RNAseq Analysis of Livers from Pigs Treated with Testosterone and Nandrolone Esters: Selection and Field Validation of Transcriptional Biomarkers
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.1.1. Preclinical Trial
2.1.2. Clinical Trial
2.2. Field Survey
- grower pigs with weight ranges lower than experimental animals (50–60 kg)
- medium-size pigs intended for fresh pork meat production (from 90 kg up to 110 kg)
- heavy-weight pigs intended for cured meat and production of sausages (from 150 kg up to 180 kg b.w.).
2.3. Molecular Analysis
2.3.1. Sample Preparation
2.3.2. RNA Sequencing Analysis
2.3.3. Biomarkers Validation Study
2.4. Experimental Samples Residues Analysis
2.4.1. Chemicals and Reagents
2.4.2. Blood Sample Preparation
2.4.3. LC-MS/MS Analysis
2.5. Analysis of Field Sample Residues
2.5.1. Chemicals and Reagents
2.5.2. Blood Sample Preparation
2.5.3. LC-MS/MS Analysis
2.6. Data Analysis
3. Results
3.1. Animal Trial and Collection of Field Samples
3.2. Quality Check of RNA Samples
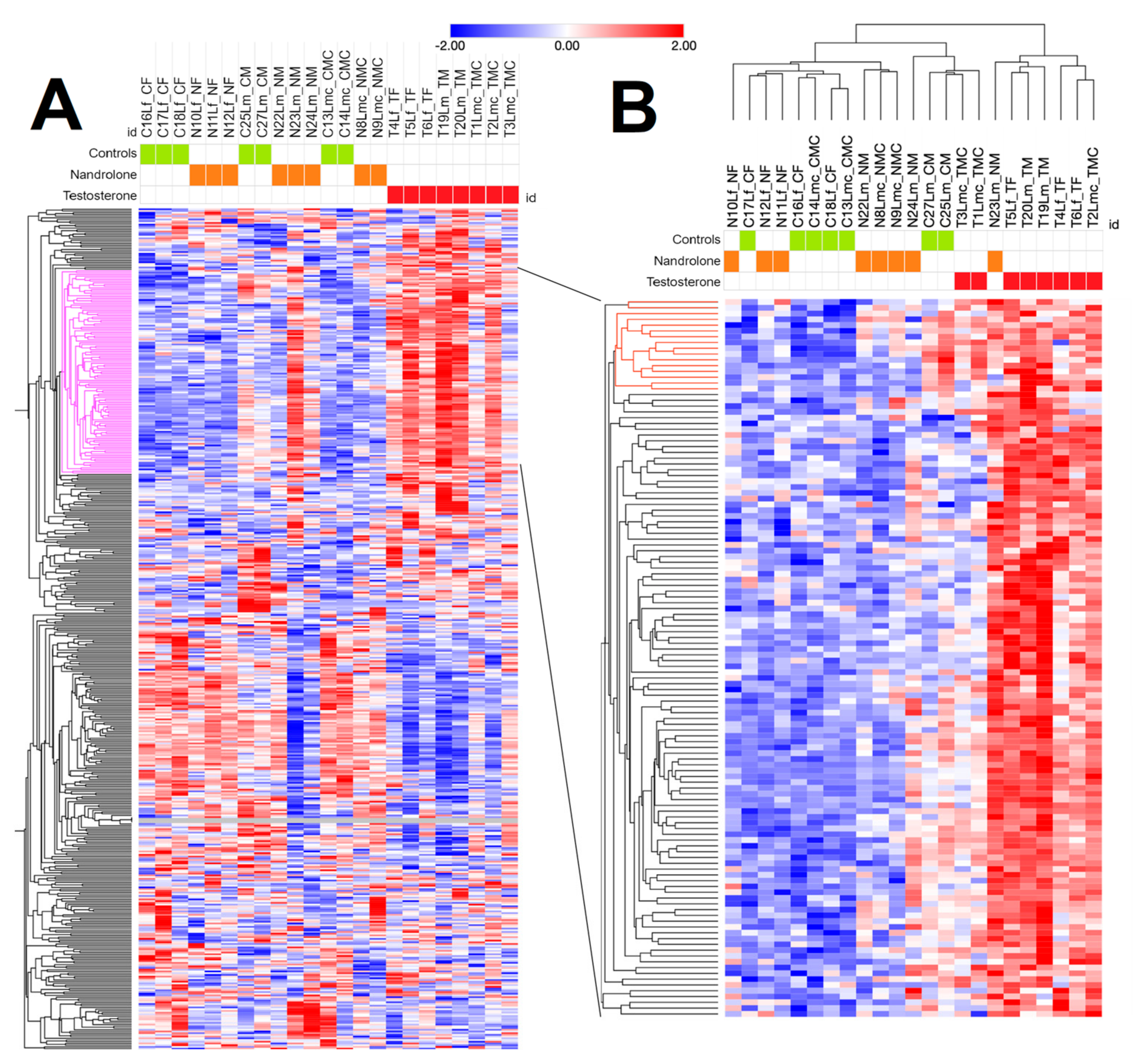
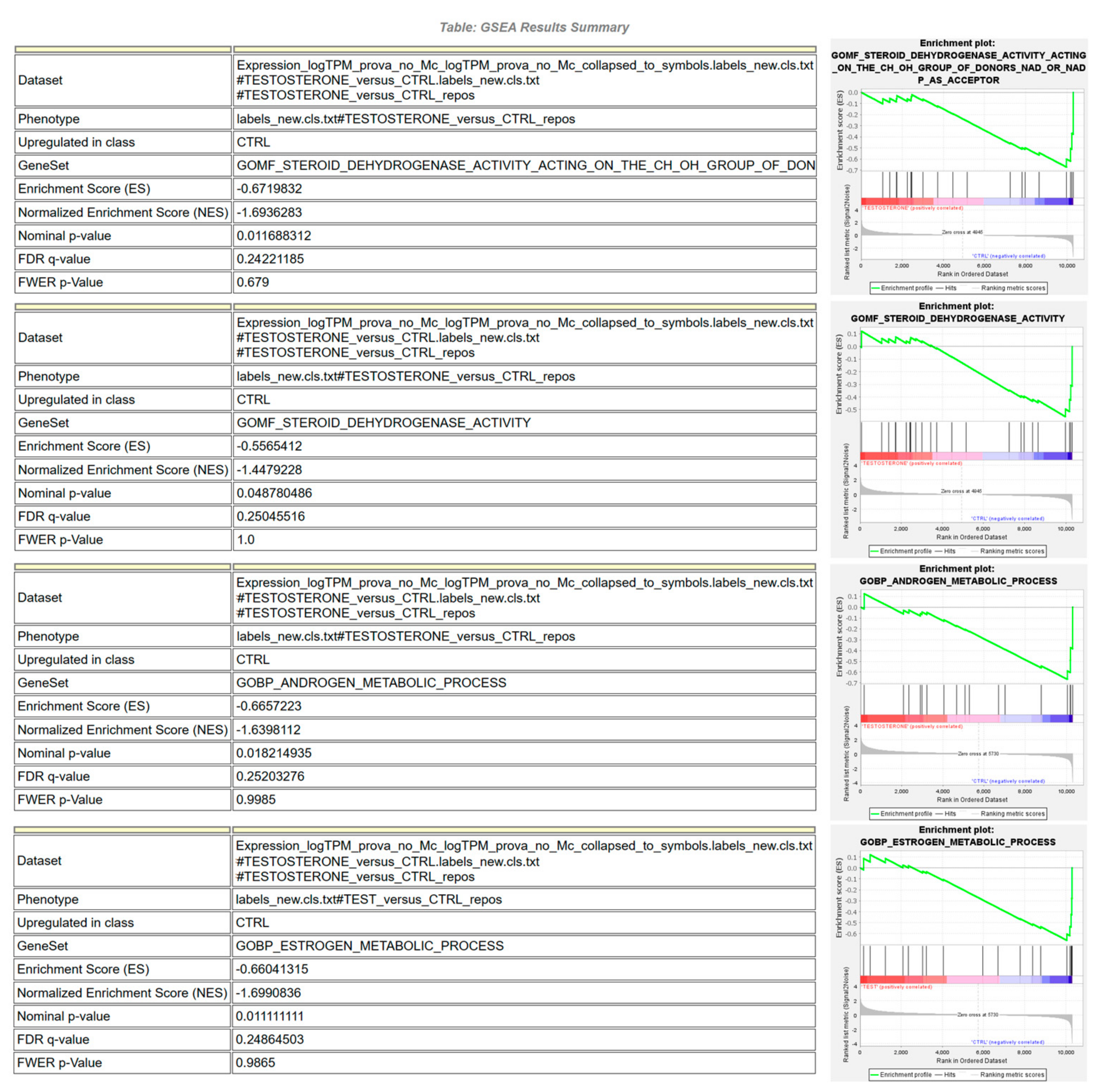
3.3. RNAseq Analysis
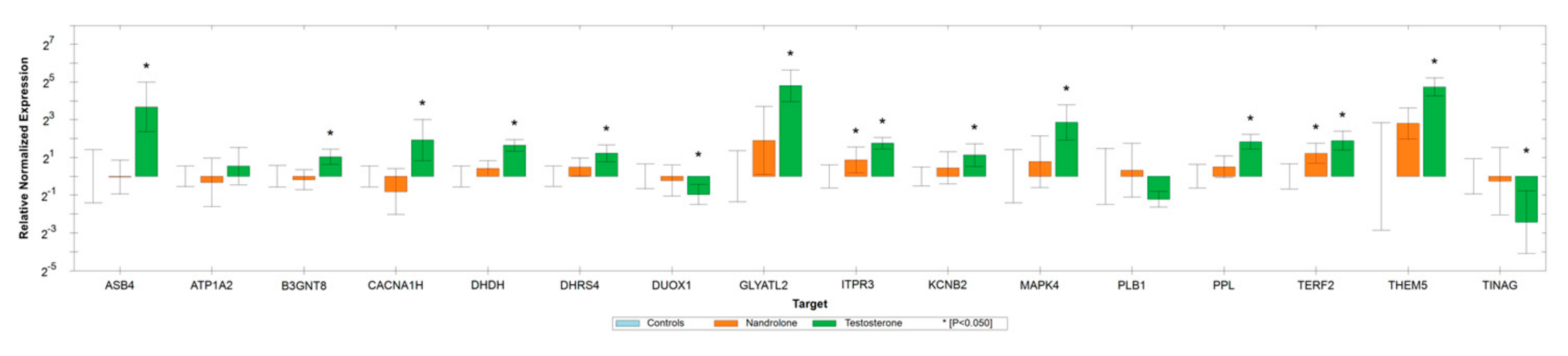
3.4. Real-Time PCR Validation Study
3.5. Analysis of Steroid Residues (Experimental Animals)
3.6. Analysis of Steroid Residues (Field Animals)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nachman, K.E.; Smith, T.J.S. Hormone Use in Food Animal Production: Assessing Potential Dietary Exposures and Breast Cancer Risk. Curr. Environ. Heal. Rep. 2015, 2, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Qaid, M.M.; Abdoun, K.A. Safety and concerns of hormonal application in farm animal production: A review. J. Appl. Anim. Res. 2022, 50, 426–439. [Google Scholar] [CrossRef]
- Passantino, A. Steroid Hormones in Food Producing Animals. In A Bird’s-Eye View of Veterinary Medicine; IntechOpen: London, UK, 2012. [Google Scholar]
- European Food Safety Authority. Report for 2018 on the results from the monitoring of veterinary medicinal product residues and other substances in live animals and animal products. EFSA Support. Publ. 2020, 17, 1775E. [Google Scholar] [CrossRef]
- European Food Safety Authority. Report for 2019 on the results from the monitoring of veterinary medicinal product residues and other substances in live animals and animal products. EFSA Support. Publ. 2021, 18, 1997E. [Google Scholar] [CrossRef]
- Marclay, F.; Mangin, P.; Margot, P.; Saugy, M. Perspectives for Forensic Intelligence in anti-doping: Thinking outside of the box. Forensic Sci. Int. 2013, 229, 133–144. [Google Scholar] [CrossRef] [PubMed]
- EUROPOL. Keeping Sport Safe and Fair: 3.8 Million Doping Substances and Fake Medicines Seized Worldwide. Available online: https://www.europol.europa.eu/media-press/newsroom/news/keeping-sport-safe-and-fair-38-million-doping-substances-and-fake-medicines-seized-worldwide (accessed on 11 June 2023).
