Thyreophagus tauricus sp. n., a New Subcortical Mite Species (Acari: Acaridae), with a COX1 DNA Sequence Analysis of Several Economically Important Species of Thyreophagus †
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Molecular Identification
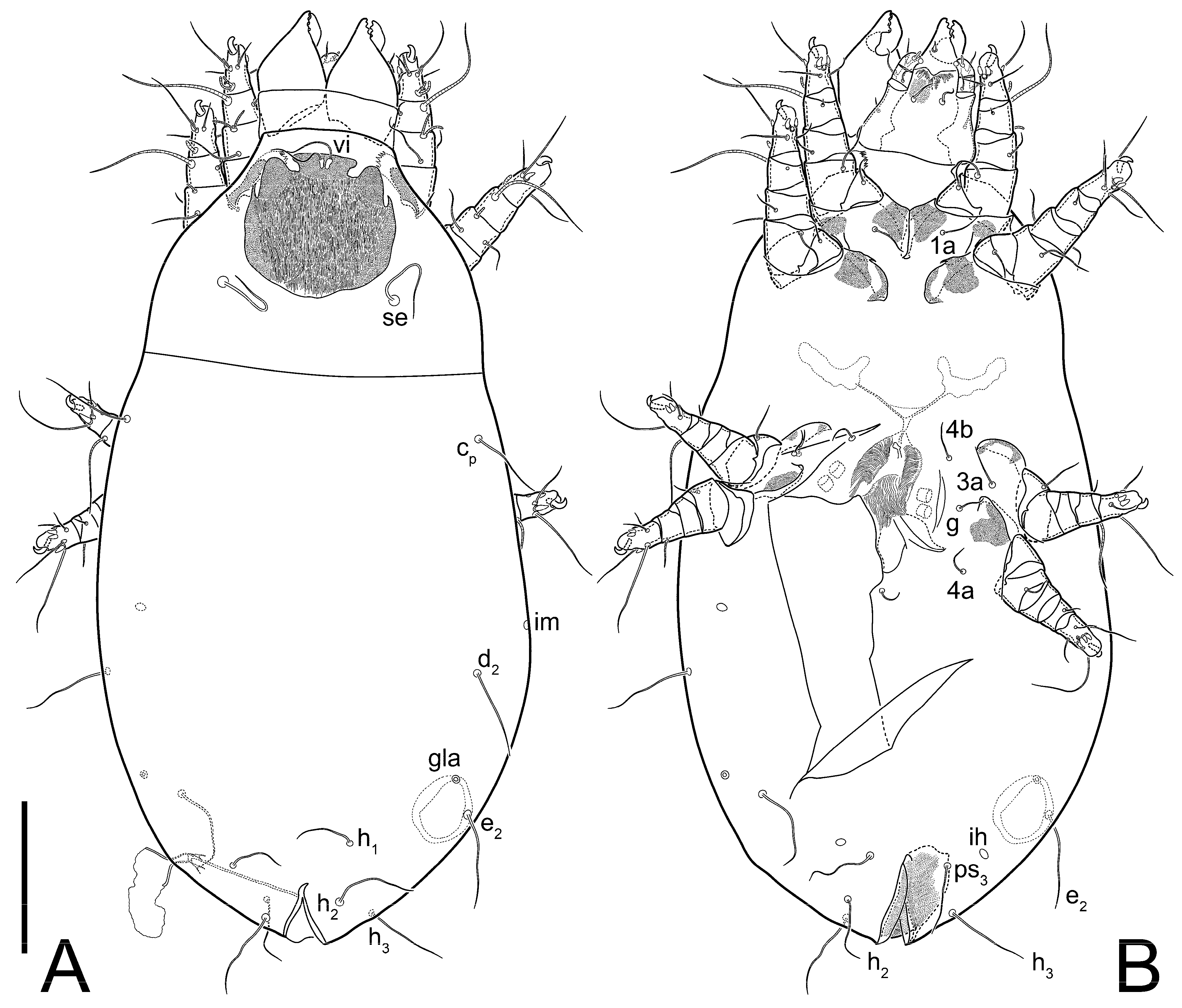
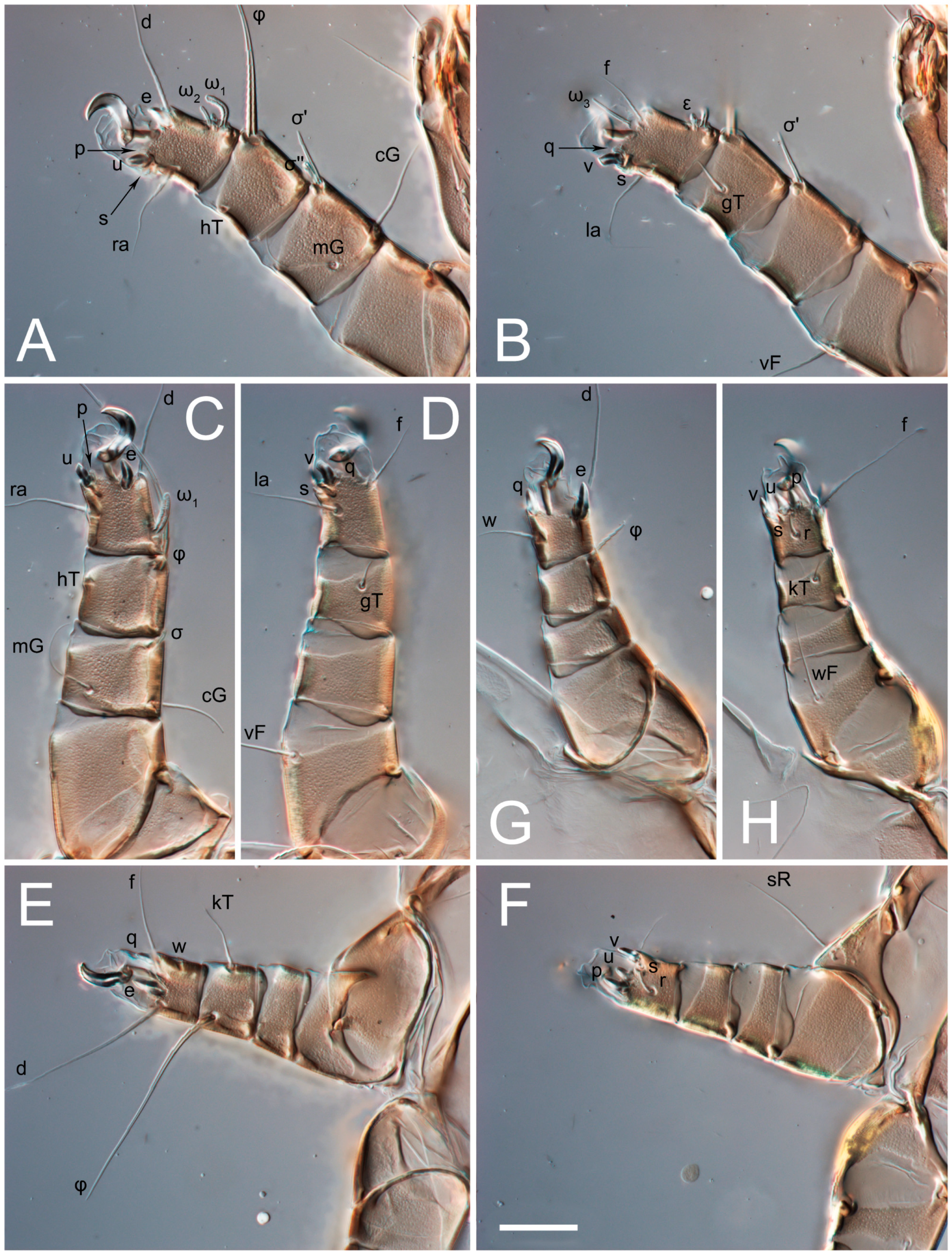
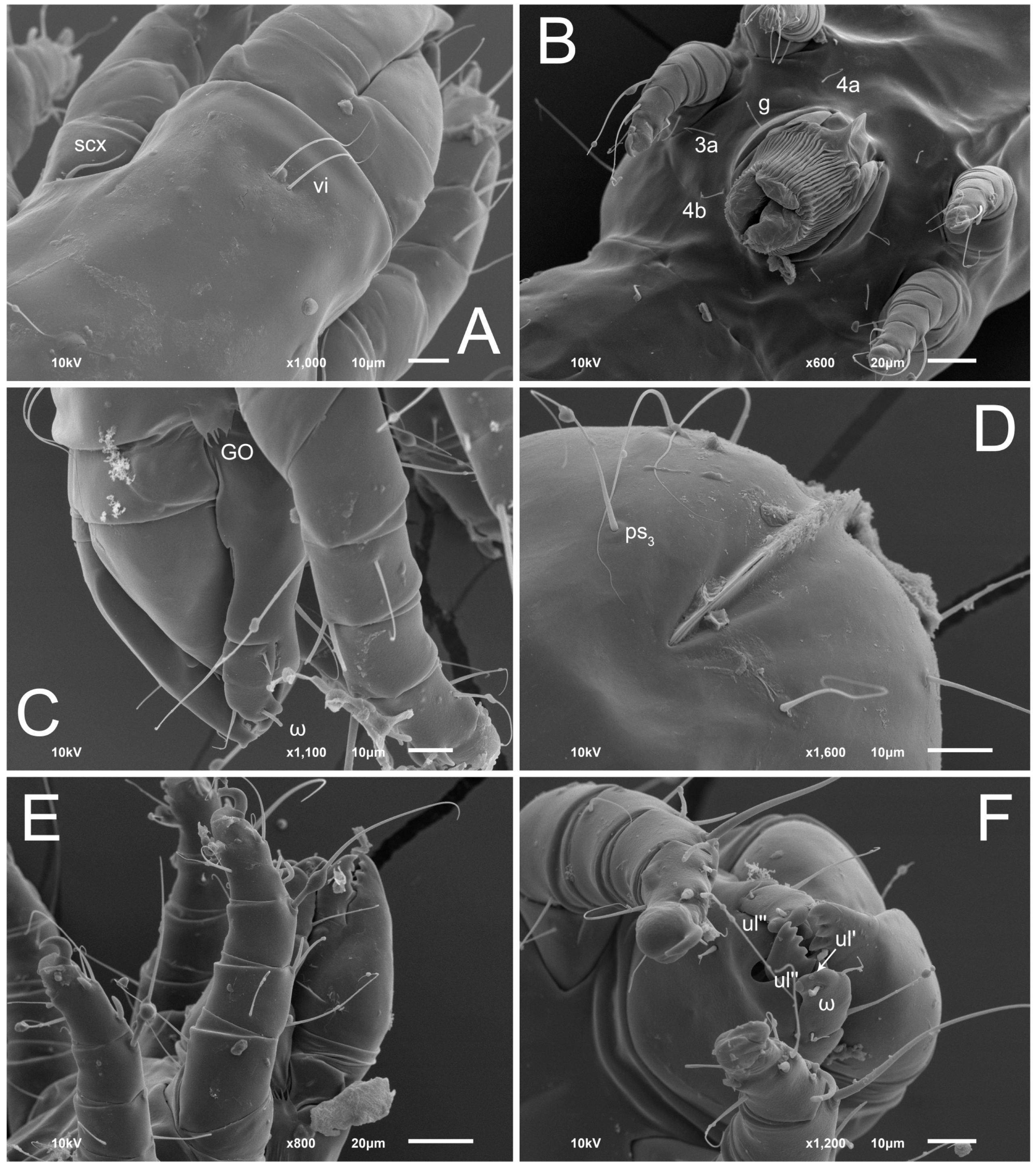
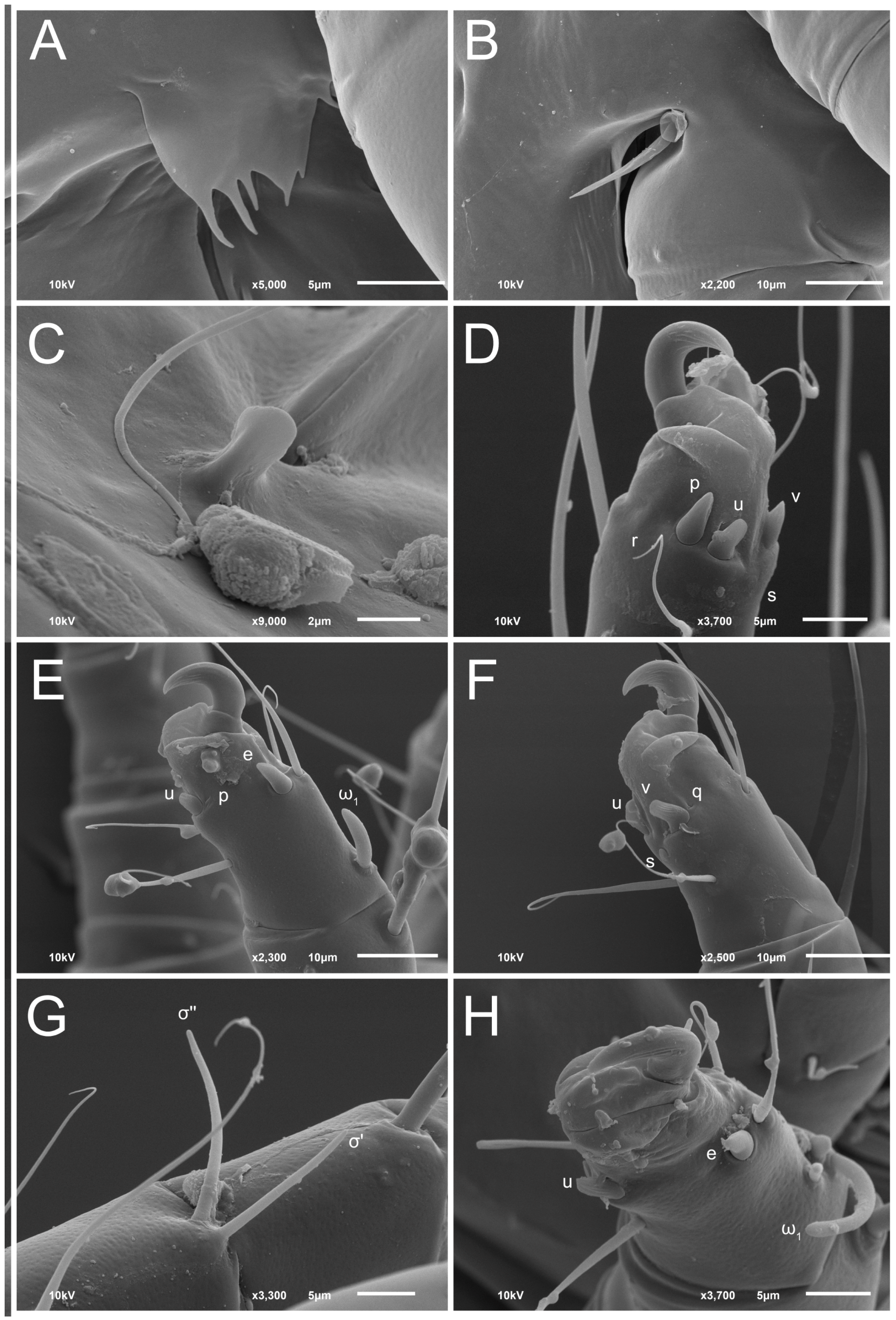
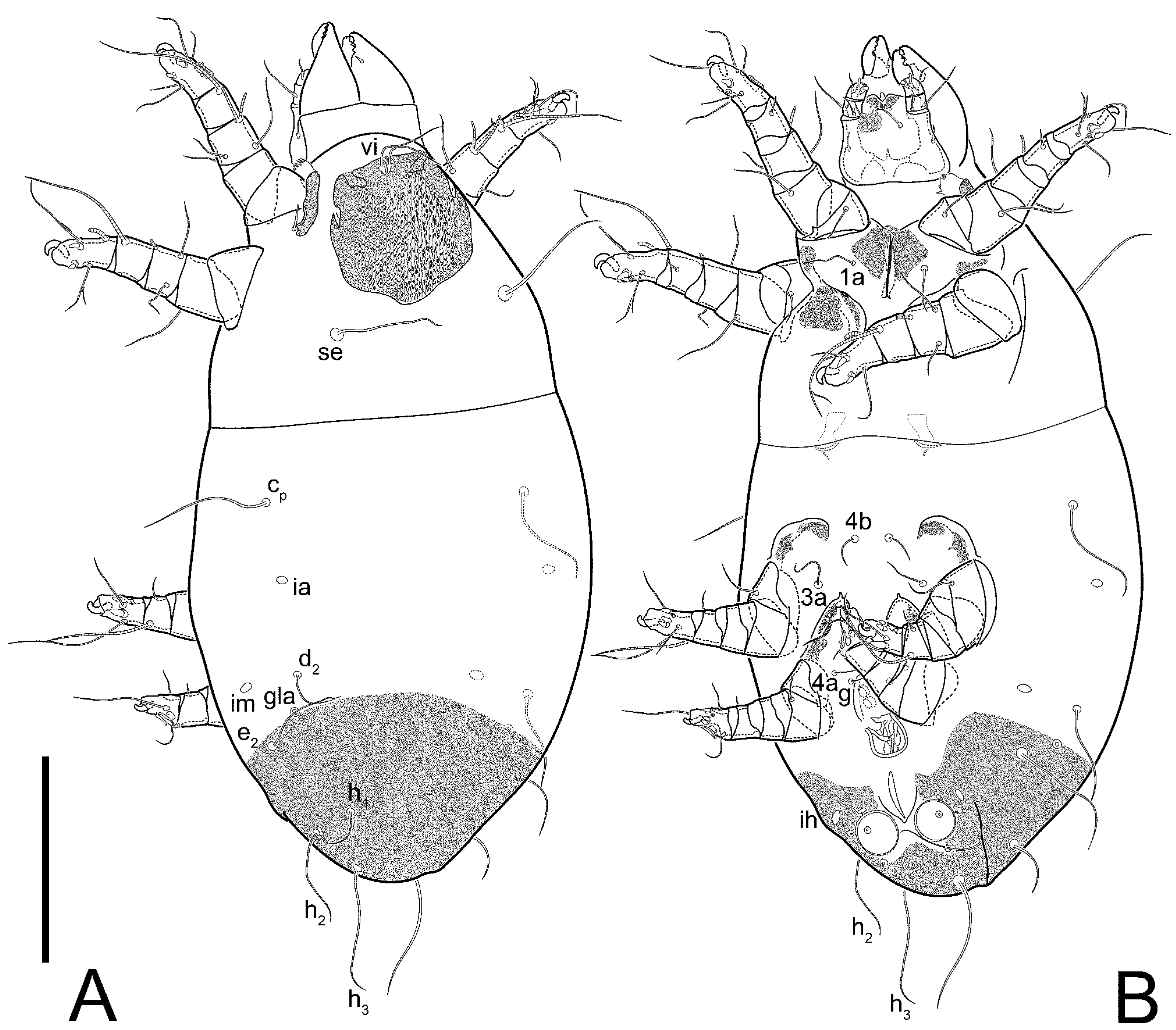
