Effects of Multiple Environmental Stressors on Zoobenthos Communities in Shallow Lakes: Evidence from a Mesocosm Experiment
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
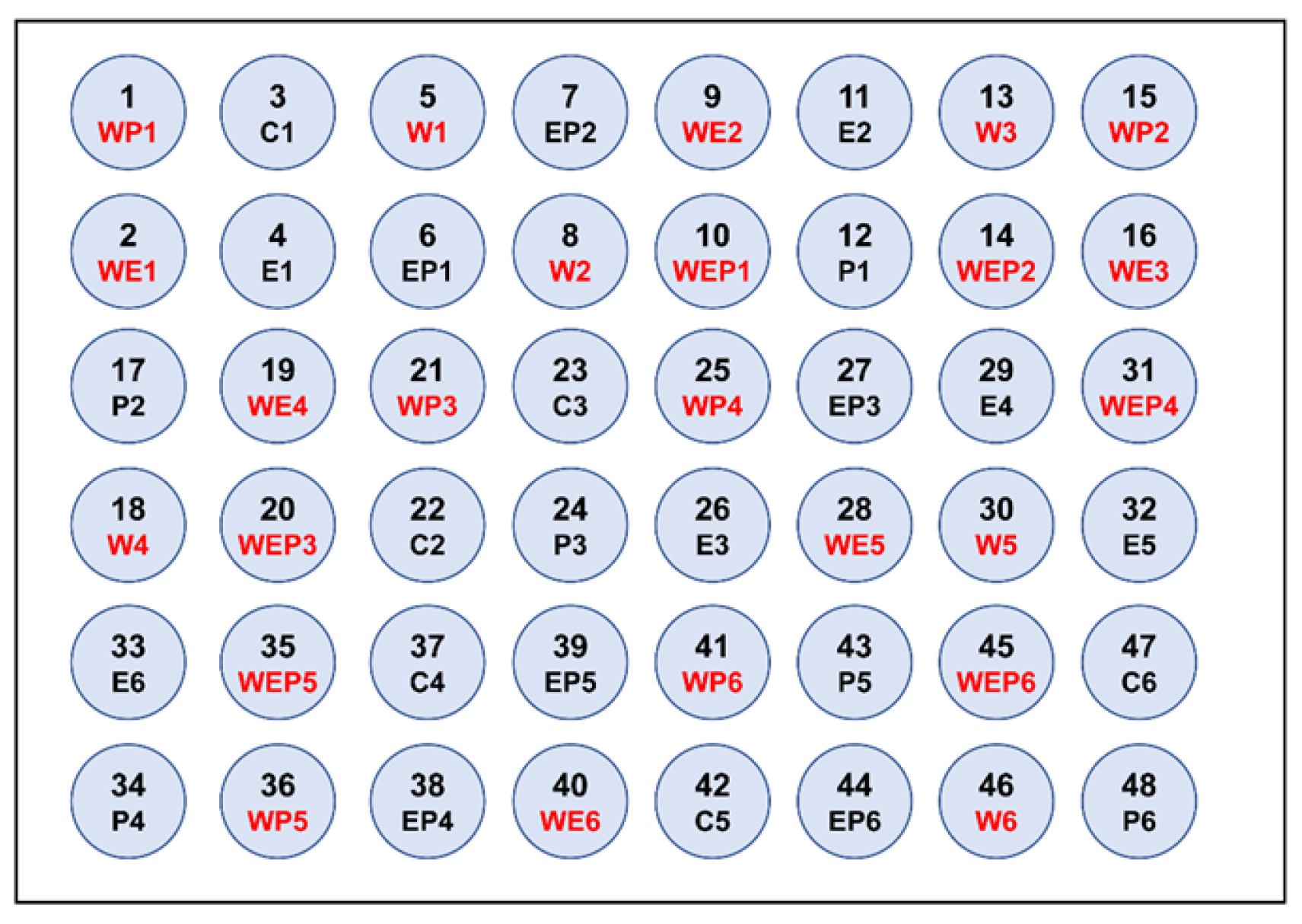
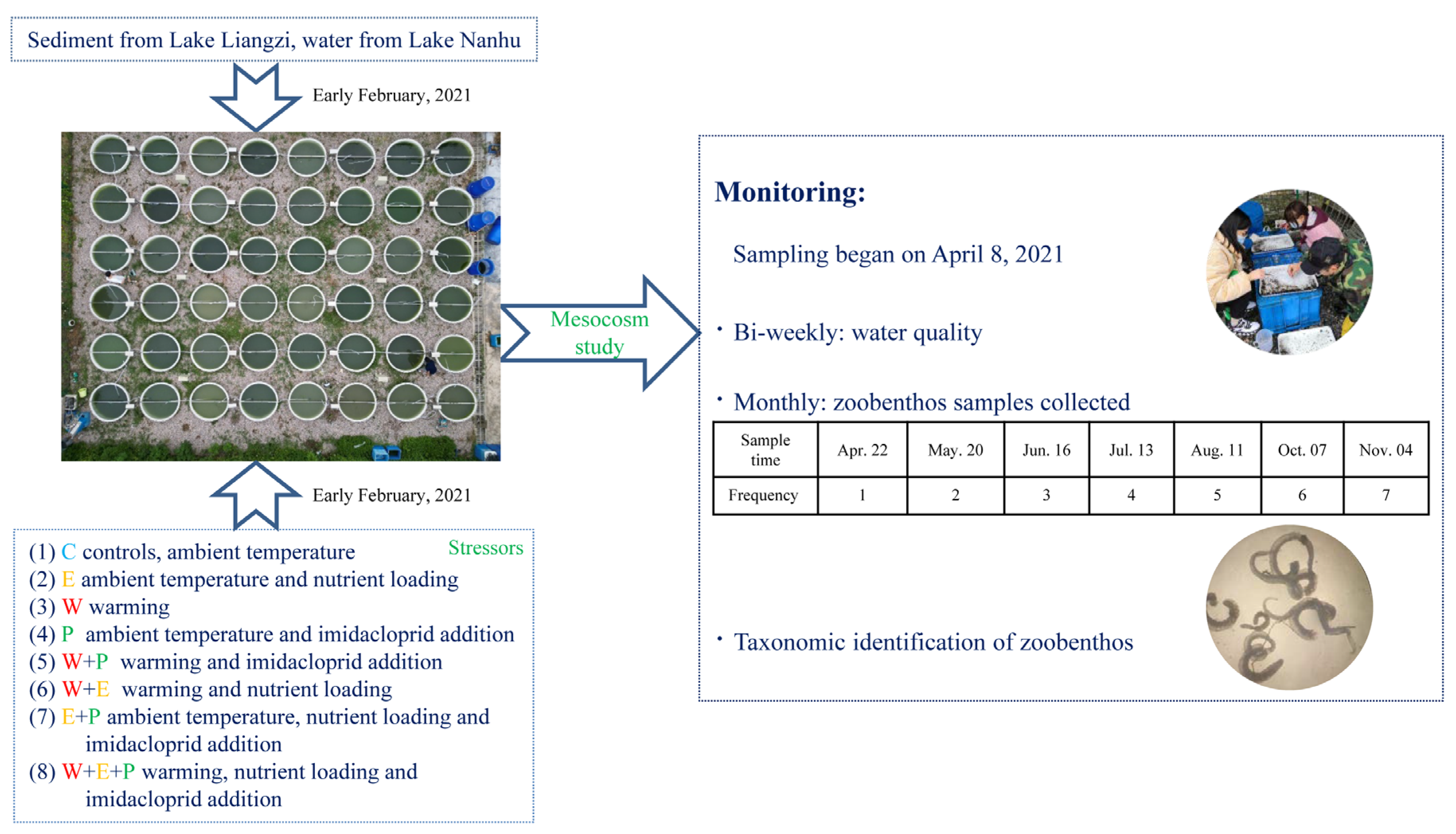
2.1. Experiment Design
2.2. Experiment Set-Up
2.3. Sampling Strategy
2.4. Data Analysis
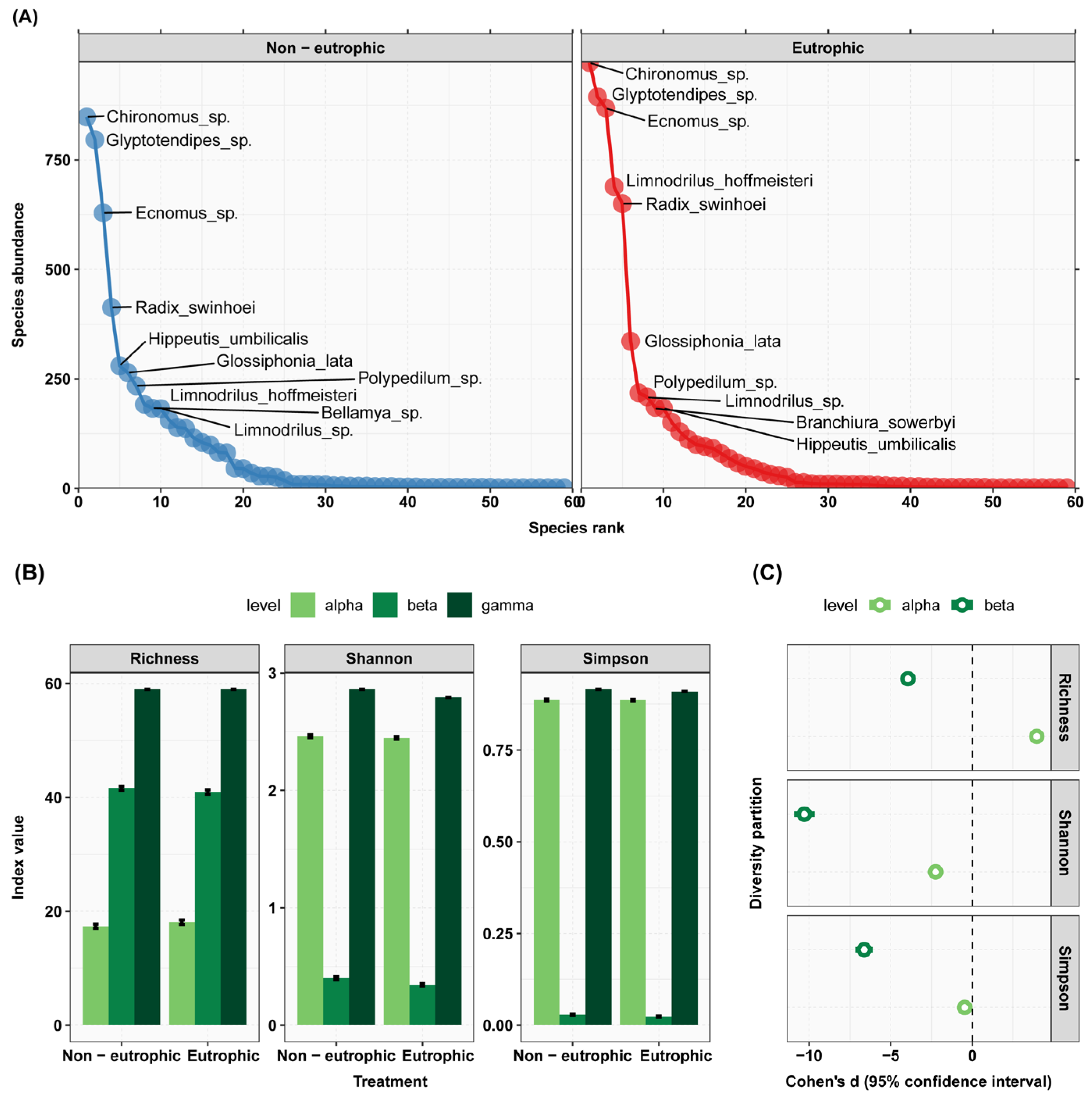
3. Results
3.1. Warming Effects on Zoobenthos Diversity
3.2. Eutrophication Effects on Zoobenthos Diversity
3.3. Pesticide Effects on Zoobenthos Diversity
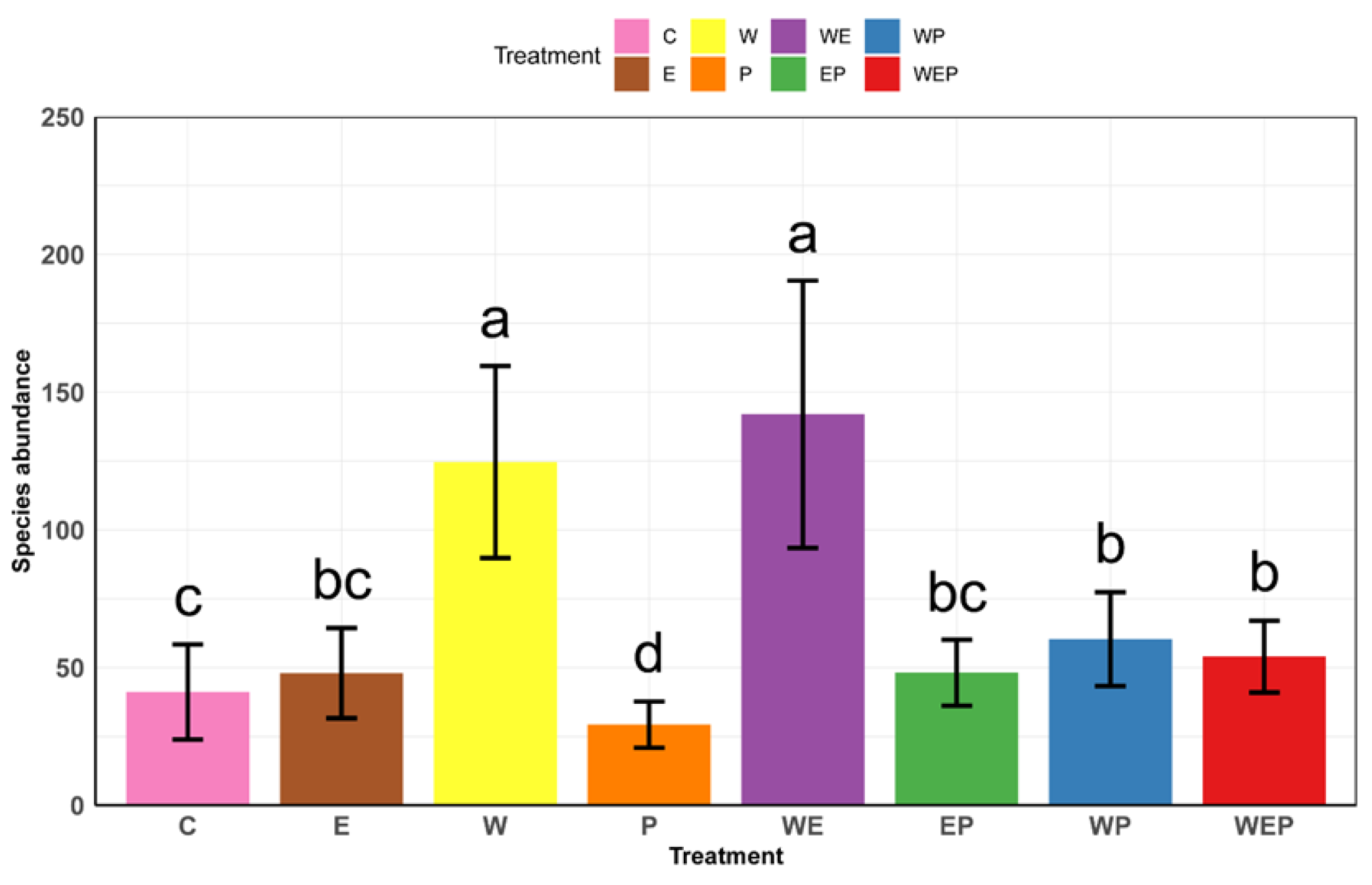
3.4. All Treatments’ Effect on Zoobenthos Diversity and the Response of Zoobenthos Abundance to the Treatments
4. Discussion
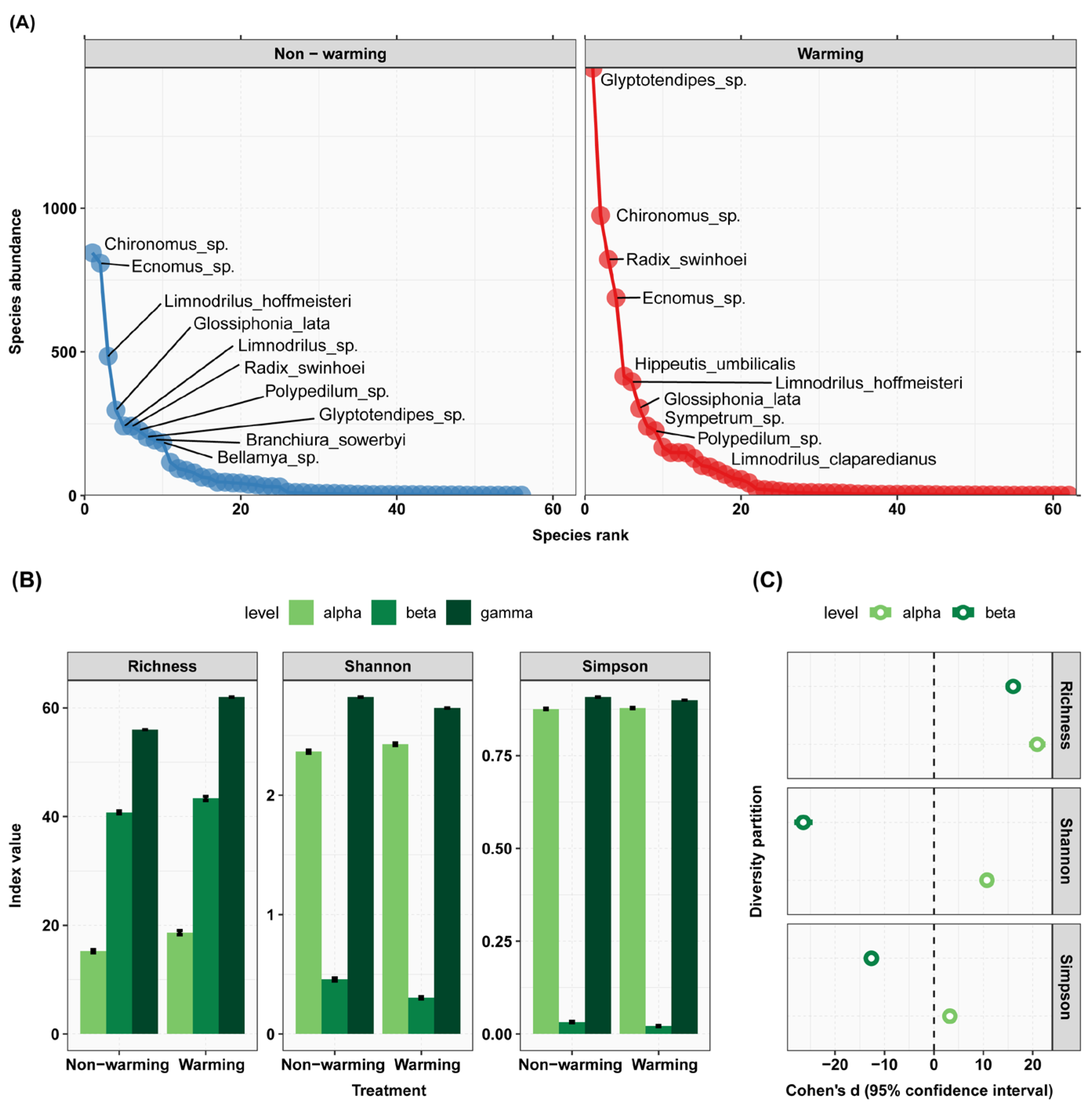
4.1. The Effects of Different Treatment Conditions on the α Diversity of Zoobenthos
4.2. The Effects of Different Treatment Conditions on the β-Diversity of Zoobenthos
4.3. The Sensitivity of Different Zoobenthic Species to Various Treatment Conditions
4.4. Issues in the Study and Future Exploration
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andrade, C.; Ríos, C.; Gerdes, D.; Brey, T. Trophic structure of shallow-water benthic communities in the sub-Antarctic Strait of Magellan. Polar Biol. 2016, 39, 2281–2297. [Google Scholar] [CrossRef]
- Mao, Z.; Gu, X.; Cao, Y.; Luo, J.; Zeng, Q.; Chen, H.; Jeppesen, E. Pelagic energy flow supports the food web of a shallow lake following a dramatic regime shift driven by water level changes. Sci. Total Environ. 2021, 756, 143642. [Google Scholar] [CrossRef] [PubMed]
- Bruschetti, M. Role of reef-building, ecosystem engineering polychaetes in shallow water ecosystems. Diversity 2019, 11, 168. [Google Scholar] [CrossRef]
- Cheng, S.; Tan, S.Y.; Li, Z. Ecological interaction between submerged macrophytes and zoobenthos. J. Earth Sci. Environ. Stud. 2017, 2, 173–182. [Google Scholar] [CrossRef]
- Sarkar, U.K.; Mishal, P.; Borah, S.; Karnatak, G.; Chandra, G.; Kumari, S.; Meena, D.; Debnath, D.; Yengkokpam, S.; Das, P. Status, potential, prospects, and issues of floodplain wetland fisheries in India: Synthesis and review for sustainable management. Rev. Fish. Sci. Aquac. 2020, 29, 1–32. [Google Scholar] [CrossRef]
- Liu, X.; Yang, W.; Fu, X.; Li, X. Determination of the ecological water levels in shallow lakes based on regime shifts: A case study of China’s Baiyangdian Lake. Ecohydrol. Hydrobiol. 2023, in press. [Google Scholar] [CrossRef]
- Wang, J.; Ding, L.; Tao, J.; Ding, C.; He, D. The effects of dams on macroinvertebrates: Global trends and insights. River Res. Appl. 2019, 35, 702–713. [Google Scholar] [CrossRef]
- Burlakova, L.E.; Karatayev, A.Y.; Pennuto, C.; Mayer, C. Changes in Lake Erie benthos over the last 50 years: Historical perspectives, current status, and main drivers. J. Great Lakes Res. 2014, 40, 560–573. [Google Scholar] [CrossRef]
- Martínez-Megías, C.; Rico, A. Biodiversity impacts by multiple anthropogenic stressors in Mediterranean coastal wetlands. Sci. Total Environ. 2022, 818, 151712. [Google Scholar] [CrossRef]
- Wang, H.; Molinos, J.G.; Heino, J.; Zhang, H.; Zhang, P.; Xu, J. Eutrophication causes invertebrate biodiversity loss and decreases cross-taxon congruence across anthropogenically-disturbed lakes. Environ. Int. 2021, 153, 106494. [Google Scholar] [CrossRef]
- Lande, R. Statistics and partitioning of species diversity, and similarity among multiple communities. Oikos 1996, 76, 5–13. [Google Scholar] [CrossRef]
- Ribeiro, D.B.; Prado, P.I.; Brown, K.S., Jr.; Freitas, A.V. Additive partitioning of butterfly diversity in a fragmented landscape: Importance of scale and implications for conservation. Divers. Distrib. 2008, 14, 961–968. [Google Scholar] [CrossRef]