- Gadaj, A.; Ventura, E.; Ripoche, A.; Mooney, M.H. Monitoring of selective androgen receptor modulators in bovine muscle tissue by ultra-high performance liquid chromatography-tandem mass spectrometry. Food Chem. X 2019, 4, 100056. [Google Scholar] [CrossRef] [PubMed]
- Gheddar, L.; Ameline, A.; Raul, J.S.; Kintz, P. Designer anabolic steroids: A challenge for toxicologists. Toxicol. Anal. Clin. 2019, 31, 293–297. [Google Scholar] [CrossRef]
- Poelmans, S.; De Wasch, K.; Noppe, H.; Van Hoof, N.; Van Cruchten, S.; Le Bizec, B.; Deceuninck, Y.; Sterk, S.; Van Rossum, H.J.; Hoffman, M.K.; et al. Endogenous occurrence of some anabolic steroids in swine matrices. Food Addit. Contam. 2005, 22, 808–815. [Google Scholar] [CrossRef]
- Hartmann, S.; Lacorn, M.; Steinhart, H. Natural occurrence of steroid hormones in food. Food Chem. 1998, 62, 7–20. [Google Scholar] [CrossRef]
- Nielen, M.W.F.; Nijrolder, A.W.J.M.; Hooijerink, H.; Stolker, A.A.M. Feasibility of desorption electrospray ionization mass spectrometry for rapid screening of anabolic steroid esters in hair. Anal. Chim. Acta 2011, 700, 63–69. [Google Scholar] [CrossRef]
- Janssens, G.; Courtheyn, D.; Mangelinckx, S.; Prévost, S.; Bichon, E.; Monteau, F.; De Poorter, G.; De Kimpe, N.; Le Bizec, B. Use of isotope ratio mass spectrometry to differentiate between endogenous steroids and synthetic homologues in cattle: A review. Anal. Chim. Acta 2013, 772, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Pezzolato, M.; Baioni, E.; Maurella, C.; Benedetto, A.; Biasibetti, E.; Bozzetta, E. The Italian strategy to fight illegal treatment with growth promoters: Results of the 2017–2019 histological monitoring plan. Ital. J. Food Saf. 2022, 10. [Google Scholar] [CrossRef] [PubMed]
- Benedetto, A.; Pezzolato, M.; Biasibetti, E.; Bozzetta, E. Omics applications in the fight against abuse of anabolic substances in cattle: Challenges, perspectives and opportunities. Curr. Opin. Food Sci. 2021, 40, 112–120. [Google Scholar] [CrossRef]
- Skoupá, K.; Šťastný, K.; Sládek, Z. Anabolic Steroids in Fattening Food-Producing Animals—A Review. Animals 2022, 12, 2115. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Bi, Y.; Bingga, G.; Li, X.; Zhang, S.; Li, J.; Li, H.; Ding, S.; Xia, X. Metabolomic analysis of swine urine treated with β2-agonists by ultra-high performance liquid chromatography-quadrupole time-of-flight mass spectrometry. J. Chromatogr. A 2015, 1400, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Fu, Y.; Han, X.; Li, X.; Li, C. Metabolomic investigation of porcine muscle and fatty tissue after Clenbuterol treatment using gas chromatography/mass spectrometry. J. Chromatogr. A 2016, 1456, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Stastny, K.; Putecova, K.; Leva, L.; Franek, M.; Dvorak, P.; Faldyna, M. Profiling of metabolomic changes in plasma and urine of pigs caused by illegal administration of testosterone esters. Metabolites 2020, 10, 307. [Google Scholar] [CrossRef]
- Peng, T.; Royer, A.L.; Guitton, Y.; Le Bizec, B.; Dervilly-Pinel, G. Serum-based metabolomics characterization of pigs treated with ractopamine. Metabolomics 2017, 13, 77. [Google Scholar] [CrossRef]
- Beccuti, M.; Cordero, F.; Arigoni, M.; Panero, R.; Amparore, E.G.; Donatelli, S.; Calogero, R.A. SeqBox: RNAseq/ChIPseq reproducible analysis on a consumer game computer. Bioinformatics 2018, 34, 871–872. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Raudvere, U.; Kolberg, L.; Kuzmin, I.; Arak, T.; Adler, P.; Peterson, H.; Vilo, J. G:Profiler: A web server for functional enrichment analysis and conversions of gene lists (2019 update). Nucleic Acids Res. 2019, 47, W191–W198. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef] [PubMed]
- Grätz, C.; Bui, M.L.U.; Thaqi, G.; Kirchner, B.; Loewe, R.P.; Pfaffl, M.W. Obtaining Reliable RT-qPCR Results in Molecular Diagnostics—MIQE Goals and Pitfalls for Transcriptional Biomarker Discovery. Life 2022, 12, 386. [Google Scholar] [CrossRef] [PubMed]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, 1–12. [Google Scholar] [CrossRef]
- Markham, N.R. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 2003, 31, 3406–3415. [Google Scholar]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Pezzolato, M.; Biasibetti, E.; Gili, M.; Maurella, C.; Benedetto, A.; Marturella, M.; Ostorero, F.; Bozzo, G.; Bellino, C.; D’angelo, A.; et al. Assessment of the Physiological Values and the Reference Histological Profile Related to Sex Steroids in Veal Calves. Agriculture 2023, 13, 1145. [Google Scholar] [CrossRef]
- Tereszkiewicz, K.; Kulig, Ł.; Antos, P.; Kowalczyk, K. Influence of the Level of Sex Hormones in the Blood of Gilts on Slaughter Characteristics and Meat Quality. Animals 2023, 13, 267. [Google Scholar] [CrossRef]
- Kress, K.; Weiler, U.; Schmucker, S.; Čandek-Potokar, M.; Vrecl, M.; Fazarinc, G.; Škrlep, M.; Batorek-Lukač, N.; Stefanski, V. Influence of housing conditions on reliability of immunocastration and consequences for growth performance of male pigs. Animals 2020, 10, 27. [Google Scholar] [CrossRef]
- Arrizabalaga Larranaga, A.; Groot, M.J.; Blokland, M.H.; Barbu, I.M.; Smits, N.G.E.; Sterk, S.S. EURL Reflection Paper 2.0: Natural Growth Promoting Substances in Biological Samples: Presence—and Formation—of Hormones and Other Growth Promoting Substances in Food Producing Animals. 2022. Available online: https://edepot.wur.nl/606740 (accessed on 11 June 2023).
