Effects of Broad Bean Diet on the Growth Performance, Muscle Characteristics, Antioxidant Capacity, and Intestinal Health of Nile Tilapia (Oreochromis niloticus)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Experimental Diet
2.3. Experimental Fish and Feeding Management
2.4. Sampling and Analyzing
2.4.1. Proximate Composition Analysis
2.4.2. Sampling
2.4.3. Detection of Serum Biochemical Parameters
2.4.4. Histological Observation
2.4.5. Muscle Texture Profile Analysis (TPA)
2.5. Intestinal Microbiome Analysis
2.6. Statistical Analysis
3. Results
3.1. Growth Performance
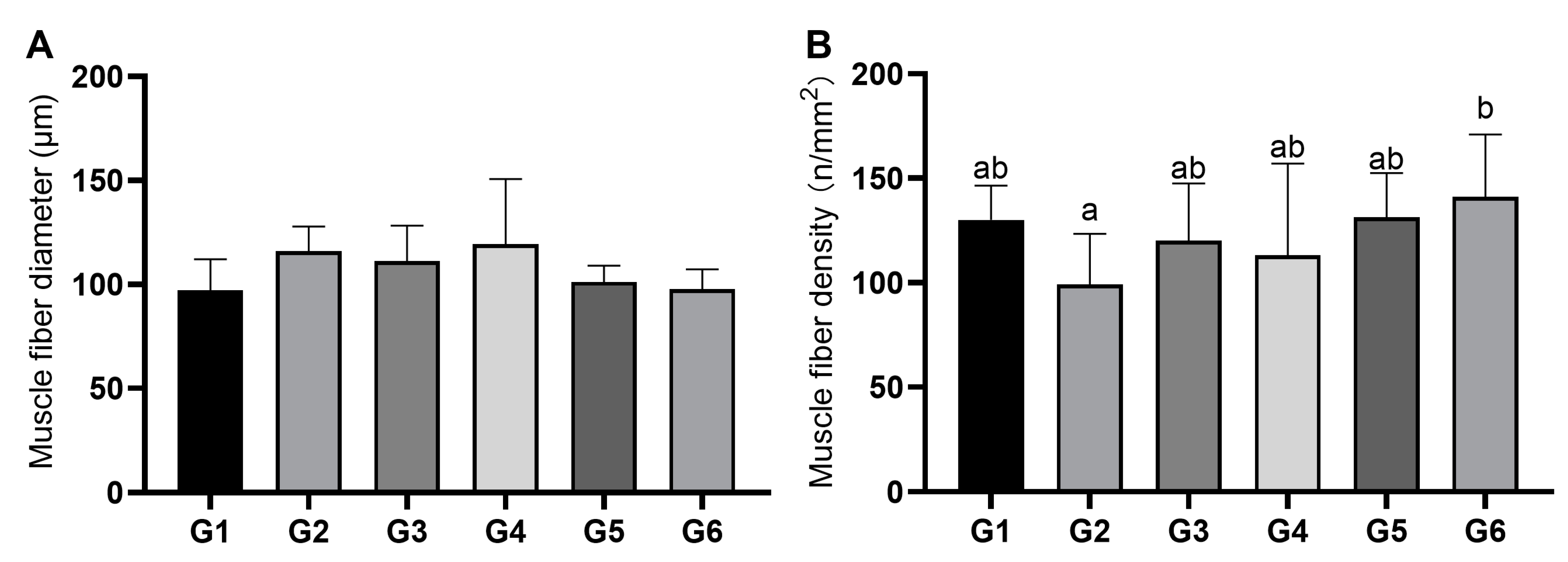
3.2. Characteristics of Muscle Fibers
3.3. Muscle Texture Profile
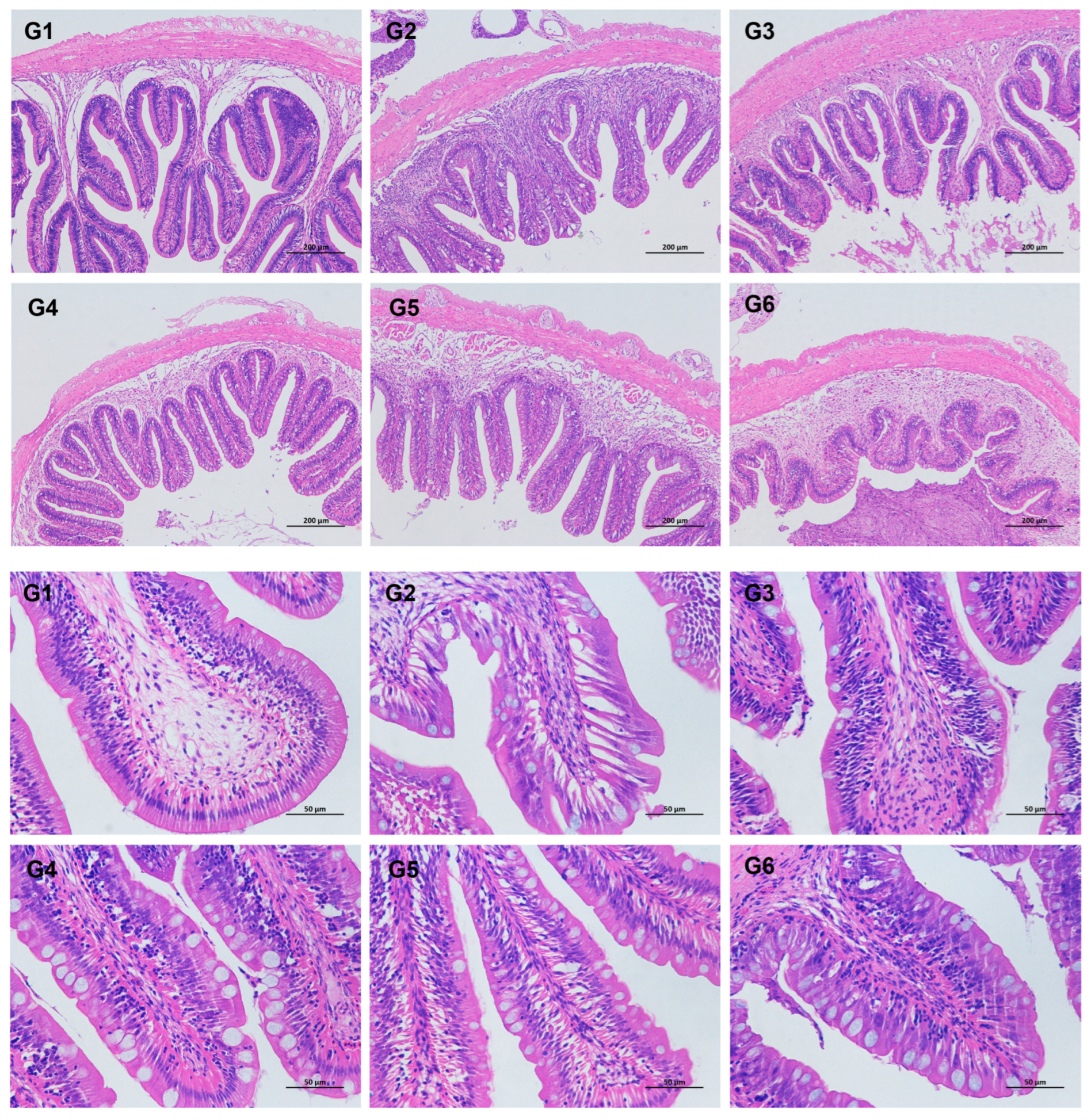
3.4. Intestinal Histomorphological Parameters
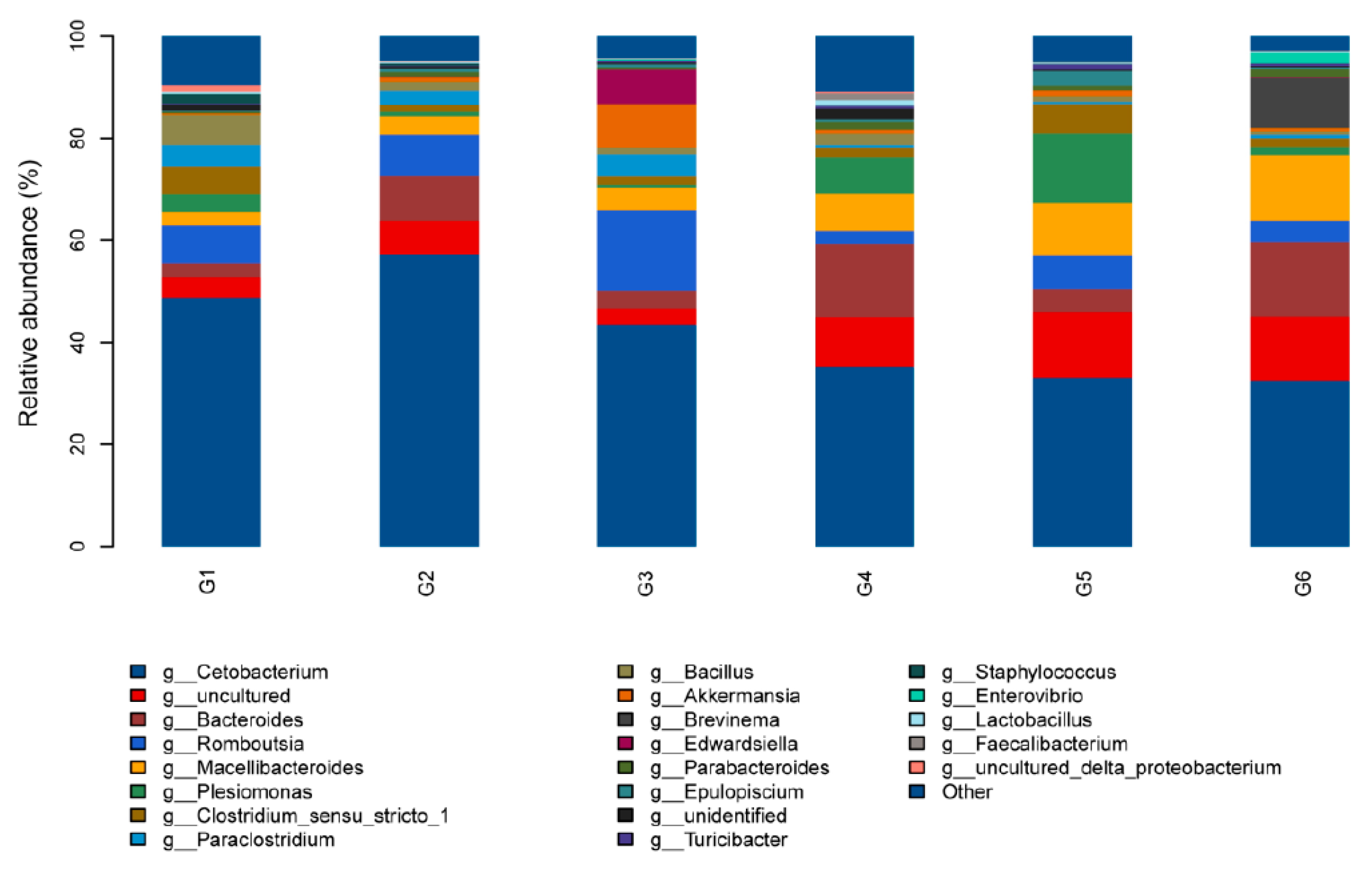
3.5. Intestinal Microbiota
4. Discussion
4.1. Effects of Broad Bean Diets on the Growth of Nile Tilapia
4.2. Effects of Broad Bean Diets on the Serum Indices of Nile Tilapia
4.3. Effects of Broad Bean Diets on the Flesh Quality of Nile Tilapia
4.4. Effects of Broad Bean Diets on the Intestine of Tilapia
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xiong, W.; Guo, C.; Gozlan, R.E.; Liu, J. Tilapia introduction in China: Economic boom in aquaculture versus ecological threats to ecosystems. Rev. Aquac. 2022, 15, 179–197. [Google Scholar] [CrossRef]
- Canonico, G.C.; Arthington, A.; McCrary, J.K.; Thieme, M.L. The effects of introduced tilapias on native biodiversity. Aquat. Conserv. Mar. Freshw. Ecosyst. 2005, 15, 463–483. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture, 2020; FAO: Rome, Italy, 2020. [Google Scholar]
- Wang, B.; Thompson, K.D.; Wangkahart, E.; Yamkasem, J.; Bondad-Reantaso, M.G.; Tattiyapong, P.; Jian, J.; Surachetpong, W. Strategies to enhance tilapia immunity to improve their health in aquaculture. Rev. Aquacult. 2023, 15, 41–56. [Google Scholar] [CrossRef]
- Mungkung, R.; Aubin, J.; Prihadi, T.H.; Slembrouck, J.; van der Werf, H.M.; Legendre, M. Life cycle assessment for environ-mentally sustainable aquaculture management: A case study of combined aquaculture systems for carp and tilapia. J. Clean. Prod. 2013, 57, 249–256. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Fu, B.; Xie, J.; Wang, G.; Tian, J.; Xia, Y.; Yu, E. Textural quality, growth parameters and oxidative responses in Nile tilapia (Oreochromis niloticus) fed broad bean water extract diet. PeerJ 2022, 10, e13048. [Google Scholar] [CrossRef] [PubMed]
- Lie, Ø. Flesh quality—The role of nutrition. Aquac. Res. 2001, 32, 341–348. [Google Scholar] [CrossRef]
- Fu, B.; Xie, J.; Kaneko, G.; Wang, G.; Yang, H.; Tian, J.; Xia, Y.; Li, Z.; Gong, W.; Zhang, K. MicroRNA-dependent regulation of targeted mRNAs for improved muscle texture in crisp grass carp fed with broad bean. Food Res. Int. 2022, 155, 111071. [Google Scholar] [CrossRef]