- Acosta-González, G.; Rodríguez-Zaragoza, F.A.; Hernández-Landa, R.C.; Arias-González, J.E. Additive diversity partitioning of fish in a Caribbean coral reef undergoing shift transition. PLoS ONE 2013, 8, e65665. [Google Scholar] [CrossRef]
- Silva, C.B.; Dias, J.D.; Bialetzki, A. Fish larvae diversity in a conservation area of a neotropical floodplain: Influence of temporal and spatial scales. Hydrobiologia 2017, 787, 141–152. [Google Scholar] [CrossRef]
- Latham, R.E. Measuring Biological Diversity. J. Veg. Sci. 2004, 15, 854. [Google Scholar] [CrossRef]
- Whittaker, R.H. Vegetation of the Siskiyou mountains, Oregon and California. Ecol. Monogr. 1960, 30, 279–338. [Google Scholar] [CrossRef]
- Wagner, H.H.; Wildi, O.; Ewald, K.C. Additive partitioning of plant species diversity in an agricultural mosaic landscape. Landsc. Ecol. 2000, 15, 219–227. [Google Scholar] [CrossRef]
- Hautmann, M. Diversification and diversity partitioning. Paleobiology 2014, 40, 162–176. [Google Scholar] [CrossRef]
- Kuznetsova, N.; Saraeva, A. Beta-diversity partitioning approach in soil zoology: A case of Collembola in pine forests. Geoderma 2018, 332, 142–152. [Google Scholar] [CrossRef]
- Hale, S.S.; Cicchetti, G.; Deacutis, C.F. Eutrophication and hypoxia diminish ecosystem functions of benthic communities in a New England estuary. Front. Mar. Sci. 2016, 3, 249. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, L.; Li, K.; Zhang, L.; Cai, Y.; Wang, X.; Heino, J. Nutrient enrichment homogenizes taxonomic and functional diversity of benthic macroinvertebrate assemblages in shallow lakes. Limnol. Oceanogr. 2019, 64, 1047–1058. [Google Scholar] [CrossRef]
- Oertli, B.; Parris, K.M. Toward management of urban ponds for freshwater biodiversity. Ecosphere 2019, 10, e02810. [Google Scholar] [CrossRef]
- Reynolds, J.; Souty-Grosset, C.; Richardson, A. Ecological roles of crayfish in freshwater and terrestrial habitats. Freshw. Crayfish 2013, 19, 197–218. [Google Scholar] [CrossRef]
- Viitasalo, M.; Bonsdorff, E. Global climate change and the Baltic Sea ecosystem: Direct and indirect effects on species, communities and ecosystem functioning. Earth Syst. Dyn. 2022, 13, 711–747. [Google Scholar] [CrossRef]
- Goldenberg Vilar, A.; van Dam, H.; van Loon, E.E.; Vonk, J.A.; van Der Geest, H.G.; Admiraal, W. Eutrophication decreases distance decay of similarity in diatom communities. Freshw. Biol. 2014, 59, 1522–1531. [Google Scholar] [CrossRef]
- Isabwe, A.; Yang, J.R.; Wang, Y.; Liu, L.; Chen, H.; Yang, J. Community assembly processes underlying phytoplankton and bacterioplankton across a hydrologic change in a human-impacted river. Sci. Total Environ. 2018, 630, 658–667. [Google Scholar] [CrossRef]
- Jiao, C.; Zhao, D.; Huang, R.; He, F.; Yu, Z. Habitats and seasons differentiate the assembly of bacterial communities along a trophic gradient of freshwater lakes. Freshw. Biol. 2021, 66, 1515–1529. [Google Scholar] [CrossRef]
- Chase, J.M. Drought mediates the importance of stochastic community assembly. Proc. Natl. Acad. Sci. USA 2007, 104, 17430–17434. [Google Scholar] [CrossRef]
- Wisnoski, N.I.; Leibold, M.A.; Lennon, J.T. Dormancy in metacommunities. Am. Nat. 2019, 194, 135–151. [Google Scholar] [CrossRef]
- Wernberg, T.; Smale, D.A.; Tuya, F.; Thomsen, M.S.; Langlois, T.J.; De Bettignies, T.; Bennett, S.; Rousseaux, C.S. An extreme climatic event alters marine ecosystem structure in a global biodiversity hotspot. Nat. Clim. Chang. 2013, 3, 78–82. [Google Scholar] [CrossRef]
- Huang, Q.; Li, N.; Li, Y. Long-term trend of heat waves and potential effects on phytoplankton blooms in Lake Qiandaohu, a key drinking water reservoir. Environ. Sci. Pollut. Res. 2021, 28, 68448–68459. [Google Scholar] [CrossRef] [PubMed]
