169–182. [Google Scholar]
- Garrison, B.A.; Humphrey, J.M.; Laymon, S.A. Bank Swallow distribution and nesting ecology on the Sacramento River, California. West. Birds 1987, 18, 71–76. [Google Scholar]
- Spencer, S.J. A Study of the Physical Characteristics of Nesting Sites Used by Bank Swallows. Ph.D. Thesis, The Pennsylvania State University, State College, PA, USA, 1962. [Google Scholar]
- Ghent, A.W. Regular spatial patterns of bank swallow (Riparia riparia) tunnel entrances, with some possible evolutionary implications. Am. Midl. Nat. 2001, 146, 414–423. [Google Scholar] [CrossRef]
- Norman, D.; Peach, W.J. Density-dependent survival and recruitment in a long-distance Palaearctic migrant, the Sand Martin Riparia riparia. Ibis 2013, 155, 284–296. [Google Scholar] [CrossRef]
- Environment Canada. North American Breeding Bird Survey—Canadian Trends Website, Data-Version 2011; Environment Canada: Gatineau, QC, Canada, 2013. [Google Scholar]
- Campbell, R.W.; Dawe, N.K.; McTaggart-Cowan, I.; Cooper, J.M.; Kaiser, G.W.; McNall, M.C.E.; Smith, G.E.J. The Birds of British Columbia; Royal British Columbia Museum: Victoria, BC, Canada, 1990; Volume 2. [Google Scholar]
- Persson, C. Sand Martin (Riparia riparia) populations in south-west Scania, Sweden, 1964 to 1984. J. Zool. Lond. 1987, 1, 619–637. [Google Scholar] [CrossRef]
- Falconer, M.; Richardson, K.; Heagy, A.; Tozer, D.; Stewart, B.; McCracken, J.; Reid, R. Recovery Strategy for the Bank Swallow (Riparia riparia) in Ontario; Ontario Recovery Strategy Series; Ontario Ministry of Natural Resources and Forestry: Peterborough, ON, Canada, 2016.
- Bryant, D.M.; Turner, A.K. Central place foraging by swallows (Hirundinidae): The question of load size. Anim. Behav. 1982, 30, 845–856. [Google Scholar] [CrossRef]
- Moffatt, K.C.; Crone, E.E.; Holl, K.D.; Schlorff, R.W.; Garrison, B.A. Importance of hydrologic and landscape heterogeneity for restoring Bank Swallow (Riparia riparia) colonies along the Sacramento River, California. Restor. Ecol. 2005, 13, 391–402. [Google Scholar] [CrossRef]
- Garrison, B.A.; Turner, A. Bank Swallow (Riparia riparia), version 1.0. In Birds of the World; Billerman, S.M., Ed.; Cornell Lab of Ornithology: Ithaca, NY, USA, 2020. [Google Scholar] [CrossRef]
- Cadman, M.D.; Sutherland, D.A.; Beck, G.G.; Lepage, D.; Couturier, A. (Eds.) Atlas of the Breeding Birds of Ontario, 2001–2005; Bird Studies Canada, Environment Canada, Ontario Field Ornithologists, Ontario Ministry of Natural Resources, and Ontario Nature: Toronto, ON, Canada, 2007; p. xxii,706.
- Saldanha, S. Foraging and Roosting Habitat Use of Nesting Bank Swallows in Sackville, NB. Master’s Thesis, Dalhousie University, Halifax, NS, Canada, 2016. [Google Scholar]
- Brennan, L.A.; Kuvlesky, W.P., Jr. North American grassland birds: An unfolding conservation crisis? J. Wildl. Manag. 2005, 69, 1–13. [Google Scholar] [CrossRef]
- Svensson, S. Hackningsbiologiska studier i en koloni av backsvala, Riparia riparia, vid Ammarnas ar 1968. Vår Fågelvarld 1969, 28, 236–240, (In Swedish with an English Summary). [Google Scholar]
- Hopkins, L.; Officer, A.C. Best Practice Guidelines. Artificial Bank Creation for Sand Martins and Kingfishers; The Environment Agency: Rotherham, UK, 2001.