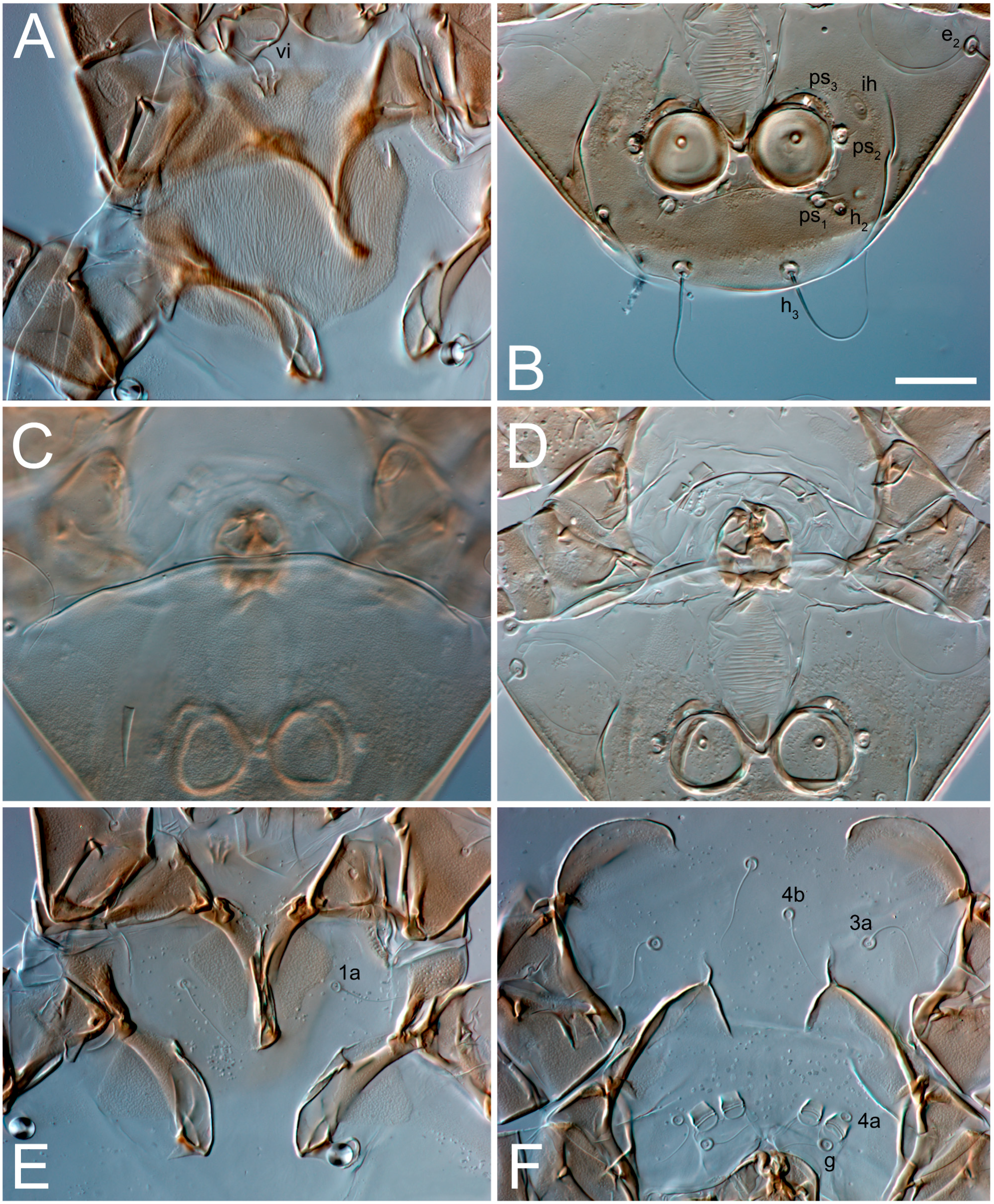
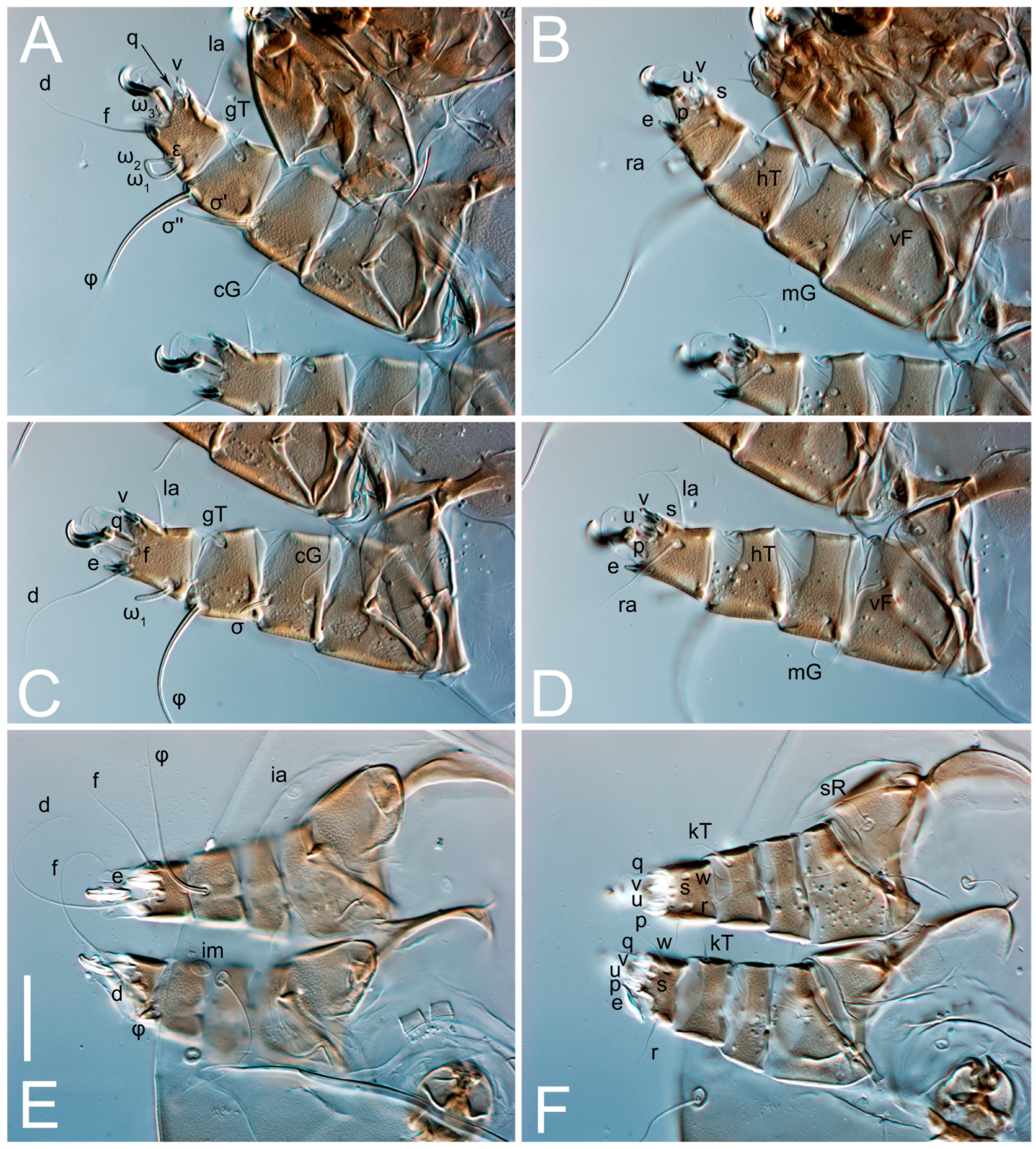
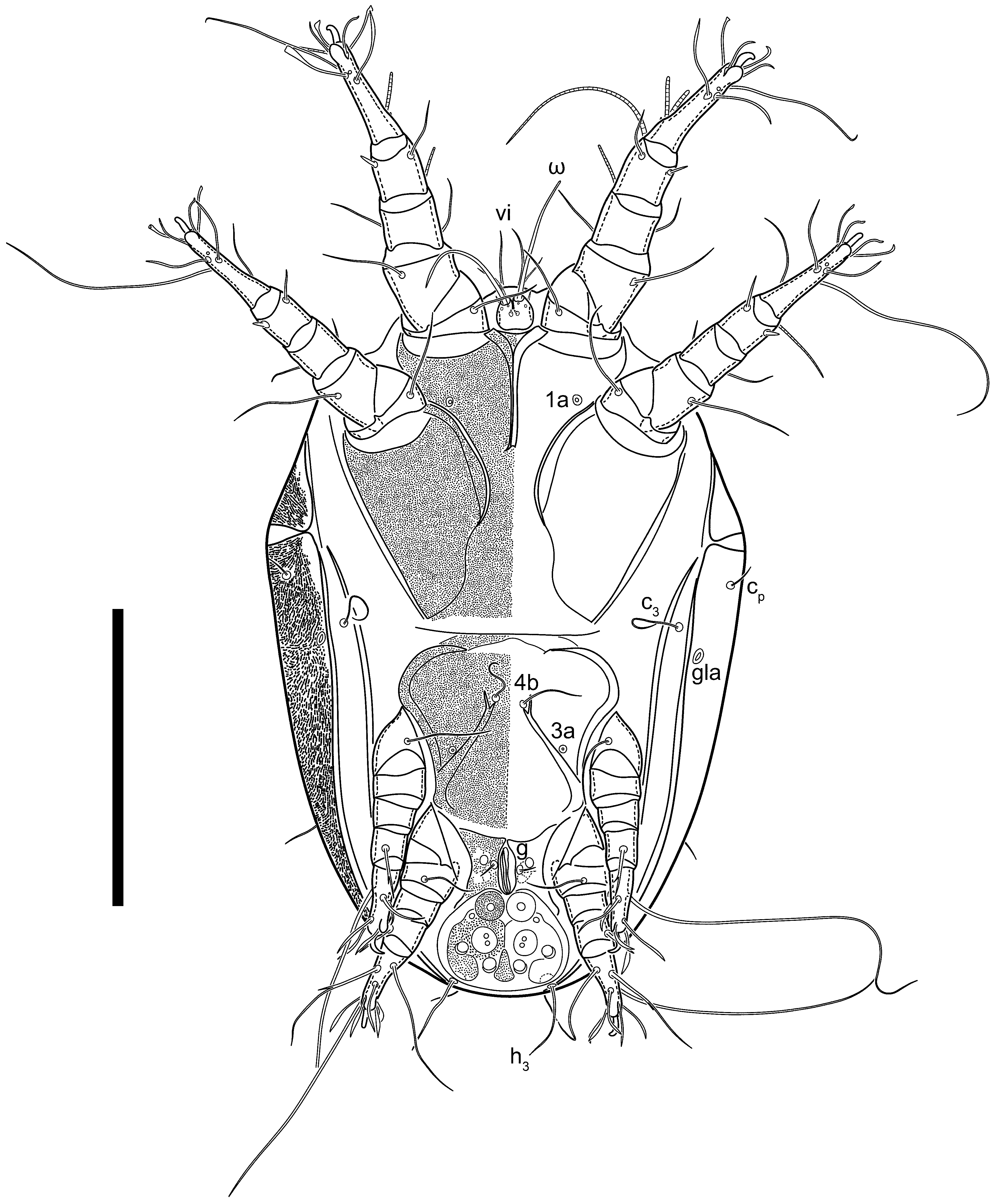
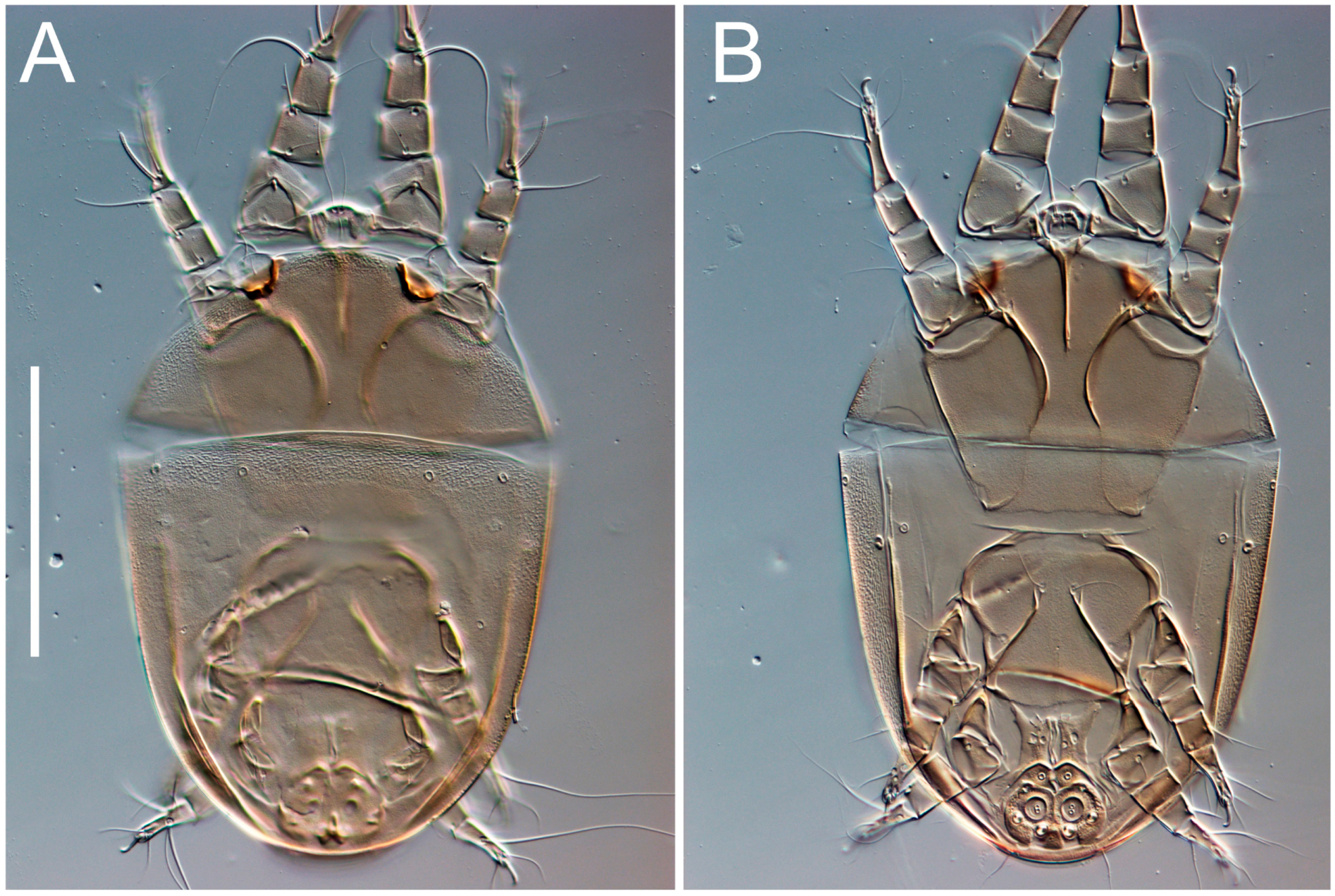
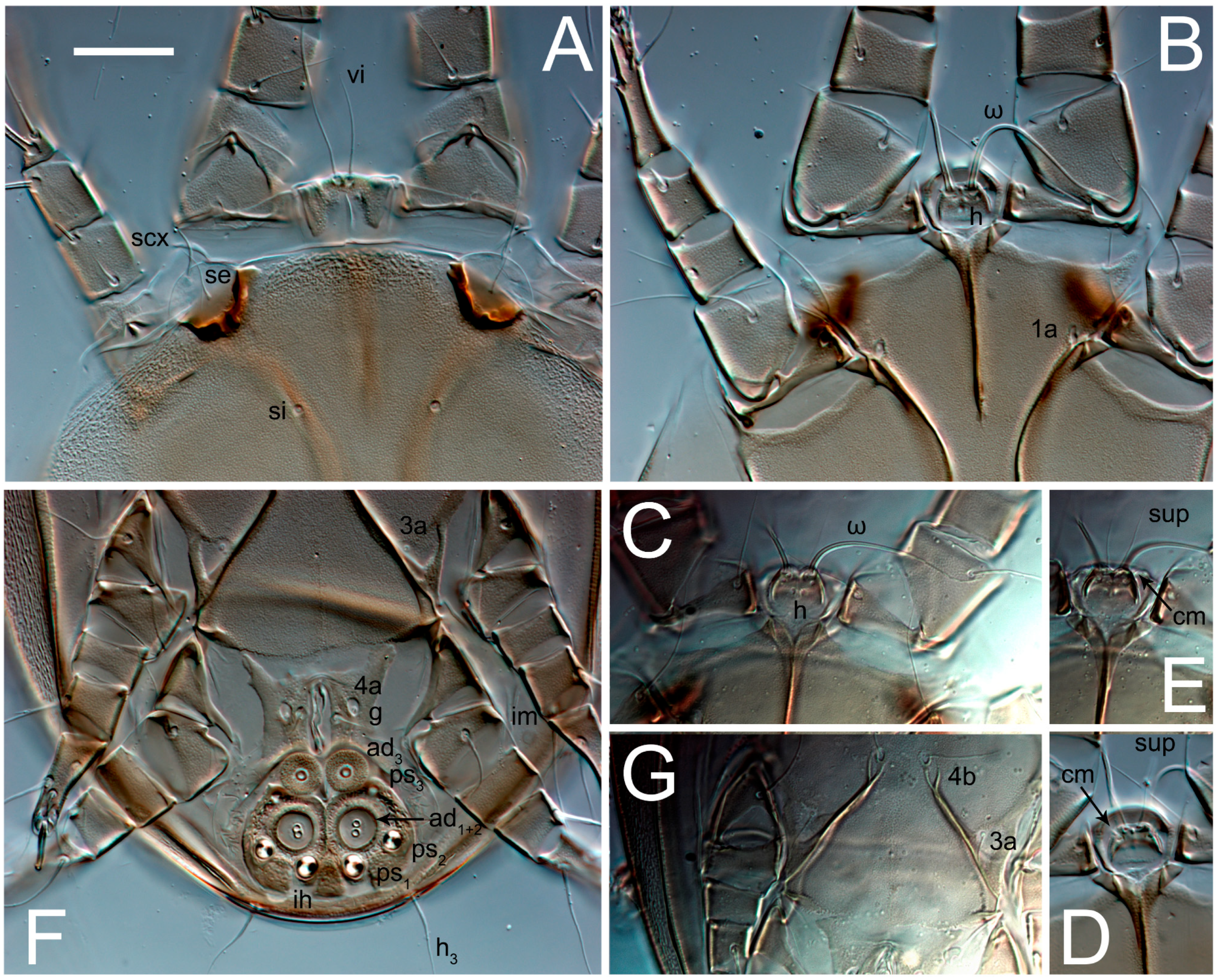
3.2. Morphological Description
3.2.1. Genus Thyreophagus Rondani, 1874
3.2.2. Thyreophagus tauricus sp. n.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Klimov, P.B.; Demard, E.P.; Stinson, C.S.A.; Duarte, M.V.A.; Wäckers, F.L.; Vangansbeke, D. Thyreophagus