- Törnroos, A.; Pecuchet, L.; Olsson, J.; Gårdmark, A.; Blomqvist, M.; Lindegren, M.; Bonsdorff, E. Four decades of functional community change reveals gradual trends and low interlinkage across trophic groups in a large marine ecosystem. Glob. Chang. Biol. 2019, 25, 1235–1246. [Google Scholar] [CrossRef] [PubMed]
- Hölker, F.; Vanni, M.J.; Kuiper, J.J.; Meile, C.; Grossart, H.-P.; Stief, P.; Adrian, R.; Lorke, A.; Dellwig, O.; Brand, A. Tube-dwelling invertebrates: Tiny ecosystem engineers have large effects in lake ecosystems. Ecol. Monogr. 2015, 85, 333–351. [Google Scholar] [CrossRef]
- Hassall, C. Time stress and temperature explain continental variation in damselfly body size. Ecography 2013, 36, 894–903. [Google Scholar] [CrossRef]
- Socolar, J.B.; Epanchin, P.N.; Beissinger, S.R.; Tingley, M.W. Phenological shifts conserve thermal niches in North American birds and reshape expectations for climate-driven range shifts. Proc. Natl. Acad. Sci. USA 2017, 114, 12976–12981. [Google Scholar] [CrossRef] [PubMed]
- Carreira, B.; Segurado, P.; Orizaola, G.; Gonçalves, N.; Pinto, V.; Laurila, A.; Rebelo, R. Warm vegetarians? Heat waves and diet shifts in tadpoles. Ecology 2016, 97, 2964–2974. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.M.; Friedges, C.; Lozinski, L.; Nyquist, C.; Durnin, T.; Ferrington, L.C., Jr. Longevity and oviposition of winter-emerging Chironomidae (Insecta: Diptera) at varying low temperatures. Aquat. Insects 2023, 44, 52–63. [Google Scholar] [CrossRef]
- Nelson, D.; Benstead, J.P.; Huryn, A.D.; Cross, W.F.; Hood, J.M.; Johnson, P.W.; Junker, J.R.; Gíslason, G.M.; Ólafsson, J.S. Shifts in community size structure drive temperature invariance of secondary production in a stream-warming experiment. Ecology 2017, 98, 1797–1806. [Google Scholar] [CrossRef]
- Prather, C.M.; Pelini, S.L.; Laws, A.; Rivest, E.; Woltz, M.; Bloch, C.P.; Del Toro, I.; Ho, C.K.; Kominoski, J.; Newbold, T.S. Invertebrates, ecosystem services and climate change. Biol. Rev. 2013, 88, 327–348. [Google Scholar] [CrossRef]
- Floury, M.; Usseglio-Polatera, P.; Ferreol, M.; Delattre, C.; Souchon, Y. Global climate change in large E uropean rivers: Long-term effects on macroinvertebrate communities and potential local confounding factors. Glob. Chang. Biol. 2013, 19, 1085–1099. [Google Scholar] [CrossRef]
- Reid, A.J.; Carlson, A.K.; Creed, I.F.; Eliason, E.J.; Gell, P.A.; Johnson, P.T.; Kidd, K.A.; MacCormack, T.J.; Olden, J.D.; Ormerod, S.J. Emerging threats and persistent conservation challenges for freshwater biodiversity. Biol. Rev. 2019, 94, 849–873. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhu, X.; Peng, Q.; Wang, Y.; Ge, J.; Yang, G.; Wang, X.; Cai, L.; Shen, W. Multi-level ecotoxicological effects of imidacloprid on earthworm (Eisenia fetida). Chemosphere 2019, 219, 923–932. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Cui, D.; Zhong, G.; Liu, J. Microbial technologies employed for biodegradation of neonicotinoids in the agroecosystem. Front. Microbiol. 2021, 12, 759439. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Halim, K.Y.; Osman, S.R. Cytotoxicity and oxidative stress responses of imidacloprid and glyphosate in human prostate epithelial WPM-Y. 1 cell line. J. Toxicol. 2020, 2020, 4364650. [Google Scholar] [CrossRef] [PubMed]
- Kundoo, A.A.; Dar, S.A.; Mushtaq, M.; Bashir, Z.; Dar, M.S.; Gul, S.; Ali, M.T.; Gulzar, S. Role of neonicotinoids in insect pest management: A review. J. Entomol. Zool. Stud. 2018, 6, 333–339. [Google Scholar]