- Stella, R.; Bovo, D.; Mastrorilli, E.; Manuali, E.; Pezzolato, M.; Bozzetta, E.; Lega, F.; Angeletti, R.; Biancotto, G. A Novel Tool to Screen for Treatments with Clenbuterol in Bovine: Identification of two Hepatic Markers by Metabolomics Investigation. Food Chem. 2021, 353, 129366. [Google Scholar] [CrossRef]
- De Wasch, K.; Le Bizec, B.; De Brabander, H.; Andr, F.; Impens, S. Consequence of boar edible tissue consumption on urinary profiles of nandrolone metabolites. II. Identification and quantification of 19-norsteroids responsible for 19-norandrosterone and 19-noretiocholanolone excretion in human urine. Rapid Commun. Mass Spectrom. 2001, 15, 1442–1447. [Google Scholar] [CrossRef] [PubMed]
- Hülsemann, F.; Fußhöller, G.; Lehn, C.; Thevis, M. Excretion of 19-norandrosterone after consumption of boar meat. Drug Test. Anal. 2020, 12, 1581–1586. [Google Scholar] [CrossRef] [PubMed]
- Elgendy, R.; Giantin, M.; Montesissa, C.; Dacasto, M. The transcriptome of muscle and liver is responding differently to a combined trenbolone acetate and estradiol implant in cattle. Steroids 2016, 106, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Riedmaier, I.; Pfaffl, M.W.; Meyer, H.H.D. The physiological way: Monitoring RNA expression changes as new approach to combat illegal growth promoter application. Drug Test. Anal. 2012, 4, 70–74. [Google Scholar] [CrossRef]
- Benedetto, A.; Pezzolato, M.; Robotti, E.; Biasibetti, E.; Poirier, A.; Dervilly, G.; Le Bizec, B.; Marengo, E.; Bozzetta, E. Profiling of transcriptional biomarkers in FFPE liver samples: PLS-DA applications for detection of illicit administration of sex steroids and clenbuterol in veal calves. Food Control 2021, 128, 108149. [Google Scholar] [CrossRef]
- Benedetto, A.; Biasibetti, E.; Robotti, E.; Marengo, E.; Audino, V.; Bozzetta, E.; Pezzolato, M. Transcriptional Biomarkers and Immunohistochemistry for Detection of Illicit Dexamethasone Administration in Veal Calves. Foods 2022, 11, 1810. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Yang, S.; Cheng, Y.; Hou, C.; You, X.; Zhao, J.; Zhang, Y.; He, W. Identification of transcriptional biomarkers by RNA-sequencing for improved detection of β2-agonists abuse in goat skeletal muscle. PLoS ONE 2017, 12, e0181695. [Google Scholar] [CrossRef] [PubMed]
- Dervilly-Pinel, G.; Royer, A.L.; Bozzetta, E.; Pezzolato, M.; Herpin, L.; Prevost, S.; Le Bizec, B. When LC-HRMS metabolomics gets ISO17025 accredited and ready for official controls–application to the screening of forbidden compounds in livestock. Food Addit. Contam.—Part A Chem. Anal. Control. Expo. Risk Assess 2018, 35, 1948–1958. [Google Scholar] [CrossRef]
- Directorate-General for Health and Food. Safety Guidelines on EU Requirements for Entry of Animals and Products of Animal Origin Control Plans for Residues of Veterinary Medicines, Pesticides and Contaminants; Directorate-General for Health and Food: Brussel, Belgium, 2023. [Google Scholar]