- Wang, X.; Qian, Y.; Gu, T.; Zhang, Z.; Wang, J.; Lv, H.; Zhang, M.; Qiao, F.; Du, Z. Dietary tea polyphenols change flesh quality with dose-related manner in the GIFT tilapia fed with a high-fat diet. Aquac. Nutr. 2020, 27, 519–532. [Google Scholar] [CrossRef]
- Dhull, S.B.; Kidwai, M.K.; Noor, R.; Chawla, P.; Rose, P.K. A review of nutritional profile and processing of broad bean (Vicia faba L.). Legume Sci. 2022, 4, e129. [Google Scholar] [CrossRef]
- Wang, Y.; Ji, H.; Chen, H.; Yu, E.; Xie, J. Effects of feeding broad bean on muscle texture characteristics, lipid accumulation and tissue fatty acid composition of grass carp (Ctenopharynodon idellus). J. South. Agricult. 2015, 46, 2040–2045. [Google Scholar]
- Yu, E.-M.; Ma, L.-L.; Ji, H.; Li, Z.-F.; Wang, G.-J.; Xie, J.; Yu, D.-G.; Kaneko, G.; Tian, J.-J.; Zhang, K. Smad4-dependent regulation of type I collagen expression in the muscle of grass carp fed with broad bean. Gene 2019, 685, 32–41. [Google Scholar] [CrossRef]
- Yu, E.M.; Liu, B.H.; Wang, G.J.; Yu, D.G.; Xie, J.; Xia, Y.; Gong, W.B.; Wang, H.H.; Li, Z.F.; Wei, N. Molecular cloning of type I collagen cDNA and nutritional regulation of type I collagen mRNA expression in grass carp. J. Anim. Physiol. Anim. Nutr. 2013, 98, 755–765. [Google Scholar] [CrossRef] [PubMed]
- Yu, E.; Fu, B.; Wang, G.; Li, Z.; Ye, D.; Jiang, Y.; Ji, H.; Wang, X.; Yu, D.; Ehsan, H. Proteomic and metabolomic basis for im-proved textural quality in crisp grass carp (Ctenopharyngodon idellus C. et V) fed with a natural dietary pro-oxidant. Food Chem. 2020, 325, 126906. [Google Scholar] [CrossRef] [PubMed]
- Fontaínhas-Fernandes, A.; Gomes, E.; Reis-Henriques, M.A.; Coimbra, J. Replacement of fish meal by plant proteins in the diet of nile tilapia: Digestibility and growth performance. Aquac. Int. 1999, 7, 57–67. [Google Scholar] [CrossRef]
- Gaber, M.M. Partial and complete replacement of fish meal by broad bean meal in feeds for Nile tilapia, Oreochromis niloticus, L., fry. Aquac. Res. 2006, 37, 986–993. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists (AOAC). Official Methods of Association of Official Analytical Chemists International; Association of Official Analytical Chemists: Ann Arbor, MI, USA, 1995. [Google Scholar]
- Multari, S.; Stewart, D.; Russell, W.R. Potential of fava bean as future protein supply to partially replace meat intake in the human diet. Compr. Rev. Food Sci. Food Saf. 2015, 14, 511–522. [Google Scholar] [CrossRef]
- Warsame, A.O.; O’Sullivan, D.M.; Tosi, P. Seed storage proteins of broad bean (Vicia faba L): Current status and prospects for genetic improvement. J. Agricult. Food Chem. 2018, 66, 12617–12626. [Google Scholar] [CrossRef]