- Mondain-Monval, T.O.; Sharp, S.P. Burrow depth, carbon dioxide and reproductive success in Sand Martins Riparia riparia. Bird Study 2018, 65, 123–131. [Google Scholar] [CrossRef]
- Yuan, H.W.; Brent Burt, D.; Wang, L.P.; Chang, W.L.; Wang, M.K.; Chiou, C.R.; Ding, T.S. Colony site choice of blue-tailed bee-eaters: Influences of soil, vegetation, and water quality. J. Nat. Hist. 2006, 40, 485–493. [Google Scholar] [CrossRef]
- Thomas, G.W. Soil pH and soil acidity. Methods Soil Anal. 2018, 5, 475–490. [Google Scholar] [CrossRef]
- Francisca, F.M.; Glatstein, D.A. Long term hydraulic conductivity of compacted soils permeated with landfill leachate. Appl. Clay Sci. 2010, 49, 187–193. [Google Scholar] [CrossRef]
- Heneberg, P. Soil particle composition affects the physical characteristics of Sand Martin Riparia riparia holes. Ibis 2003, 145, 392–399. [Google Scholar] [CrossRef]
- Meisner, K.; Sunde, P.; Clausen, K.K.; Clausen, P.; Faelled, C.C.; Hoelgaard, M. Foraging ecology and spatial behaviour of the red fox (Vulpes vulpes) in a wet grassland ecosystem. Acta Theriol. 2014, 59, 377–389. [Google Scholar] [CrossRef]
- Persson, C. Population processes in south-west Scanian Sand martins (Riparia riparia). J. Zool. 1987, 1, 671–691. [Google Scholar] [CrossRef]
- Rendell, W.B.; Robertson, R.J. Cavity-Entrance Orientation and Nest-Site Use by Secondary Hole-Nesting Birds. J. Field Ornithol. 1994, 65, 27–35. [Google Scholar]
- Huhta, E.; Jokimäki, J. Breeding occupancy and success of two hole-nesting passerines: The impact of fragmentation caused by forestry. Ecography 2001, 24, 431–440. [Google Scholar] [CrossRef]
- Huhta, E.; Jokimäki, J.; Rahko, P. Distribution and reproductive success of the pied fly-catcher (Ficedula hypoleuca) in relation to forest patch size and vegetation characteristics; the effect of scale. Ibis 1998, 140, 214–222. [Google Scholar] [CrossRef]
- Jokimäki, J.; Huhta, E. Artificial nest predation and abundance of birds along an urban gradient. Condor 2000, 102, 838–847. [Google Scholar] [CrossRef]
- Rohrer, Z.; Rebollo, S.; Andivia, E.; Franco Goyena, J.; Rodriguez Urquia, C. Restoration and management for cliff-nesting birds in Mediterranean mining sites: The Sand Martin case study. Restor. Ecol. 2020, 28, 706–716. [Google Scholar] [CrossRef]
- Brown, C.R.; Brown, M.B. Ectoparasitism as a cause of natal dispersal in cliff swallows. Ecology 1992, 73, 1718–1723. [Google Scholar] [CrossRef]
- Ganter, B.; Cooke, F. Colonial nesters in a deteriorating habitat: Site fidelity and colony dynamics of lesser snow geese. Auk 1998, 115, 642–652. [Google Scholar]
- Augustin, J.; Blomqvist, D.; Szép, T.; Szabó, Z.D.; Wagner, R.H. No evidence of genetic benefits from extra-pair fertilisations in female sand martins (Riparia riparia). J. Ornithol. 2007, 148, 189–198. [Google Scholar] [CrossRef]
- Heneberg, P. Decision making in burrowing birds: Sediment properties in conflict with biological variables. Quat. Int. 2013, 296, 227–230. [Google Scholar] [CrossRef]
- Szép, T.; Für, J.; Molnár, E. A high level of nest predation observed in a large Sand Martin colony. Ornis Hung. 2016, 24, 46–53. [Google Scholar] [CrossRef]
- Brown, C.R.; Hoogland, J.L. Risk in mobbing for solitary and colonial swallows. Anim. Behav. 1986, 34, 1319–1323. [Google Scholar] [CrossRef]
- Hoogland, J.L.; Sherman, P.W. Advantages and disadvantages of bank swallow (Riparia riparia) coloniality. Ecol. Monogr. 1976, 46, 33–58. [Google Scholar] [CrossRef]
- Shurtliff, Q.R.; Whiting, J.C. 2009 Breeding Bird Surveys on the Idaho National Laboratory Site. 2010. U.S. Department of Energy-Idaho Operations Office Environmental Surveillance, Education, and Research Program. Available online: https://inl.gov/content/uploads/2023/08/2009BBSReport.pdf (accessed on 21 September 2023).