- Xiang, D.; Xu, X.; Zhou, Q.; Yan, R.; Chen, M.; Guo, Y.; Zhu, G. The expression of soluble functional α7-nicotinic acetylcholine receptors in E. coli and its high-affinity binding to neonicotinoid pesticides. Pestic. Biochem. Physiol. 2020, 164, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, K.; Ihara, M.; Sattelle, D.B. Neonicotinoid insecticides: Molecular targets, resistance, and toxicity. Annu. Rev. Pharmacol. Toxicol. 2020, 60, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Pisa, L.W.; Amaral-Rogers, V.; Belzunces, L.P.; Bonmatin, J.-M.; Downs, C.A.; Goulson, D.; Kreutzweiser, D.P.; Krupke, C.; Liess, M.; McField, M. Effects of neonicotinoids and fipronil on non-target invertebrates. Environ. Sci. Pollut. Res. 2015, 22, 68–102. [Google Scholar] [CrossRef]
- Van der Sluijs, J.P.; Amaral-Rogers, V.; Belzunces, L.P.; Bijleveld van Lexmond, M.F.; Bonmatin, J.-M.; Chagnon, M.; Downs, C.; Furlan, L.; Gibbons, D.W.; Giorio, C. Conclusions of the Worldwide Integrated Assessment on the risks of neonicotinoids and fipronil to biodiversity and ecosystem functioning. Environ. Sci. Pollut. Res. 2015, 22, 148–154. [Google Scholar] [CrossRef]
- Botías, C.; David, A.; Hill, E.M.; Goulson, D. Contamination of wild plants near neonicotinoid seed-treated crops, and implications for non-target insects. Sci. Total Environ. 2016, 566, 269–278. [Google Scholar] [CrossRef]
- Hallmann, C.A.; Foppen, R.P.; Van Turnhout, C.A.; De Kroon, H.; Jongejans, E. Declines in insectivorous birds are associated with high neonicotinoid concentrations. Nature 2014, 511, 341–343. [Google Scholar] [CrossRef] [PubMed]
- Gunstone, T.; Cornelisse, T.; Klein, K.; Dubey, A.; Donley, N. Pesticides and soil invertebrates: A hazard assessment. Front. Environ. Sci. 2021, 9, 122. [Google Scholar] [CrossRef]
- Anderson, J.; Dubetz, C.; Palace, V. Neonicotinoids in the Canadian aquatic environment: A literature review on current use products with a focus on fate, exposure, and biological effects. Sci. Total Environ. 2015, 505, 409–422. [Google Scholar] [CrossRef]
- Cavallaro, M.C.; Morrissey, C.A.; Headley, J.V.; Peru, K.M.; Liber, K. Comparative chronic toxicity of imidacloprid, clothianidin, and thiamethoxam to Chironomus dilutus and estimation of toxic equivalency factors. Environ. Toxicol. Chem. 2017, 36, 372–382. [Google Scholar] [CrossRef]
- Yao, K.-S.; Li, D.; Lei, H.-J.; Van den Brink, P.J.; Ying, G.-G. Imidacloprid treatments induces cyanobacteria blooms in freshwater communities under sub-tropical conditions. Aquat. Toxicol. 2021, 240, 105992. [Google Scholar] [CrossRef]
- Amoatey, P.; Baawain, M.S. Effects of pollution on freshwater aquatic organisms. Water Environ. Res. 2019, 91, 1272–1287. [Google Scholar] [CrossRef]
- Pestana, J.L.; Loureiro, S.; Baird, D.J.; Soares, A.M. Fear and loathing in the benthos: Responses of aquatic insect larvae to the pesticide imidacloprid in the presence of chemical signals of predation risk. Aquat. Toxicol. 2009, 93, 138–149. [Google Scholar] [CrossRef]
- Van Dijk, T.C.; Van Staalduinen, M.A.; Van der Sluijs, J.P. Macro-invertebrate decline in surface water polluted with imidacloprid. PLoS ONE 2013, 8, e62374. [Google Scholar] [CrossRef]
- Hewitt, J.E.; Ellis, J.I.; Thrush, S.F. Multiple stressors, nonlinear effects and the implications of climate change impacts on marine coastal ecosystems. Glob. Chang. Biol. 2016, 22, 2665–2675. [Google Scholar] [CrossRef]
- Carrier-Belleau, C.; Drolet, D.; McKindsey, C.W.; Archambault, P. Environmental stressors, complex interactions and marine benthic communities’ responses. Sci. Rep. 2021, 11, 4194. [Google Scholar] [CrossRef] [PubMed]
- Hansson, L.-A.; Brodersen, J.; Chapman, B.B.; Ekvall, M.K.; Hargeby, A.; Hulthén, K.; Nicolle, A.; Nilsson, P.A.; Skov, C.; Brönmark, C. A lake as a microcosm: Reflections on developments in aquatic ecology. Aquat. Ecol. 2013, 47, 125–135. [Google Scholar] [CrossRef]