- Rahate, K.A.; Madhumita, M.; Prabhakar, P.K. Nutritional composition, anti-nutritional factors, pretreatments-cum-processing impact and food formulation potential of broad bean (Vicia faba L.): A comprehensive review. Lwt 2021, 138, 110796. [Google Scholar] [CrossRef]
- Rochfort, S.; Panozzo, J. Phytochemicals for health, the role of pulses. J. Agric. Food Chem. 2007, 55, 7981–7994. [Google Scholar] [CrossRef]
- Khazaei, H.; Purves, R.W.; Hughes, J.; Link, W.; O’Sullivan, D.M.; Schulman, A.H.; Björnsdotter, E.; Geu-Flores, F.; Nadzieja, M.; Andersen, S.U. Eliminating vicine and convicine, the main anti-nutritional factors restricting broad bean usage. Trends Food Sci. Technol. 2019, 91, 549–556. [Google Scholar] [CrossRef]
- Ali, E.; Awadelkareem, A.M.; Gasim, S.; Yousif, N. Nutritional composition and anti-nutrients of two broad bean (Vicia faba L.) LINES. Int. J. Adv. Res. 2014, 2, 538–544. [Google Scholar]
- Meng, Z.; Liu, Q.; Zhang, Y.; Chen, J.; Sun, Z.; Ren, C.; Zhang, Z.; Cheng, X.; Huang, Y. Nutritive value of faba bean (Vicia faba L.) as a feedstuff resource in livestock nutrition: A review. Food Sci. Nutr. 2021, 9, 5244–5262. [Google Scholar] [CrossRef]
- Francis, G.; Makkar, H.P.S.; Becker, K. Antinutritional factors present in plant-derived alternate fish feed ingredients and their effects in fish. Aquaculture 2001, 199, 197–227. [Google Scholar] [CrossRef]
- Soltanzadeh, S.; Esmaeili Fereidouni, A.; Ouraji, H.; Khalili, K.J. Growth performance, body composition, hematological, and serum biochemical responses of beluga (Huso huso) juveniles to different dietary inclusion levels of broad bean (Vicia faba) meal. Aquacult. Int. 2016, 24, 395–413. [Google Scholar] [CrossRef]
- Gan, L.; Li, X.X.; Pan, Q.; Wu, S.L.; Feng, T.; Ye, H. Effects of replacing soybean meal with broad bean meal on growth, feed utilization and antioxidant status of juvenile grass carp, Ctenopharyngodon idella. Aquacult. Nutr. 2017, 23, 192–200. [Google Scholar] [CrossRef]
- Öner, M.; Atli, G.; Canli, M. Changes in serum biochemical parameters of freshwater fish Oreochromis niloticus following pro-longed metal (Ag, Cd, Cr, Cu, Zn) exposures. Environ. Toxicol. Chem. Int. J. 2008, 27, 360–366. [Google Scholar] [CrossRef]
- Yüksel, S.; Yiğit, A.A. Malondialdehyde and nitric oxide levels and catalase, superoxide dismutase, and glutathione peroxidase levels in maternal blood during different trimesters ofpregnancy and in the cord blood of newborns. Turk. J. Med. Sci. 2015, 45, 454–459. [Google Scholar] [CrossRef]
- Yusof, Y.A.M.; Abdul-Aziz, A. Effects of Zingiber officinale on superoxide dismutase, glutathione peroxidase, catalase, glu-tathione and malondialdehyde content in HepG2 cell line. Malays. J. Biochem. Mol. Biol. 2005, 11, 36–41. [Google Scholar]