- Silver, M.; Griffin, C.R. Nesting habitat characteristics of bank swallows and belted king-fishers on the Connecticut River. Northeast Nat. 2009, 16, 519–534. [Google Scholar] [CrossRef]
- Hjertaas, D.G. Colony Site Selection in Bank Swallows. Master’s Thesis, University of Saskatchewan, Saskatchewan, SK, Canada, 1984. [Google Scholar]
- Castaño-Vázquez, F.; Merino, S.; Cuezva, S.; Sánchez-Moral, S. Nest gasses as a potential attraction cue for biting flying insects and other ectoparasites of cavity nesting birds. Front. Ecol. Evol. 2020, 8, 258. [Google Scholar] [CrossRef]
- Chaisson, K.E.; Hallem, E.A. Chemosensory behaviors of parasites. Trends Parasitol. 2012, 28, 427–436. [Google Scholar] [CrossRef]
- McLaren, C.M.; Sealy, S.G. Factors influencing susceptibility of host nests to brood parasitism. Ethol. Ecol. Evol. 2003, 15, 343–353. [Google Scholar] [CrossRef]
- Garcia, D. Spatial and Temporal Patterns of the Bank Swallow on the Sacramento River. Master’s Thesis, California State University, Chico, CA, USA, 2009. [Google Scholar]
- John, R.D. Observations on soil requirements for nesting bank swallows, Riparia riparia. Can. Field-Nat. 1991, 105, 251–254. [Google Scholar]