- Zhang, H.; Urrutia-Cordero, P.; He, L.; Geng, H.; Chaguaceda, F.; Xu, J.; Hansson, L.A. Life-history traits buffer against heat wave effects on predator–prey dynamics in zooplankton. Glob. Chang. Biol. 2018, 24, 4747–4757. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, T.; Zhang, H.; Wang, H.; Hilt, S.; Shi, P.; Cheng, H.; Feng, M.; Pan, M.; Guo, Y. Heat waves rather than continuous warming exacerbate impacts of nutrient loading and herbicides on aquatic ecosystems. Environ. Int. 2022, 168, 107478. [Google Scholar] [CrossRef]
- Wang, T.; Xu, J.; Molinos, J.G.; Li, C.; Hu, B.; Pan, M.; Zhang, M. A dynamic temperature difference control recording system in shallow lake mesocosm. MethodsX 2020, 7, 100930. [Google Scholar] [CrossRef]
- Xu, J.; Wang, T.; Molinos, J.G.; Li, C.; Hu, B.; Pan, M.; Zhang, M. Effects of warming, climate extremes and phosphorus enrichment on the growth, sexual reproduction and propagule carbon and nitrogen stoichiometry of Potamogeton crispus L. Environ. Int. 2020, 137, 105502. [Google Scholar] [CrossRef]
- Jackson, M.C.; Pawar, S.; Woodward, G. The temporal dynamics of multiple stressor effects: From individuals to ecosystems. Trends Ecol. Evol. 2021, 36, 402–410. [Google Scholar] [CrossRef]
- Jeppesen, E.; Søndergaard, M.; Meerhoff, M.; Lauridsen, T.L.; Jensen, J.P. Shallow lake restoration by nutrient loading reduction—Some recent findings and challenges ahead. In Shallow Lakes in a Changing World, Proceedings of the 5th International Symposium on Shallow Lakes, Dalfsen, The Netherlands, 5–9 June 2005; Springer: Dordrecht, The Netherlands, 2007; pp. 239–252. [Google Scholar] [CrossRef]
- Morrissey, C.A.; Mineau, P.; Devries, J.H.; Sanchez-Bayo, F.; Liess, M.; Cavallaro, M.C.; Liber, K. Neonicotinoid contamination of global surface waters and associated risk to aquatic invertebrates: A review. Environ. Int. 2015, 74, 291–303. [Google Scholar] [CrossRef]
- Gong, Z.; Li, Y.; Xie, P. Population dynamics and production of Bellamya aeruginosa (reeve)(Mollusca: Viviparidae) in Lake Donghu, Wuhan. J. Lake Sci. 2009, 21, 401–407. [Google Scholar] [CrossRef]
- Zhi, Y.; Liu, Y.; Li, W.; Cao, Y. Responses of four submerged macrophytes to freshwater snail density (Radix swinhoei) under clear-water conditions: A mesocosm study. Ecol. Evol. 2020, 10, 7644–7653. [Google Scholar] [CrossRef]
- Yu, J.; Zhen, W.; Kong, L.; He, H.; Zhang, Y.; Yang, X.; Chen, F.; Zhang, M.; Liu, Z.; Jeppesen, E. Changes in pelagic fish community composition, abundance, and biomass along a productivity gradient in subtropical lakes. Water 2021, 13, 858. [Google Scholar] [CrossRef]
- Chinese National Standards. Chinese National Standards for Surface Water Quality Analysis; Standards Press of China: Beijing, China, 1996. [Google Scholar]
- Brock, T.C.; Crum, S.; Van Wijngaarden, R.; Budde, B.; Tijink, J.; Zuppelli, A.; Leeuwangh, P. Fate and effects of the insecticide Dursban® 4E in indoor Elodea-dominated and macrophyte-free freshwater model ecosystems: I. Fate and primary effects of the active ingredient chlorpyrifos. Arch. Environ. Contam. Toxicol. 1992, 23, 69–84. [Google Scholar] [CrossRef]
- Lenth, R.; Buerkner, P.; Herve, M.; Love, J.; Miguez, F.; Riebl, H.; Singmann, H. Emmeans: Estimated Marginal Means, Aka Least-Squares Means, R package version 1.7. 2; R Foundation for Statistical Computing: Vienna, Austria, 2022.
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.; O’hara, R.; Simpson, G.; Solymos, P. vegan: Community Ecology Package, R package version 2.5-7; 3.1-152; Statistical Computing: Vienna, Austria, 2020.
- Roswell, M.; Dushoff, J.; Winfree, R. A conceptual guide to measuring species diversity. Oikos 2021, 130, 321–338. [Google Scholar] [CrossRef]
- Torchiano, M. Effsize: Efficient Effect Size Computation, R package version 0.8; R Foundation for Statistical Computing: Vienna, Austria, 2020; Volume 1.