- Sarhat, E.R. Evaluation of Serum Malondialdehyde, Glutathione peroxidase, Superoxide dismutase, and Catalase levels in Hormonal Contraceptives in Tikrit City. Tikrit Med. J. 2018, 24, 10–20. [Google Scholar]
- Gopčević, K.R.; Rovčanin, B.R.; Tatić, S.B.; Krivokapić, Z.V.; Gajić, M.M.; Dragutinović, V.V. Activity of superoxide dismutase, catalase, glutathione peroxidase, and glutathione reductase in different stages of colorectal carcinoma. Dig. Dis. Sci. 2013, 58, 2646–2652. [Google Scholar] [CrossRef]
- İnal, M.E.; Kanbak, G.; Sunal, E. Antioxidant enzyme activities and malondialdehyde levels related to aging. Clin. Chim. Acta 2001, 305, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Weifen, L.; Xiaoping, Z.; Wenhui, S.; Bin, D.; Quan, L.; Luoqin, F.; Jiajia, Z.; Yue, W.; Dongyou, Y. Effects of Bacillus prepara-tions on immunity and antioxidant activities in grass carp(Ctenopharyngodon idellus). Fish Physiol. Biochem. 2012, 38, 1585–1592. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, R.M.; Solís, R.V. Hepatoprotective and inhibition of oxidative stress in liver of prostechea michuacana. Rec. Nat. Prod. 2009, 3, 46–51. [Google Scholar]
- Cristalli, G.; Costanzi, S.; Lambertucci, C.; Lupidi, G.; Vittori, S.; Volpini, R.; Camaioni, E. Adenosine deaminase: Functional implications and different classes of inhibitors. Med. Res. Rev. 2001, 21, 105–128. [Google Scholar] [CrossRef] [PubMed]
- Osserman, E. Lysozyme; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Zhang, Q.; Hu, Z.; Xu, Z.; Zhang, P.; Jiang, Y.; Fu, D. Quality Evaluation and Freshness Prediction of Grass Carp (Ctenopha-ryngodon Idellus) Fillets During Storage at 0 Degrees C Using Near-Infrared Spectroscopy. 2023. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=4532550 (accessed on 5 August 2023).
- Cong, J.; Zhang, L.; Li, J.; Wang, S.; Gao, F.; Zhou, G. Effects of dietary supplementation with carnosine on growth performance, meat quality, antioxidant capacity and muscle fiber characteristics in broiler chickens. J. Sci. Food Agric. 2017, 97, 3733–3741. [Google Scholar] [CrossRef]
- Ma, L.-L.; Kaneko, G.; Wang, X.-J.; Xie, J.; Tian, J.-J.; Zhang, K.; Wang, G.-J.; Yu, D.-G.; Li, Z.-F.; Gong, W.-B. Effects of four broad bean extracts on growth parameters, textural quality, oxidative responses, and gut characteristics in grass carp. Aquaculture 2020, 516, 734620. [Google Scholar] [CrossRef]
- Song, D.; Yun, Y.; Mi, J.; Luo, J.; Jin, M.; Nie, G.; Zhou, Q. Effects of broad bean on growth performance and fillet texture of Yellow River carp, Cyprinus carpio haematopterus. Aquacult. Rep. 2020, 17, 100379. [Google Scholar]
- Moreno, H.; Montero, M.; Gómez-Guillén, M.; Fernández-Martín, F.; Mørkøre, T.; Borderías, J. Collagen characteristics of farmed Atlantic salmon with firm and soft fillet texture. Food Chem. 2012, 134, 678–685. [Google Scholar] [CrossRef]