calusorum sp. n. (Acari, Acaridae), a new parthenogenetic species from the USA, with a checklist of Thyreophagus species of the world. Syst. Appl. Acarol. 2022, 27, 1920–1956. [Google Scholar] [CrossRef]
- Fain, A. Revision des genres Thyreophagus Rondani, 1874 et Michaelopus Fain & Johnston, 1974 (Acari, Acaridae) avec description de neuf especes nouvelles. Bull. L’institut R. Sci. Nat. Belg. Entomol. 1982, 54, 1–47. [Google Scholar]
- Ostojá-Starzewski, J.C. Michaelopus spinitarsis Fain (Acari: Acaridae): A first record in the British Isles. Br. J. Entomol. Nat. Hist. 2001, 14, 217–221. [Google Scholar]
- Portus, M.; Gomez, M.S. Thyreophagus gallegoi a new mite from flour and house dust in Spain (Acaridae, Sarcoptiformes). Acarologia 1980, 21, 477–481. [Google Scholar] [PubMed]
- Chmielewski, W. Stored products mites (Acaroidea) in Polish bee hives. In Modern Acarology, Volume I: Proceedings of the 8 International Congress of Acarology, Ceske Budejovice, Czechoslovakia, 6–11 August 1990; SPB Academic Publishing: The Hague, The Netherlands, 1991; pp. 615–619. [Google Scholar]
- Okabe, K.; OConnor, B.M. Thelytokous reproduction in the family Acaridae (Astigmata). In Acarology: Proceedings of the 10th International Congress; Halliday, R.B., Walter, D.E., Proctor, H.C., Norton, R.A., Colloff, M.J., Eds.; CSIRO Publishing: Melbourne, Australia, 2001; pp. 170–175. [Google Scholar]
- Barbosa, M.F.D.C.; OConnor, B.M.; De Moraes, G.J. A new species of Thyreophagus (Acari: Acaridae) from Brazil, with notes on species associated with stored food and human habitats and a key to species of this genus. Zootaxa 2016, 4088, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Klimov, P.B.; Mwangi, E.; Merckx, J.; Duarte, M.V.A.; Wäckers, F.L.; Vangansbeke, D. Thyreophagus plocepasseri sp. n., a new parthenogenetic species of acarid mites (Acariformes: Acaridae) from Kenya. Syst. Appl. Acarol. 2020, 25, 2250–2262. [Google Scholar] [CrossRef]
- Fidgett, M.J.; Stinson, C.S.A. A method of rearing Amblyseius predatory mites using Thyreophagus entomophagus as prey. Patent CA2658292A1, 7 February 2008. Available online: https://patentimages.storage.googleapis.com/e7/4f/61/612fa7977fb51a/CA2658292A1.pdf (accessed on 10 November 2023).