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Dell, A.I.; Pawar, S.; Savage, V.M. Systematic variation in the temperature dependence of physiological and ecological traits. Proc. Natl. Acad. Sci. USA 2011, 108, 10591–10596. [Google Scholar] [CrossRef]
- Brighenti, S.; Tolotti, M.; Bruno, M.C.; Wharton, G.; Pusch, M.T.; Bertoldi, W. Ecosystem shifts in Alpine streams under glacier retreat and rock glacier thaw: A review. Sci. Total Environ. 2019, 675, 542–559. [Google Scholar] [CrossRef]
- Clarke, A.; Fraser, K. Why does metabolism scale with temperature? Funct. Ecol. 2004, 18, 243–251. [Google Scholar] [CrossRef]
- Rall, B.C.; Vucic-Pestic, O.; Ehnes, R.B.; Emmerson, M.; Brose, U. Temperature, predator–prey interaction strength and population stability. Glob. Chang. Biol. 2010, 16, 2145–2157. [Google Scholar] [CrossRef]
- Borer, E.T.; Seabloom, E.W.; Tilman, D. Plant diversity controls arthropod biomass and temporal stability. Ecol. Lett. 2012, 15, 1457–1464. [Google Scholar] [CrossRef]
- Jiang, X.; Sun, X.; Alahuhta, J.; Heino, J.; Xie, Z. Responses of multiple facets of macroinvertebrate alpha diversity to eutrophication in floodplain lakes. Environ. Pollut. 2022, 306, 119410. [Google Scholar] [CrossRef]
- Scholer, J.; Krischik, V. Chronic exposure of imidacloprid and clothianidin reduce queen survival, foraging, and nectar storing in colonies of Bombus impatiens. PLoS ONE 2014, 9, e91573. [Google Scholar] [CrossRef]
- Rao, K.; Zhang, X.; Yi, X.-J.; Li, Z.-S.; Wang, P.; Huang, G.-W.; Guo, X.-X. Interactive effects of environmental factors on phytoplankton communities and benthic nutrient interactions in a shallow lake and adjoining rivers in China. Sci. Total Environ. 2018, 619, 1661–1672. [Google Scholar] [CrossRef]
- Dillon, M.E.; Wang, G.; Huey, R.B. Global metabolic impacts of recent climate warming. Nature 2010, 467, 704–706. [Google Scholar] [CrossRef]
- Macaulay, S.J.; Hageman, K.J.; Piggott, J.J.; Juvigny-Khenafou, N.P.; Matthaei, C.D. Warming and imidacloprid pulses determine macroinvertebrate community dynamics in experimental streams. Glob. Chang. Biol. 2021, 27, 5469–5490. [Google Scholar] [CrossRef]
- Dinh, K.V.; Konestabo, H.S.; Borgå, K.; Hylland, K.; Macaulay, S.J.; Jackson, M.C.; Verheyen, J.; Stoks, R. Interactive effects of warming and pollutants on marine and freshwater invertebrates. Curr. Pollut. Rep. 2022, 8, 341–359. [Google Scholar] [CrossRef]
- Su, H.; Feng, Y.; Chen, J.; Chen, J.; Ma, S.; Fang, J.; Xie, P. Determinants of trophic cascade strength in freshwater ecosystems: A global analysis. Ecology 2021, 102, e03370. [Google Scholar] [CrossRef]
- Birk, S.; Chapman, D.; Carvalho, L.; Spears, B.M.; Andersen, H.E.; Argillier, C.; Auer, S.; Baattrup-Pedersen, A.; Banin, L.; Beklioğlu, M. Impacts of multiple stressors on freshwater biota across spatial scales and ecosystems. Nat. Ecol. Evol. 2020, 4, 1060–1068. [Google Scholar] [CrossRef]
- Jackson, M.C.; Loewen, C.J.; Vinebrooke, R.D.; Chimimba, C.T. Net effects of multiple stressors in freshwater ecosystems: A meta-analysis. Glob. Chang. Biol. 2016, 22, 180–189. [Google Scholar] [CrossRef]
- Hermann, M.; Peeters, E.T.; Van den Brink, P.J. Heatwaves, elevated temperatures, and a pesticide cause interactive effects on multi-trophic levels of a freshwater ecosystem. Environ. Pollut. 2023, 327, 121498. [Google Scholar] [CrossRef]
- Heino, J.; Virkkala, R.; Toivonen, H. Climate change and freshwater biodiversity: Detected patterns, future trends and adaptations in northern regions. Biol. Rev. 2009, 84, 39–54. [Google Scholar] [CrossRef]
- Pecl, G.T.; Araújo, M.B.; Bell, J.D.; Blanchard, J.; Bonebrake, T.C.; Chen, I.-C.; Clark, T.D.; Colwell, R.K.; Danielsen, F.; Evengård, B. Biodiversity redistribution under climate change: Impacts on ecosystems and human well-being. Science 2017, 355, eaai9214. [Google Scholar] [CrossRef]