- Rašković, B.; Stanković, M.; Marković, Z.; Poleksić, V. Histological methods in the assessment of different feed effects on liver and intestine of fish. J. Agric. Sci. 2011, 56, 87–100. [Google Scholar]
- Forstner, J. Intestinal mucins in health and disease. Digestion 1978, 17, 234–263. [Google Scholar] [CrossRef]
- Yang, S.; Yu, M. Role of Goblet Cells in Intestinal Barrier and Mucosal Immunity. J. Inflamm. Res. 2021, 14, 3171–3183. [Google Scholar] [CrossRef] [PubMed]
- De Santis, C.; Ruohonen, K.; Tocher, D.; Martin, S.; Król, E.; Secombes, C.; Bell, J.; El-Mowafi, A.; Crampton, V. Atlantic salmon (Salmo salar) parr as a model to predict the optimum inclusion of air classified faba bean protein concentrate in feeds for seawater salmon. Aquaculture 2015, 444, 70–78. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, H.; Chen, T.; Shi, L.; Wang, D.; Tang, D. Regulatory role of short-chain fatty acids in inflammatory bowel disease. Cell Commun. Signal. 2022, 20, 64. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhao, L.-P.; Shen, Y.-Q. A systematic review of advances in intestinal microflora of fish. Fish Physiol. Biochem. 2021, 47, 2041–2053. [Google Scholar] [CrossRef]
- Ingerslev, H.-C.; Jørgensen, L.v.G.; Strube, M.L.; Larsen, N.; Dalsgaard, I.; Boye, M.; Madsen, L. The development of the gut microbiota in rainbow trout (Oncorhynchus mykiss) is affected by first feeding and diet type. Aquaculture 2014, 424–425, 24–34. [Google Scholar] [CrossRef]
- Liu, J.; Wang, K.; Wang, Y.; Chen, W.; Jin, Z.; Yao, Z.; Zhang, D. Strain-specific changes in the gut microbiota profiles of the white shrimp Litopenaeus vannamei in response to cold stress. Aquaculture 2019, 503, 357–366. [Google Scholar] [CrossRef]
- Xiong, F.; Wu, S.; Qin, L.; Shi, M.; Li, W.; Zou, H.; Li, M.; Wang, G. Transcriptome analysis of grass carp provides insights into disease-related genes and novel regulation pattern of bile acid feedback in response to lithocholic acid. Aquaculture 2018, 500, 613–621. [Google Scholar] [CrossRef]
- Zhou, L.; Lin, K.-T.; Gan, L.; Sun, J.-J.; Guo, C.-J.; Liu, L.; Huang, X.-D. Intestinal microbiota of grass carp fed broad beans: A comparative study. Microorganisms 2019, 7, 465. [Google Scholar] [CrossRef]
- Jalili, R.; Agh, N.; Noori, F.; Mohseni, M.; Emani, A. Effects of replacing fish meal with plant protein on growth performance, feed utilization and muscle fatty acid profile of rainbow trout (Oncorhynshus mykiss). J. Anim. Environ. 2012, 3, 33–42. [Google Scholar]
- Mackie, R.I.; Sghir, A.; Gaskins, H.R. Developmental microbial ecology of the neonatal gastrointestinal tract. Am. J. Clin. Nutr. 1999, 69, 1035s–1045s. [Google Scholar] [CrossRef]