- Iglesias-Souto, J.; Sánchez-Machín, I.; Iraola, V.; Poza, P.; González1, R.; Matheu, V. Oral mite anaphylaxis by Thyreophagus entomophagus in a child: Acase report. Clin. Mol. Allergy 2009, 7, 1–3. [Google Scholar] [CrossRef]
- Simoni, S.; Nannelli, R.; Roversi, P.F.; Turchetti, T.; Bouneb, M. Thyreophagus corticalis as a vector of hypovirulence in Cryphonectria parasitica in chestnut stands. Exp. Appl. Acarol. 2014, 62, 363–375. [Google Scholar] [CrossRef]
- Barbosa, M.F.D.C.; de Moraes, G.J. Evaluation of astigmatid mites as factitious food for rearing four predaceous phytoseiid mites (Acari: Astigmatina, Phytoseiidae). Biol. Control 2015, 91, 22–26. [Google Scholar] [CrossRef]
- Bouneb, M.; Turchetti, T.; Nannelli, R.; Roversi, P.F.; Paoli, F.; Danti, R.; Simoni, S. Occurrence and transmission of mycovirus Cryphonectria hypovirus 1 from dejecta of Thyreophagus corticalis (Acari, Acaridae). Fungal Biol. 2016, 120, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Knapp, M.; van Houten, Y.; van Baal, E.; Groot, T. Use of predatory mites in commercial biocontrol: Current status and future prospects. Acarologia 2018, 58, 72–82. [Google Scholar] [CrossRef]
- Stinson, C.S.A.; Klimov, P.B.; Demard, E.P.; Dunham, J.L.; Duarte, M.V.A.; Vangansbeke, D.; Bolckmans, K.J.F.; Wäckers, F.L. Mite Composition and Method for Feeding Predatory Mites. Patent WO/2023/170145, 8 March 2023. [Google Scholar]
- Clark, J.M. A new Thyreophagus mite from honeydew scale insects on black beech (Nothofagus). Rec. Canterb. Mus. 2009, 23, 1–9. [Google Scholar]
- Fain, A.; Johnston, D. Three new species of hypopi phoretic on springtails (Collembola) in England (Acari: Acarididae). J. Nat. Hist. 1974, 8, 411–420. [Google Scholar] [CrossRef]
- Fain, A.; Knülle, W.; Wurst, E. First description of the hypopial stage of Thyreophagus entomophagus (Laboulbène, 1852) (Acari Acaridae). Bull. Soc. R. Belg. Entomol. 2000, 136, 153–156. [Google Scholar]
- Griffiths, D.A.; Atyeo, W.T.; Norton, R.A.; Lynch, C.A. The idiosomal chaetotaxy of astigmatid mites. J. Zool. 1990, 220, 1–32. [Google Scholar] [CrossRef]
- Norton, R.A. Morphological evidence for the evolutionary origin of Astigmata (Acari: Acariformes). Exp. Appl. Acarol. 1998, 22, 559–594. [Google Scholar] [CrossRef]
- Grandjean, F. Au sujet de l’organe de Claparède, des eupathidies multiples et des taenidies mandibulaires chez les Acariens actinochitineux. Arch. Sci. Phys. Nat. 5ème Période 1946, 28, 63–87. [Google Scholar]
- Grandjean, F. La chaetotaxie des pattes chez les Acaridiae. Bull. Société Zool. Fr. 1939, 64, 50–60. [Google Scholar]
- Matthews, A.E.; Klimov, P.B.; Proctor, H.C.; Dowling, A.P.G.; Diener, L.; Hager, S.B.; Larkin, J.L.; Raybuck, D.W.; Fiss, C.J.; McNeil, D.J.; et al. Cophylogenetic assessment of New World warblers (Parulidae) and their symbiotic feather mites (Proctophyllodidae). J. Avian Biol. 2018, 49, 1–17. [Google Scholar] [CrossRef]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A new genome assembly algorithm and Its applications to single-cell sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef] [PubMed]
- Swofford, D.L. PAUP*. Phylogenetic Analysis Using Parsimony (*and other methods). Version 4.0a168, 2022. Distributed by the author. Available online: https://paup.phylosolutions.com (accessed on 10 November 2023).
- Mcmurtry, J.A.; De Moraes, G.J.; Sourassou, N.F. Revision of the lifestyles of phytoseiid mites (Acari: Phytoseiidae) and implications for biological control strategies. Syst. Appl. Acarol. 2013, 18, 297–320. [Google Scholar] [CrossRef]
- McMurtry, J.A.; Croft, B.A. Life-styles of phytoseiid mites and their role in biological control. Annu. Rev. Entomol. 1997, 42, 291–321. [Google Scholar] [CrossRef] [PubMed]
- Klimov, P.B.; Skoracki, M.; Bochkov, A.V. Cox1 barcoding versus multilocus species delimitation: Validation of two mite species with contrasting effective population sizes. Parasites Vectors 2019, 12, 8. [Google Scholar] [CrossRef] [PubMed]











| Th. “entomophagus” | Th. calusorum | Th. entomophagus | Th. corticalis | Th. tauricus | |
|---|---|---|---|---|---|
| Th. “entomophagus” China NC 066986.1 | - | 0.1686 | 0.1834 | 0.1699 | 0.2059 |
| Th. calusorum OR640973 | 0.1929 | - | 0.1705 | 0.1705 | 0.1905 |
| Th. entomophagus OR640974 | 0.2116 | 0.1942 | - | 0.1538 | 0.1750 |
| Th. corticalis OR640975 | 0.1934 | 0.1984 | 0.1732 | - | 0.1725 |
| Th. tauricus OR640976 | 0.2428 | 0.2216 | 0.2008 | 0.1980 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klimov, P.B.; Kolesnikov, V.B.; Khaustov, V.A.; Khaustov, A.A. Thyreophagus tauricus sp. n., a New Subcortical Mite Species (Acari: Acaridae), with a COX1 DNA Sequence Analysis of Several Economically Important Species of Thyreophagus. Animals 2023, 13, 3546. https://doi.org/10.3390/ani13223546
Klimov PB, Kolesnikov VB, Khaustov VA, Khaustov AA. Thyreophagus tauricus sp. n., a New Subcortical Mite Species (Acari: Acaridae), with a COX1 DNA Sequence Analysis of Several Economically Important Species of Thyreophagus. Animals. 2023; 13(22):3546. https://doi.org/10.3390/ani13223546
Chicago/Turabian StyleKlimov, Pavel B., Vasiliy B. Kolesnikov, Vladimir A. Khaustov, and Alexander A. Khaustov. 2023. "Thyreophagus tauricus sp. n., a New Subcortical Mite Species (Acari: Acaridae), with a COX1 DNA Sequence Analysis of Several Economically Important Species of Thyreophagus" Animals 13, no. 22: 3546. https://doi.org/10.3390/ani13223546