- Ovaskainen, O.; Weigel, B.; Potyutko, O.; Buyvolov, Y. Long-term shifts in water quality show scale-dependent bioindicator responses across Russia—Insights from 40 year-long bioindicator monitoring program. Ecol. Indic. 2019, 98, 476–482. [Google Scholar] [CrossRef]
- Peralta, E.M.; Batucan Jr, L.S.; De Jesus, I.B.B.; Triño, E.M.C.; Uehara, Y.; Ishida, T.; Kobayashi, Y.; Ko, C.-Y.; Iwata, T.; Borja, A.S. Nutrient loadings and deforestation decrease benthic macroinvertebrate diversity in an urbanised tropical stream system. Limnologica 2020, 80, 125744. [Google Scholar] [CrossRef]
- Takeshita, K.M.; Hayashi, T.I.; Yokomizo, H. Evaluation of interregional consistency in associations between neonicotinoid insecticides and functions of benthic invertebrate communities in rivers in urban rice-paddy areas. Sci. Total Environ. 2020, 743, 140627. [Google Scholar] [CrossRef] [PubMed]
- Polazzo, F.; Roth, S.K.; Hermann, M.; Mangold-Döring, A.; Rico, A.; Sobek, A.; Van den Brink, P.J.; Jackson, M.C. Combined effects of heatwaves and micropollutants on freshwater ecosystems: Towards an integrated assessment of extreme events in multiple stressors research. Glob. Chang. Biol. 2022, 28, 1248–1267. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Bayo, F. Indirect effect of pesticides on insects and other arthropods. Toxics 2021, 9, 177. [Google Scholar] [CrossRef] [PubMed]
- Rumschlag, S.L.; Mahon, M.B.; Hoverman, J.T.; Raffel, T.R.; Carrick, H.J.; Hudson, P.J.; Rohr, J.R. Consistent effects of pesticides on community structure and ecosystem function in freshwater systems. Nat. Commun. 2020, 11, 6333. [Google Scholar] [CrossRef]
- Li, F.; Guo, F.; Gao, W.; Cai, Y.; Zhang, Y.; Yang, Z. Environmental DNA biomonitoring reveals the interactive effects of dams and nutrient enrichment on aquatic multitrophic communities. Environ. Sci. Technol. 2022, 56, 16952–16963. [Google Scholar] [CrossRef]
- Burdon, F.J.; Bai, Y.; Reyes, M.; Tamminen, M.; Staudacher, P.; Mangold, S.; Singer, H.; Räsänen, K.; Joss, A.; Tiegs, S.D. Stream microbial communities and ecosystem functioning show complex responses to multiple stressors in wastewater. Glob. Chang. Biol. 2020, 26, 6363–6382. [Google Scholar] [CrossRef]
- Li, B.; Tan, W.; Wen, L.; Zhao, X.; Peng, B.; Yang, J.; Lu, C.; Wang, Y.; Lei, G. Anthropogenic habitat alternation significantly decreases α-and β-diversity of benthopelagic metacommunity in a large floodplain lake. Hydrobiologia 2020, 847, 293–307. [Google Scholar] [CrossRef]
- Cunha, E.J.; Juen, L. Impacts of oil palm plantations on changes in environmental heterogeneity and Heteroptera (Gerromorpha and Nepomorpha) diversity. J. Insect Conserv. 2017, 21, 111–119. [Google Scholar] [CrossRef]
- Cook, S.C.; Housley, L.; Back, J.A.; King, R.S. Freshwater eutrophication drives sharp reductions in temporal beta diversity. Ecology 2018, 99, 47–56. [Google Scholar] [CrossRef]
- Statzner, B.; Beche, L.A. Can biological invertebrate traits resolve effects of multiple stressors on running water ecosystems? Freshw. Biol. 2010, 55, 80–119. [Google Scholar] [CrossRef]
- Navarro-Ortega, A.; Acuna, V.; Bellin, A.; Burek, P.; Cassiani, G.; Choukr-Allah, R.; Dolédec, S.; Elosegi, A.; Ferrari, F.; Ginebreda, A. Managing the effects of multiple stressors on aquatic ecosystems under water scarcity. The GLOBAQUA project. Sci. Total Environ. 2015, 503, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Sabater, S.; Elosegi, A.; Ludwig, R. Multiple Stressors in River Ecosystems: Status, Impacts and Prospects for the Future; Elsevier: Amsterdam, The Netherlands, 2018; Available online: https://lib.ugent.be/catalog/ebk01:4100000006374808 (accessed on 30 August 2023).