- Lan, Y.; Verstegen, M.; Tamminga, S.; Williams, B. The role of the commensal gut microbial community in broiler chickens. World’s Poult. Sci. J. 2005, 61, 95–104. [Google Scholar] [CrossRef]
- Romero, J.; Ringø, E.; Merrifield, D.L. The Gut Microbiota of Fish. Aquaculture Nutrition: Gut Health, Probiotics and Prebiotics; John Wiley & Sons: Hoboken, NJ, USA, 2014; pp. 75–100. [Google Scholar]
- Konig, H. Bacillus species in the intestine of termites and other soil invertebrates. J. Appl. Microbiol. 2006, 101, 620–627. [Google Scholar] [CrossRef] [PubMed]
- Kabiri, L.; Alum, A.; Rock, C.; McLain, J.E.; Abbaszadegan, M. Isolation of Bacteroides from fish and human fecal samples for identification of unique molecular markers. Can. J. Microbiol. 2013, 59, 771–777. [Google Scholar] [CrossRef] [PubMed]
- Gullón, P.; Gullón, B.; Tavaria, F.; Vasconcelos, M.; Gomes, A.M. In vitro fermentation of lupin seeds (Lupinus albus) and broad beans (Vicia faba): Dynamic modulation of the intestinal microbiota and metabolomic output. Food Funct. 2016, 6, 3316–3322. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.L.; Zhang, J.M.; Kaneko, G.; Xie, J.; Sun, J.H.; Wang, G.J.; Tian, J.J.; Zhang, K.; Li, Z.F.; Gong, W.B. Growth performance, intestinal microbiota and immune response of grass carp fed isonitrogenous and isoenergetic diets containing faba bean extracts. Aquacult. Rep. 2022, 22, 100924. [Google Scholar] [CrossRef]
- Diether, N.E.; Willing, B.P. Microbial fermentation of dietary protein: An important factor in diet–microbe–host interaction. Microorganisms 2019, 7, 19. [Google Scholar] [CrossRef]
- Wu, G.D.; Chen, J.; Hoffmann, C.; Bittinger, K.; Chen, Y.-Y.; Keilbaugh, S.A.; Bewtra, M.; Knights, D.; Walters, W.A.; Knight, R.; et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 2011, 334, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.; Ingale, S.; Kim, J.; Kim, K.; Park, Y.; Lee, S.; Kwon, I.; Chae, B. Effects of dietary supplementation of synthetic antimicrobial peptide-A3 and P5 on growth performance, apparent total tract digestibility of nutrients, fecal and intestinal microflora and intestinal morphology in weanling pigs. Livest. Sci. 2013, 159, 53–60. [Google Scholar] [CrossRef]
- Kimura, I.; Inoue, D.; Maeda, T.; Hara, T.; Ichimura, A.; Miyauchi, S.; Kobayashi, M.; Hirasawa, A.; Tsujimoto, G. Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41). Proc. Natl. Acad. Sci. USA 2011, 108, 8030–8035. [Google Scholar] [CrossRef]
- Sivieri, K.; Bassan, J.; Peixoto, G.; Monti, R. Gut microbiota and antimicrobial peptides. Curr. Opin. Food Sci. 2017, 13, 56–62. [Google Scholar] [CrossRef]
- Chassaing, B.; Gewirtz, A.T. Gut microbiota, low-grade inflammation, and metabolic syndrome. Toxicol. Pathol. 2014, 42, 49–53. [Google Scholar] [CrossRef] [PubMed]




| Experimental Diets (Treatments) | ||||||