- Farschtschi, S.; Riedmaier-Sprenzel, I.; Phomvisith, O.; Gotoh, T.; Pfaffl, M.W. The successful use of -omic technologies to achieve the ‘One Health’ concept in meat producing animals. Meat Sci. 2022, 193, 108949. [Google Scholar] [CrossRef]



| Analytes | DP | EP | Parent Ion | Product Ion | CE | CXP |
|---|---|---|---|---|---|---|
| 17β-estradiol-d4 | −130 | −10 | 275.2 | 147.0 | −53 | −12 |
| 187.0 | −54 | |||||
| 145.0 | −65 | |||||
| 17β-estradiol | −100 | −10 | 271.2 | 145.0 | −50 | −12 |
| 183.1 | −54 | |||||
| 143.0 | −67 | |||||
| 17β-testosterone-d3 | 90 | 10 | 292.2 | 256.4 | 25 | 12 |
| 109 | 32 | |||||
| 97.2 | 29 | |||||
| 17β-testosterone | 74 | 10 | 289.1 | 97.2 | 29 | 12 |
| 109.0 | 33 | |||||
| 79.1 | 70 | |||||
| Progesterone-d9 | 80 | 10 | 324.3 | 100.1 | 29 | 12 |
| 113.1 | 33 | |||||
| 306.4 | 25 | |||||
| Progesterone | 103 | 10 | 315.2 | 97.1 | 26 | 12 |
| 109.1 | 30 | |||||
| 297.4 | 22 |
| Group | Pig No. | Sex | Liver | Serum |
|---|---|---|---|---|
| Testosterone | 1 | barrow | T1L-Mc | S1 |
| 2 | barrow | T2L-Mc | S2 | |
| 3 | barrow | T3L-Mc | S3 | |
| 4 | gilt | T4L-F | S4 | |
| 5 | gilt | T5L-F | S5 | |
| 6 | gilt | T6L-F | S6 | |
| 19 | boar | T19L-M | S19 | |
| 20 | boar | T20L-M | S20 | |
| 21 | boar | T21L-M | S21 | |
| Nandrolone | 8 | barrow | N8L-Mc | S8 |
| 9 | barrow | N9L-Mc | S9 | |
| 10 | gilt | N10L-F | S10 | |
| 11 | gilt | N11L-F | S11 | |
| 12 | gilt | N12L-F | S12 | |
| 22 | boar | N22L-M | S22 | |
| 23 | boar | N23L-M | S23 | |
| 24 | boar | N24L-M | S24 | |
| Control | 13 | barrow | C13L-Mc | S13 |
| 14 | barrow | C14L-Mc | S14 | |
| 15 | barrow | C15L-Mc | S15 | |
| 16 | gilt | C16L-F | S16 | |
| 17 | gilt | C17L-F | S17 | |
| 18 | gilt | C18L-F | S18 | |
| 25 | boar | C25L-M | S25 | |
| 26 | boar | C26L-M | S26 | |
| 27 | boar | C27L-M | S27 |
| Group | Sample ID | Sex | Testosterone | Nandrolone | Estradiol |
|---|---|---|---|---|---|
| Concentration (ng/mL) | Concentration (ng/mL) | Concentration (ng/mL) | |||
| Testosterone | S1 | Barrow | 15.85 | 0 | 0 |
| S2 | Barrow | 13.71 | 0 | 0 | |
| S3 | Barrow | 17.48 | 0 | 0 | |
| S4 | Gilt | 16.79 | 0 | 0 | |
| S5 | Gilt | 21.20 | 0 | 0 | |
| S6 | Gilt | 41.99 | 0 | 0.014 | |
| S19 | Boar | 15.99 | 0 | 0 | |
| S20 | Boar | 20.53 | 0 | 0 | |
| S21 | Boar | 16.17 | 0 | 0 | |
| Nandrolone | S8 | Barrow | 0 | 7.32 | 0 |
| S9 | Barrow | 0 | 4.67 | 0 | |
| S10 | Gilt | 0 | 5.71 | 0 | |
| S11 | Gilt | 0 | 8.49 | 0 | |
| S12 | Gilt | 0 | 5.47 | 0 | |
| S22 | Boar | 0 | 10.72 | 0 | |
| S23 | Boar | 1.19 | 7.59 | 0 | |
| S24 | Boar | 1.17 | 12.35 | 0 | |
| Control | S13 | Barrow | 0 | 0 | 0 |
| S14 | Barrow | 0 | 0 | 0 | |
| S15 | Barrow | 0 | 0 | 0 | |
| S16 | Gilt | 0 | 0 | 0 | |
| S17 | Gilt | 0 | 0 | 0.044 | |
| S18 | Gilt | 0 | 0 | 0 | |