- Richner, H.; Heeb, P. Communal life: Honest signaling and the recruitment center hypothesis. Behav. Ecol. 1996, 7, 115–118. [Google Scholar] [CrossRef]
- Laughlin, A.J.; Sheldon, D.R.; Winkler, D.W.; Taylor, C.M. Quantifying non-breeding season occupancy patterns and the timing and drivers of autumn migration for a migratory songbird using Doppler radar. Ecography 2016, 39, 1017–1024. [Google Scholar] [CrossRef]
- Masoero, G.; Boano, G.; Alberto, T.; Caprio, E. Proper gravel management may counteract population decline of the Collared Sand Martin Riparia riparia. Avocetta 2019, 43, 139–147. [Google Scholar]


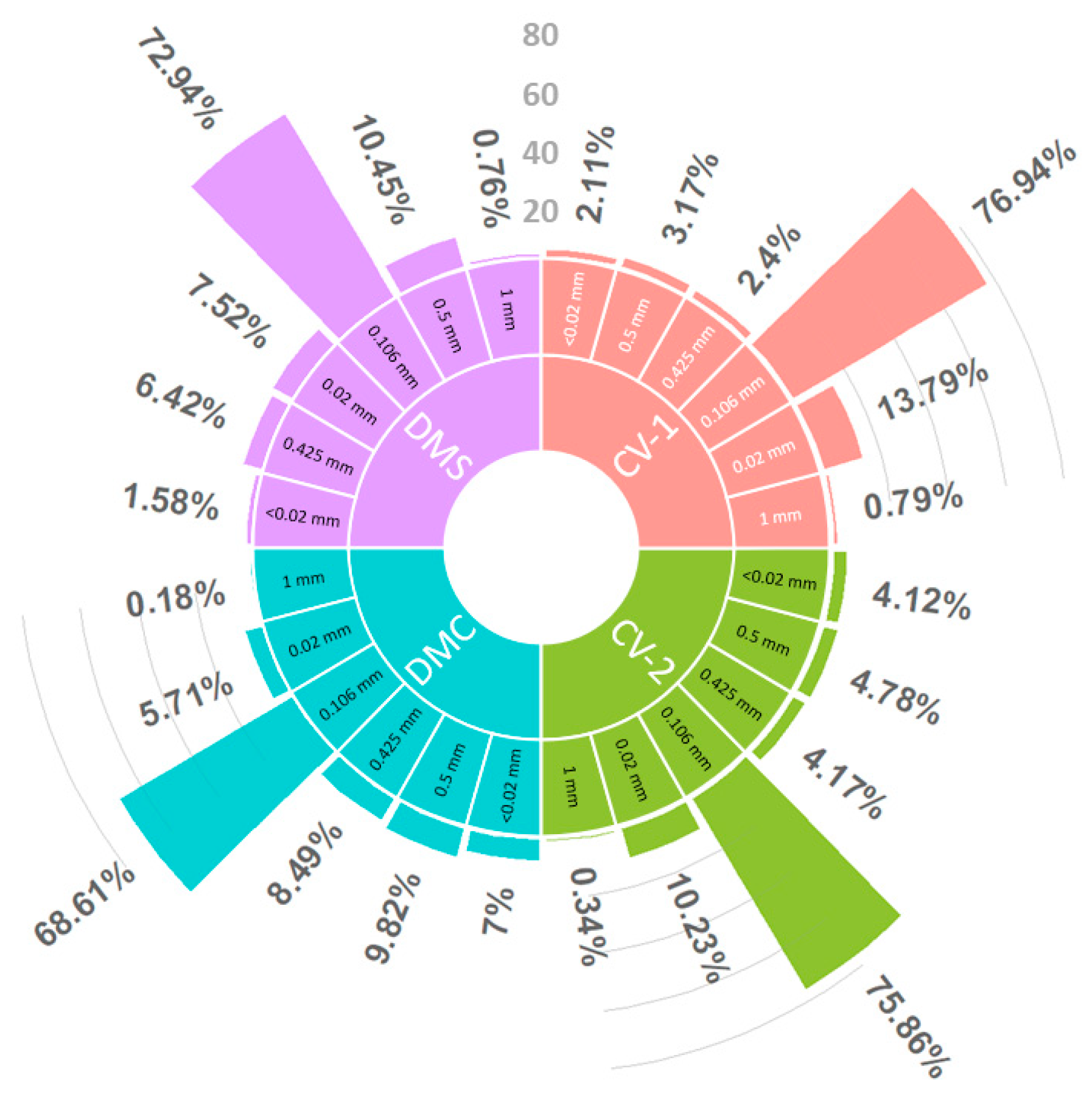
| Region | HE (cm) | LE (m) | NN (n) | DW (km) | DH (km) | DS (km) |
|---|---|---|---|---|---|---|
| CV-1 | 320 | 22 | 458 | 0.94 | 5.8 | 1.6 |
| CV-2 | 275 | 12 | 278 | 0.9 | 5.83 | 1.63 |
| DMS | 240 | 8 | 920 | 0.03 | 4.16 | 0.22 |
| DMC | 260 | 15 | 854 | 0.015 | 4.7 | 1.65 |
| β | SE | t | P | VIF | |
|---|---|---|---|---|---|
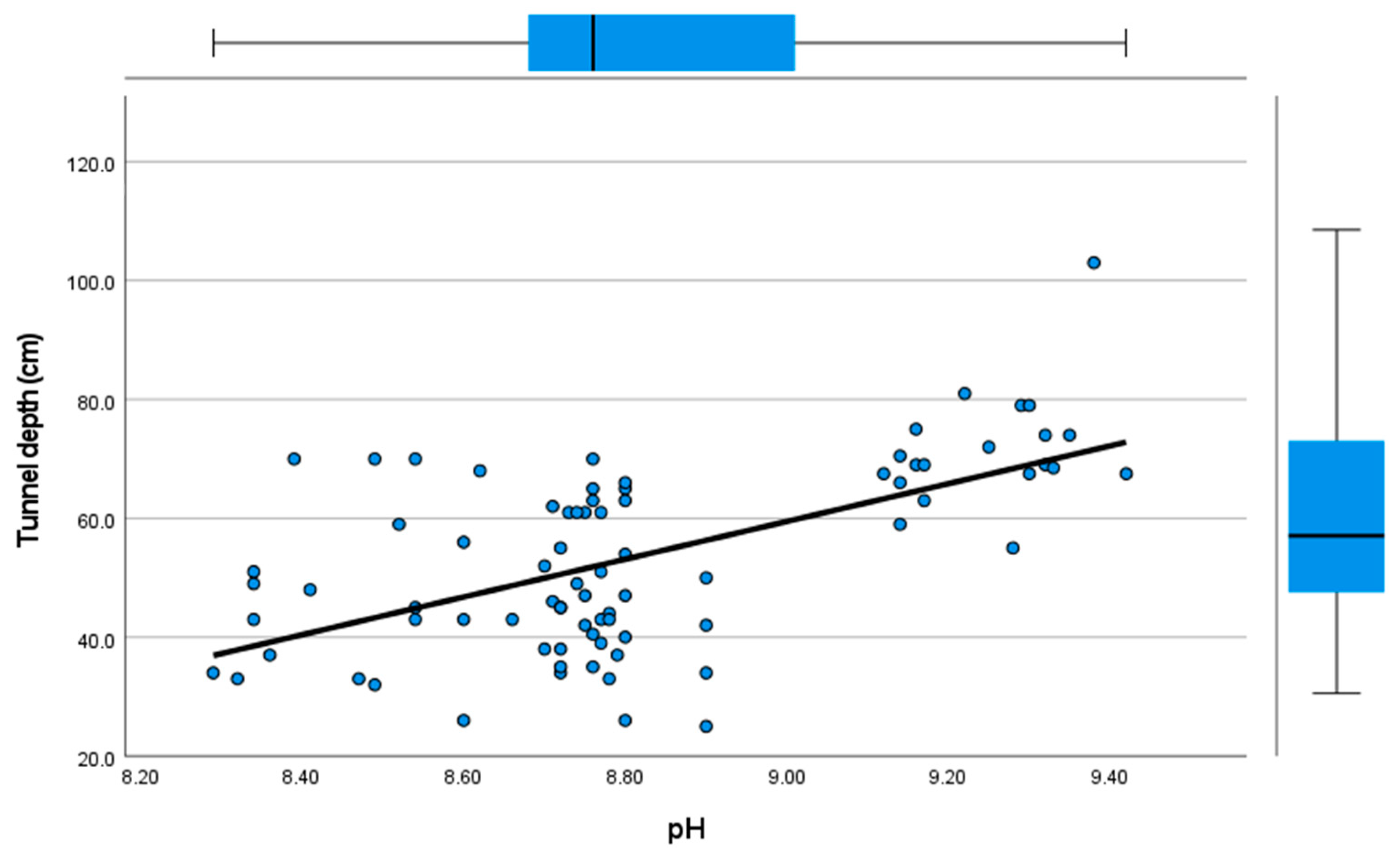
| Tunnel depth (R2 = 0.54; Adj. R2 = 0.51; SE = 0.51; F = 21.28, df = 4, 75; p < 0.001) | |||||
| Constant | −202.86 | 69.32 | −2.93 | 0.005 | |
| pH | 27.72 | 8.38 | 3.30 | 0.001 | 3.92 |