|---|---|---|---|---|---|---|
| G1 | G2 | G3 | G4 | G5 | 1 G6 | |
| Ingredient (%) | ||||||
| Fish meal | 10 | 10 | 10 | 10 | 10 | |
| Degossypolized cottonseed protein | 3 | 8 | 16.3 | 8 | 16.3 | |
| Soybean meal | 26.5 | 18.1 | 0 | 18.1 | 0 | |
| Rapeseed meal | 25.5 | 0 | 0 | 0 | 0 | |
| Rice bran | 10 | 0 | 0 | 0 | 0 | |
| Broad bean | 0 | 40 | 50 | 40 | 50 | |
| Soybean oil | 4.4 | 3.5 | 3.2 | 3.5 | 3.2 | |
| Wheat flour | 18 | 17.6 | 17.5 | 17.6 | 17.5 | |
| 2 Permix | 2.6 | 2.6 | 2.6 | 2.6 | 2.6 | |
| Lysine | 0 | 0.07 | 0.19 | 0.07 | 0.19 | |
| L-methionine | 0 | 0.19 | 0.23 | 0.19 | 0.23 | |
| 3 CFFAM | 0.5 | 0.5 | ||||
| Proximate composition (%) | ||||||
| Crude lipid | 6.39 | 6.32 | 6.32 | 6.39 | 6.39 | 6.1 |
| Crude protein | 32.79 | 32.75 | 32.75 | 32.79 | 32.79 | 32.23 |
| Lysine | 1.90 | 1.90 | 1.90 | 1.90 | 1.90 | 1.86 |
| Methionine | 0.56 | 0.56 | 0.56 | 0.56 | 0.56 | 0.52 |
| Index | Experimental Diets | |||||
|---|---|---|---|---|---|---|
| G1 | G2 | G3 | G4 | G5 | G6 | |
| IBW (g) | 616.44 ± 1.50 | 615.83 ± 0.64 | 617.25 ± 2.69 | 615.75 ± 2.33 | 619.42 ± 3.59 | 619.22 ± 4.55 |
| FBW (g) | 717.77 ± 24.75 b | 758.79 ± 17.06 a | 783.27 ± 31.06 a | 774.77 ± 34.30 a | 774.75 ± 10.70 a | 729.37 ± 15.06 b |
| WGR (%) | 16.43 ± 3.82 b | 23.22 ± 2.84 ab | 26.89 ± 4.66 a | 25.84 ± 5.89 a | 25.08 ± 1.28 a | 17.8 ± 2.87 b |
| SGR (%/day) | 0.17 ± 0.04 b | 0.23 ± 0.02 ab | 0.26 ± 0.04 a | 0.25 ± 0.05 a | 0.25 ± 0.01 a | 0.18 ± 0.03 b |
| FCR | 3.87 ± 0.99 a | 2.73 ± 0.36 b | 2.53 ± 0.31 b | 2.53 ± 0.47 b | 2.69 ± 0.27 b | 3.68 ± 0.21 a |
| SR (%) | 80.95 ± 10.91 | 87.50 ± 6.84 | 85.71 ± 5.83 | 80.36 ± 14.72 | 87.50 ± 6.84 | 76.19 ± 17.98 |
| HSI (%) | 1.31 ± 0.39 | 1.39 ± 0.40 | 1.37 ± 0.36 | 1.41 ± 0.42 | 1.47 ± 0.41 | 1.29 ± 0.45 |
| VSI (%) | 5.71 ± 0.76 b | 6.04 ± 1.23 b | 6.20 ± 1.27 a | 6.11 ± 1.22 a | 6.28 ± 1.24 a | 6.93 ± 1.81 a |
| CF (g/cm3) | 3.60 ± 0.29 | 3.55 ± 0.23 | 3.55 ± 0.28 | 3.48 ± 0.30 | 3.46 ± 0.71 | 3.58 ± 0.31 |
| Index | Experimental Diets | |||||
|---|---|---|---|---|---|---|
| G1 | G2 | G3 | G4 | G5 | G6 | |
| MDA (nmol/mL) | 9.70 ± 2.18 | 7.50 ± 0.32 | 8.30 ± 2.24 | 7.49 ± 1.24 | 8.01 ± 3.10 | 9.07 ± 2.76 |
| T-AOC (U/mL) | 1.10 ± 0.1 5 a | 0.86 ± 0.09 ab | 0.78 ± 0.10 b | 0.76 ± 0.08 b | 0.76 ± 0.24 b | 0.84 ± 0.23 ab |
| SOD (mmol/L) | 16.50 ± 0.08 a | 18.34 ± 0.12 b | 13.62 ± 0.71 c | 20.98 ± 0.61 d | 15.85 ± 0.33 a | 20.29 ± 0.12 d |
| CAT (mmol/L) | 68.72 ± 5.06 b | 61.39 ± 6.32 b | 28.26 ± 4.29 c | 152.00 ± 28.11 a | 75.52 ± 21.24 b | 157.91 ± 3.41 a |
| GSH-Px (mmol/L) | 370.55 ± 16.83 cd | 382.92 ± 11.35 c | 578.96 ± 27.44 a | 561.45 ± 11.64 a | 459.43 ± 27.18 b | 345.26 ± 19.91 d |
| ADA (U/L) | 3.61 ± 0.48 a | 2.47 ± 0.38 b | 2.16 ± 0.69 b | 2.90 ± 0.68 ab | 2.42 ± 0.30 b | 2.17 ± 0.47 b |