| S25 | Boar | 1.97 | 0 | 0.022 | |
| S26 | Boar | 1.91 | 0.90 | 0.031 | |
| S27 | Boar | 2.15 | 3.93 | 0 |
| Animal ID | Sex | Breed | Weight Range for Batch (Kg) | Weight Category | Age (Months) | Progesterone (ng/mL) | Estradiol (ng/mL) |
|---|---|---|---|---|---|---|---|
| 1 | Barrow | Black Pig of Piedmont | 90 | medium | 8 | 1.40 | 0 |
| 2 | Gilt | Black Pig of Piedmont | 90 | medium | 8 | 15.22 | 0 |
| 3 | Gilt | Large White | 150–160 | heavy | 10 | 21.76 | 0 |
| 4 | Gilt | Large White | 150–160 | heavy | 10 | 31.24 | 0 |
| 6 | Gilt | Large White | 150–160 | heavy | 10 | 2.40 | 0 |
| 7 | Barrow | Large White | 150–160 | heavy | 10 | 0 | 0 |
| 8 | Gilt | Unknown commercial crossbred | 100–110 | medium | 10 | 0.62 | 0 |
| 9 | Gilt | Unknown commercial crossbred | 100–110 | medium | 10 | 0 | 0 |
| 10 | Gilt | Unknown commercial crossbred | 100–110 | medium | 10 | 28.08 | 0 |
| 11 | Gilt | Unknown commercial crossbred | 100–110 | medium | 10 | 15.38 | 0 |
| 12 | Barrow | Large White × Landrace | 150–160 | heavy | 11 | 0 | 0 |
| 15 | Barrow | Large White × Landrace | 150–160 | heavy | 11 | 0 | 0 |
| 16 | Barrow | Large White × Landrace | 150–160 | heavy | 11 | 0 | 0 |
| 17 | Barrow | Large White × Landrace | 150–160 | heavy | 11 | 0 | 0 |
| 18 | Barrow | Large White × Landrace | 150–160 | heavy | 11 | 0 | 0 |
| 19 | Barrow | Large White × Landrace | 150–160 | heavy | 11 | 0 | 0 |
| 20 | Barrow | Large White × Landrace | 150–160 | heavy | 11 | 0 | 0 |
| 21 | Barrow | Large White × Landrace | 150–160 | heavy | 11 | 0 | 0 |
| 23 | Gilt | Large White | 110–120 | medium | 10 | 0 | 0 |
| 24 | Gilt | Large White | 110–120 | medium | 10 | 0 | 0 |
| 25 | Gilt | Large White | 110–120 | medium | 10 | 0.51 | 0 |
| 26 | Barrow | Large White | 110–120 | medium | 10 | 1.21 | 0 |
| 27 | Gilt | Black Pig of Piedmont | 90–100 | medium | 8 | 0 | 0 |
| 28 | Barrow | Black Pig of Piedmont | 90–100 | medium | 8 | 0.75 | 0 |
| 29 | Barrow | Black Pig of Piedmont | 90–100 | medium | 8 | 0.45 | 0 |
| 30 | Gilt | Black Pig of Piedmont | 90–100 | medium | 8 | 0 | 0 |
| 31 | Barrow | Black Pig of Piedmont × Large White | 100–110 | medium | 8 | 0 | 0 |
| 32 | Barrow | Black Pig of Piedmont × Large White | 100–110 | medium | 8 | 0 | 0 |
| 33 | Gilt | Black Pig of Piedmont × Large White | 100–110 | medium | 8 | 18.94 | 0 |
| 35 | Barrow | Landrace | 170–180 | heavy | 9 | 0 | 0 |
| 36 | Barrow | Landrace | 170–180 | heavy | 9 | 0 | 0 |
| 37 | Barrow | Landrace | 170–180 | heavy | 9 | 0 | 0 |
| 38 | Barrow | Landrace | 170–180 | heavy | 9 | 0 | 0 |
| 39 | Barrow | Landrace | 170–180 | heavy | 9 | 0 | 0 |
| 41 | Barrow | Landrace | 170–180 | heavy | 9 | 0 | 0 |
| 43 | Barrow | Landrace | 170–180 | heavy | 9 | 0 | 0 |
| 44 | Gilt | Unknown commercial crossbred | 150–160 | heavy | 10 | 8.04 | 0 |
| 46 | Gilt | Unknown commercial crossbred | 150–160 | heavy | 10 | 0 | 0.02 |