| EC | 0.116 | 0.03 | 3.42 | 0.001 | 3.43 |
| Location | −15.79 | 3.35 | 4.71 | <0.001 | 1.87 |
| Particle size | 0.001 | 0.01 | 0.05 | 0.962 | 1.07 |
| Distance between tunnel holes (R2 = 0.28; Adj. R2 = 0.43; SE = 2.09; F = 7.37, df = 4, 75; p < 0.001) | |||||
| Constant | 25.00 | 13.20 | 1.90 | 0.062 | |
| EC | 0.01 | 0.01 | 2.04 | 0.045 | 3.43 |
| Location | −2.53 | 0.64 | 3.96 | <0.001 | 1.87 |
| pH | −2.30 | 1.60 | −1.44 | 0.155 | 3.92 |
| Particle size | −0.001 | 0.002 | −0.33 | 0.742 | 1.02 |
| Width of the entrance opening (R2 = 0.26; Adj. R2 = 0.22; SE = 0.80; F = 6.49, df = 4;75; p < 0.001) | |||||
| Constant | 15.35 | 5.04 | 3.05 | 0.003 | |
| Particle size | 0.002 | 0.001 | 2.29 | 0.025 | 1.11 |
| Location | 0.09 | 0.24 | 3.52 | <0.001 | 1.87 |
| pH | −1.21 | 0.61 | −1.99 | 0.051 | 3.92 |
| EC | 0.004 | 0.002 | 1.49 | 0.139 | 3.43 |
| Tunnel Depth * | Distance Between Tunnel Holes | Width of the Entrance Opening | Height of the Entrance Opening | |
|---|---|---|---|---|
| Colony | ||||
| CV-1 (non-shore) | 46.90 ± 11.91 | 7.60 ± 2.10 | 5.90 ± 0.88 | 3.40 ± 0.50 |
| CV-2 (non-shore) | 55.70 ± 9.70 | 6.07 ± 2.51 | 5.40 ± 0.56 | 3.60 ± 0.73 |
| DMS (shore-site) | 71.42 ± 9.80 | 4.87 ± 1.53 | 6.32 ± 0.76 | 3.75 ± 0.57 |