| LZM (U/mL) | 280.00 ± 60.40 | 301.33 ± 12.22 | 270.00 ± 53.22 | 300.00 ± 35.48 | 316.00 ± 63.16 | 266.67 ± 44.06 |
| GPT (U/L) | 1.50 ± 0.35 | 1.66 ± 0.26 | 1.40 ± 0.12 | 1.83 ± 0.71 | 1.52 ± 0.50 | 1.70 ± 0.64 |
| GOT (U/L) | 4.25 ± 1.69 a | 1.73 ± 0.25 b | 2.26 ± 1.15 b | 1.66 ± 0.71 b | 1.88 ± 0.60 b | 1.51 ± 0.36 b |
| Index | Experimental Diets | |||||
|---|---|---|---|---|---|---|
| G1 | G2 | G3 | G4 | G5 | G6 | |
| Hardness (g) | 753.90 ± 20.04 b | 818.63 ± 130.95 ab | 1076.59 ± 368.57 ab | 933.93 ± 278.69 ab | 1155.32 ± 214.97 a | 982.84 ± 287.47 ab |
| Springiness (mm) | 0.95 ± 0.03 | 0.94 ± 0.02 | 0.93 ± 0.02 | 0.92 ± 0.03 | 0.95 ± 0.01 | 0.94 ± 0.01 |
| Adhesiveness (g) | 428.12 ± 33.57 b | 495.44 ± 129.40 ab | 599.63 ± 212.53 ab | 516.86 ± 163.38 ab | 696.95 ± 138.91 a | 567.45 ± 115.57 ab |
| Chewiness (mJ) | 408.80 ± 30.37 b | 469.37 ± 128.85 ab | 557.56 ± 186.22 ab | 476.06 ± 145.10 ab | 659.61 ± 126.65 a | 536.55 ± 114.20 ab |
| Cohesiveness | 0.57 ± 0.04 | 0.61 ± 0.05 | 0.56 ± 0.03 | 0.55 ± 0.02 | 0.60 ± 0.01 | 0.59 ± 0.05 |
| Resilience | 0.71 ± 0.08 | 0.71 ± 0.04 | 0.74 ± 0.06 | 0.74 ± 0.03 | 0.66 ± 0.05 | 0.73 ± 0.12 |
| Index | Experimental Diets | |||||
|---|---|---|---|---|---|---|
| G1 | G2 | G3 | G4 | G5 | G6 | |
| Villus epithelial length (mm) | 0.4 ± 0.08 | 0.29 ± 0.02 | 0.36 ± 0.07 | 0.29 ± 0.02 | 0.29 ± 0.04 | 0.3 ± 0.08 |
| Lamina propria thickness (mm) | 0.51 ± 0.07 | 0.4 ± 0.03 | 0.46 ± 0.07 | 0.4 ± 0.02 | 0.42 ± 0.09 | 0.42 ± 0.06 |
| Villous epithelial length (mm) | 0.73 ± 0.12 | 0.42 ± 0.09 | 0.66 ± 0.13 | 0.56 ± 0.05 | 0.52 ± 0.04 | 0.56 ± 0.13 |
| Muscular thickness (mm) | 0.12 ± 0.01 | 0.12 ± 0 | 0.13 ± 0.02 | 0.08 ± 0.01 | 0.12 ± 0.02 | 0.12 ± 0.02 |
| Number of goblet cells per unit length (ind./mm) | 23.76 ± 3.52 ab | 27.46 ± 1.43 ab | 20.93 ± 8.08 b | 28.22 ± 9.91 ab | 41.69 ± 5.17 ab | 57.73 ± 8.23 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, X.; Shu, H.; Xu, T.; Huang, Y.; Mo, J.; Ai, C. Effects of Broad Bean Diet on the Growth Performance, Muscle Characteristics, Antioxidant Capacity, and Intestinal Health of Nile Tilapia (Oreochromis niloticus). Animals 2023, 13, 3705. https://doi.org/10.3390/ani13233705
He X, Shu H, Xu T, Huang Y, Mo J, Ai C. Effects of Broad Bean Diet on the Growth Performance, Muscle Characteristics, Antioxidant Capacity, and Intestinal Health of Nile Tilapia (Oreochromis niloticus). Animals. 2023; 13(23):3705. https://doi.org/10.3390/ani13233705
Chicago/Turabian StyleHe, Xiaogang, Haoming Shu, Tian Xu, Yuting Huang, Jiajun Mo, and Chunxiang Ai. 2023. "Effects of Broad Bean Diet on the Growth Performance, Muscle Characteristics, Antioxidant Capacity, and Intestinal Health of Nile Tilapia (Oreochromis niloticus)" Animals 13, no. 23: 3705. https://doi.org/10.3390/ani13233705