- Gianuca, A.T.; Declerck, S.A.; Lemmens, P.; De Meester, L. Effects of dispersal and environmental heterogeneity on the replacement and nestedness components of β-diversity. Ecology 2017, 98, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Villéger, S.; Grenouillet, G.; Brosse, S. Decomposing functional β-diversity reveals that low functional β-diversity is driven by low functional turnover in E uropean fish assemblages. Glob. Ecol. Biogeogr. 2013, 22, 671–681. [Google Scholar] [CrossRef]
- Akamagwuna, F.C.; Odume, O.N. Ephemeroptera, Plecoptera and Trichoptera (EPT) functional feeding group responses to fine grain sediment stress in a river in the Eastern Cape, South Africa. Environ. Monit. Assess. 2020, 192, 214. [Google Scholar] [CrossRef] [PubMed]
- Chagnon, M.; Kreutzweiser, D.; Mitchell, E.A.; Morrissey, C.A.; Noome, D.A.; Van der Sluijs, J.P. Risks of large-scale use of systemic insecticides to ecosystem functioning and services. Environ. Sci. Pollut. Res. 2015, 22, 119–134. [Google Scholar] [CrossRef] [PubMed]
- Goulson, D. An overview of the environmental risks posed by neonicotinoid insecticides. J. Appl. Ecol. 2013, 50, 977–987. [Google Scholar] [CrossRef]
- Naiel, M.A.; Shehata, A.M.; Negm, S.S.; Abd El-Hack, M.E.; Amer, M.S.; Khafaga, A.F.; Bin-Jumah, M.; Allam, A.A. The new aspects of using some safe feed additives on alleviated imidacloprid toxicity in farmed fish: A review. Rev. Aquac. 2020, 12, 2250–2267. [Google Scholar] [CrossRef]
- Holzenthal, R.W.; Blahnik, R.J.; Prather, A.L.; Kjer, K.M. Order trichoptera kirby, 1813 (insecta), caddisflies. Zootaxa 2007, 1668, 639–698. [Google Scholar] [CrossRef]
- Cycoń, M.; Markowicz, A.; Borymski, S.; Wójcik, M.; Piotrowska-Seget, Z. Imidacloprid induces changes in the structure, genetic diversity and catabolic activity of soil microbial communities. J. Environ. Manag. 2013, 131, 55–65. [Google Scholar] [CrossRef]
- Shi, X.; Jiang, L.; Wang, H.; Qiao, K.; Wang, D.; Wang, K. Toxicities and sublethal effects of seven neonicotinoid insecticides on survival, growth and reproduction of imidacloprid-resistant cotton aphid, Aphis gossypii. Pest Manag. Sci. 2011, 67, 1528–1533. [Google Scholar] [CrossRef] [PubMed]
- Rix, R.R.; Ayyanath, M.M.; Christopher Cutler, G. Sublethal concentrations of imidacloprid increase reproduction, alter expression of detoxification genes, and prime Myzus persicae for subsequent stress. J. Pest Sci. 2016, 89, 581–589. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, G.; Jiang, Y.; Ma, C.; Yang, G. Sublethal effects of Imidacloprid on fecundity, apoptosis and virus transmission in the small brown planthopper Laodelphax striatellus. Insects 2021, 12, 1131. [Google Scholar] [CrossRef] [PubMed]
- Martelli, F.; Zhongyuan, Z.; Wang, J.; Wong, C.-O.; Karagas, N.E.; Roessner, U.; Rupasinghe, T.; Venkatachalam, K.; Perry, T.; Bellen, H.J. Low doses of the neonicotinoid insecticide imidacloprid induce ROS triggering neurological and metabolic impairments in Drosophila. Proc. Natl. Acad. Sci. USA 2020, 117, 25840–25850. [Google Scholar] [CrossRef]
- Carey, N.; Chester, E.T.; Robson, B.J. Loss of functionally important and regionally endemic species from streams forced into intermittency by global warming. Glob. Chang. Biol. 2023, 29, 3019–3038. [Google Scholar] [CrossRef] [PubMed]
- Belzunces, L.P.; Tchamitchian, S.; Brunet, J.-L. Neural effects of insecticides in the honey bee. Apidologie 2012, 43, 348–370. [Google Scholar] [CrossRef]
- Liu, P.; Wu, F.; Li, H.; You, J. The neonicotinoid alternative sulfoxaflor causes chronic toxicity and impairs mitochondrial energy production in Chironomus kiinensis. Aquat. Toxicol. 2021, 235, 105822. [Google Scholar] [CrossRef]
- Lv, T.; Guan, X.; Fan, S.; Han, C.; Gao, Z.; Liu, C. Snail communities increase submerged macrophyte growth by grazing epiphytic algae and phytoplankton in a mesocosm experiment. Ecol. Evol. 2022, 12, e8615. [Google Scholar] [CrossRef]
- Zhang, J.; Song, Z.; Li, Z.; Yang, J.; Xie, Z. Life history and population ecology of Radix swinhoei (Lymnaeidae) in nearshore regions of a hypereutrophic plateau lake. Ecol. Evol. 2022, 12, e9631. [Google Scholar] [CrossRef]
- Yang, X.; Zhu, J.; Hu, C.; Yang, W.; Zheng, Z. Integration of Transcriptomics and Microbiomics Reveals the Responses of Bellamya aeruginosa to Toxic Cyanobacteria. Toxins 2023, 15, 119. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, H.; Deng, Z.; Wang, J.; Liu, Z.; Chen, Y.; Ma, Y.; Li, B.; Yang, L.; Zhang, Z. Efficient removal of Imidacloprid and nutrients by algae-bacteria biofilm reactor (ABBR) in municipal wastewater: Performance, mechanisms and the importance of illumination. Chemosphere 2022, 305, 135418. [Google Scholar] [CrossRef] [PubMed]
- Hayasaka, D.; Korenaga, T.; Suzuki, K.; Saito, F.; Sánchez-Bayo, F.; Goka, K. Cumulative ecological impacts of two successive annual treatments of imidacloprid and fipronil on aquatic communities of paddy mesocosms. Ecotoxicol. Environ. Saf. 2012, 80, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Austin, P.C. The use of propensity score methods with survival or time-to-event outcomes: Reporting measures of effect similar to those used in randomized experiments. Stat. Med. 2014, 33, 1242–1258. [Google Scholar] [CrossRef] [PubMed]
- Austin, P.C. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun. Stat. Simul. Comput. 2009, 38, 1228–1234. [Google Scholar] [CrossRef]
- Silvia de Juan, S.; Hewitt, J.; Thrush, S.; Freeman, D. Standardising the assessment of Functional Integrity in benthic ecosystems. J. Sea Res. 2015, 98, 33–41. [Google Scholar] [CrossRef]


| Species Name | Rank | Abundance | Accumfreq | Warming | Eutrophic | Pesticide |
|---|---|---|---|---|---|---|
| Chironomus sp. | 1 | 1820 | 15.5 | ** (+) | NS | *** (+) |
| Glyptotendipes sp. | 2 | 1690 | 29.9 | NS | NS | * (−) |
| Ecnomus sp. | 3 | 1497 | 42.6 | NS | NS | *** (−) |
| Radix swinhoei | 4 | 1063 | 51.7 | *** (+) | * (+) | *** (+) |
| Limnodrilus hoffmeisteri | 5 | 881 | 59.2 | * (−) | ** (+) | ** (−) |
| Glossiphonia lata | 6 | 600 | 64.3 | NS | NS | * (+) |
| Hippeutis umbilicalis | 7 | 463 | 68.2 | NS | NS | * (+) |
| Polypedilum sp. | 8 | 452 | 72.1 | NS | NS | NS |
| Limnodrilus sp. | 9 | 391 | 75.4 | NS | NS | NS |
| Bellamya sp. | 10 | 334 | 78.2 | NS | NS | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, X.; Su, G.; Zhang, P.; Wang, T.; Zhao, K.; Zhang, H.; Huang, J.; Wang, H.; Kong, X.; Xu, J.; et al. Effects of Multiple Environmental Stressors on Zoobenthos Communities in Shallow Lakes: Evidence from a Mesocosm Experiment. Animals 2023, 13, 3722. https://doi.org/10.3390/ani13233722
Xu X, Su G, Zhang P, Wang T, Zhao K, Zhang H, Huang J, Wang H, Kong X, Xu J, et al. Effects of Multiple Environmental Stressors on Zoobenthos Communities in Shallow Lakes: Evidence from a Mesocosm Experiment. Animals. 2023; 13(23):3722. https://doi.org/10.3390/ani13233722
Chicago/Turabian StyleXu, Xiaoqi, Guohuan Su, Peiyu Zhang, Tao Wang, Kangshun Zhao, Huan Zhang, Jinhe Huang, Hongxia Wang, Xianghong Kong, Jun Xu, and et al. 2023. "Effects of Multiple Environmental Stressors on Zoobenthos Communities in Shallow Lakes: Evidence from a Mesocosm Experiment" Animals 13, no. 23: 3722. https://doi.org/10.3390/ani13233722
APA StyleXu, X., Su, G., Zhang, P., Wang, T., Zhao, K., Zhang, H., Huang, J., Wang, H., Kong, X., Xu, J., & Zhang, M. (2023). Effects of Multiple Environmental Stressors on Zoobenthos Communities in Shallow Lakes: Evidence from a Mesocosm Experiment. Animals, 13(23), 3722. https://doi.org/10.3390/ani13233722