| 47 | Gilt | Unknown commercial crossbred | 150-–160 | heavy | 10 | 26.08 | 0 |
| 50 | Barrow | Unknown commercial crossbred | 150–160 | heavy | 10 | 0 | 0 |
| 51 | Barrow | Unknown commercial crossbred | 150–160 | heavy | 10 | 0 | 0 |
| 52 | Gilt | Unknown commercial crossbred | 150–160 | heavy | 10 | 5.72 | 0 |
| 53 | Gilt | Duroc | 60–70 | small | 6 | 4.41 | 0 |
| 54 | Gilt | Duroc | 60–70 | small | 6 | 0 | 0 |
| 55 | Barrow | Duroc | 60–70 | small | 6 | 0 | 0 |
| 56 | Gilt | Duroc | 60–70 | small | 6 | 0.66 | 0 |
| 57 | Gilt | Duroc | 60–70 | small | 6 | 0 | 0 |
| 58 | Barrow | Black Pig of Sicily | 70–90 | medium | 8 | 0 | 0 |
| 59 | Barrow | Black Pig of Sicily | 70–90 | medium | 8 | 0 | 0 |
| 60 | Gilt | Black Pig of Sicily | 70–90 | medium | 8 | 0 | 0 |
| 61 | Barrow | Black Pig of Sicily | 70–90 | medium | 8 | 0 | 0 |
| 62 | Gilt | Black Pig of Sicily | 70–90 | medium | 8 | 0 | 0 |
| 63 | Barrow | Black Pig of Sicily | 70–90 | medium | 8 | 0 | 0 |
| 64 | Gilt | Black Pig of Sicily | 70-90 | medium | 8 | 18.97 | 0 |
| 65 | Barrow | Black Pig of Sicily | 70–90 | medium | 8 | 0 | 0 |
| 66 | Barrow | Black Pig of Sicily | 70–90 | medium | 8 | 0 | 0 |
| 67 | Barrow | Black Pig of Sicily | 70–90 | medium | 8 | 0.48 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benedetto, A.; Šťastný, K.; Giaccio, N.; Marturella, M.; Biasibetti, E.; Arigoni, M.; Calogero, R.; Gili, M.; Pezzolato, M.; Tošnerová, K.; et al. RNAseq Analysis of Livers from Pigs Treated with Testosterone and Nandrolone Esters: Selection and Field Validation of Transcriptional Biomarkers. Animals 2023, 13, 3495. https://doi.org/10.3390/ani13223495
Benedetto A, Šťastný K, Giaccio N, Marturella M, Biasibetti E, Arigoni M, Calogero R, Gili M, Pezzolato M, Tošnerová K, et al. RNAseq Analysis of Livers from Pigs Treated with Testosterone and Nandrolone Esters: Selection and Field Validation of Transcriptional Biomarkers. Animals. 2023; 13(22):3495. https://doi.org/10.3390/ani13223495
Chicago/Turabian StyleBenedetto, Alessandro, Kamil Šťastný, Nunzia Giaccio, Marianna Marturella, Elena Biasibetti, Maddalena Arigoni, Raffaele Calogero, Marilena Gili, Marzia Pezzolato, Kristína Tošnerová, and et al. 2023. "RNAseq Analysis of Livers from Pigs Treated with Testosterone and Nandrolone Esters: Selection and Field Validation of Transcriptional Biomarkers" Animals 13, no. 22: 3495. https://doi.org/10.3390/ani13223495
APA StyleBenedetto, A., Šťastný, K., Giaccio, N., Marturella, M., Biasibetti, E., Arigoni, M., Calogero, R., Gili, M., Pezzolato, M., Tošnerová, K., Hodkovicová, N., Faldyna, M., Puleio, R., Bozzo, G., & Bozzetta, E. (2023). RNAseq Analysis of Livers from Pigs Treated with Testosterone and Nandrolone Esters: Selection and Field Validation of Transcriptional Biomarkers. Animals, 13(22), 3495. https://doi.org/10.3390/ani13223495