| DMC (shore-site) | 40.37 ± 10.51 | 4.12 ± 1.86 | 6.37 ± 0.98 | 3.82 ± 0.54 |
| Kruskal–Wallis H | 44.64 | 23.26 | 16.28 | 3.47 |
| Df | 3 | 3 | 3 | 3 |
| Asymp. Sig. | 0.001 | 0.001 | 0.001 | 0.325 |
| Pairwise adjusted significant differences, Dunn test | CV2 > DMC | CV1 > DMC | DMS > CV2 | not tested |
| DMS > DMC | CV1 > DMS | DMC > CV2 | ||
| DMS > CV1 | ||||
| DMS > CV2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Çelik, E.; Durmus, A.; Jokimäki, J. Sand-Related Factors Influencing Nest Burrowing Potential of the Sand Martins. Animals 2023, 13, 3463. https://doi.org/10.3390/ani13223463
Çelik E, Durmus A, Jokimäki J. Sand-Related Factors Influencing Nest Burrowing Potential of the Sand Martins. Animals. 2023; 13(22):3463. https://doi.org/10.3390/ani13223463
Chicago/Turabian StyleÇelik, Emrah, Atilla Durmus, and Jukka Jokimäki. 2023. "Sand-Related Factors Influencing Nest Burrowing Potential of the Sand Martins" Animals 13, no. 22: 3463. https://doi.org/10.3390/ani13223463
APA StyleÇelik, E., Durmus, A., & Jokimäki, J. (2023). Sand-Related Factors Influencing Nest Burrowing Potential of the Sand Martins. Animals, 13(22), 3463. https://doi.org/10.3390/ani13223463





