Microencapsulated Sodium Butyrate Alleviates Immune Injury and Intestinal Problems Caused by Clostridium Perfringens through Gut Microbiota
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Diets
2.2. Sample Collection
2.3. Organ Index
2.4. Serum Immune Indicators
2.5. Jejunum Morphology Analysis
2.6. Volatile Fatty Acid (VFA) Analysis
2.7. Cecum Microflora
2.8. Statistical Analysis
3. Results
3.1. Microencapsulated Sodium Butyrate Alleviated C. perfringens Infection
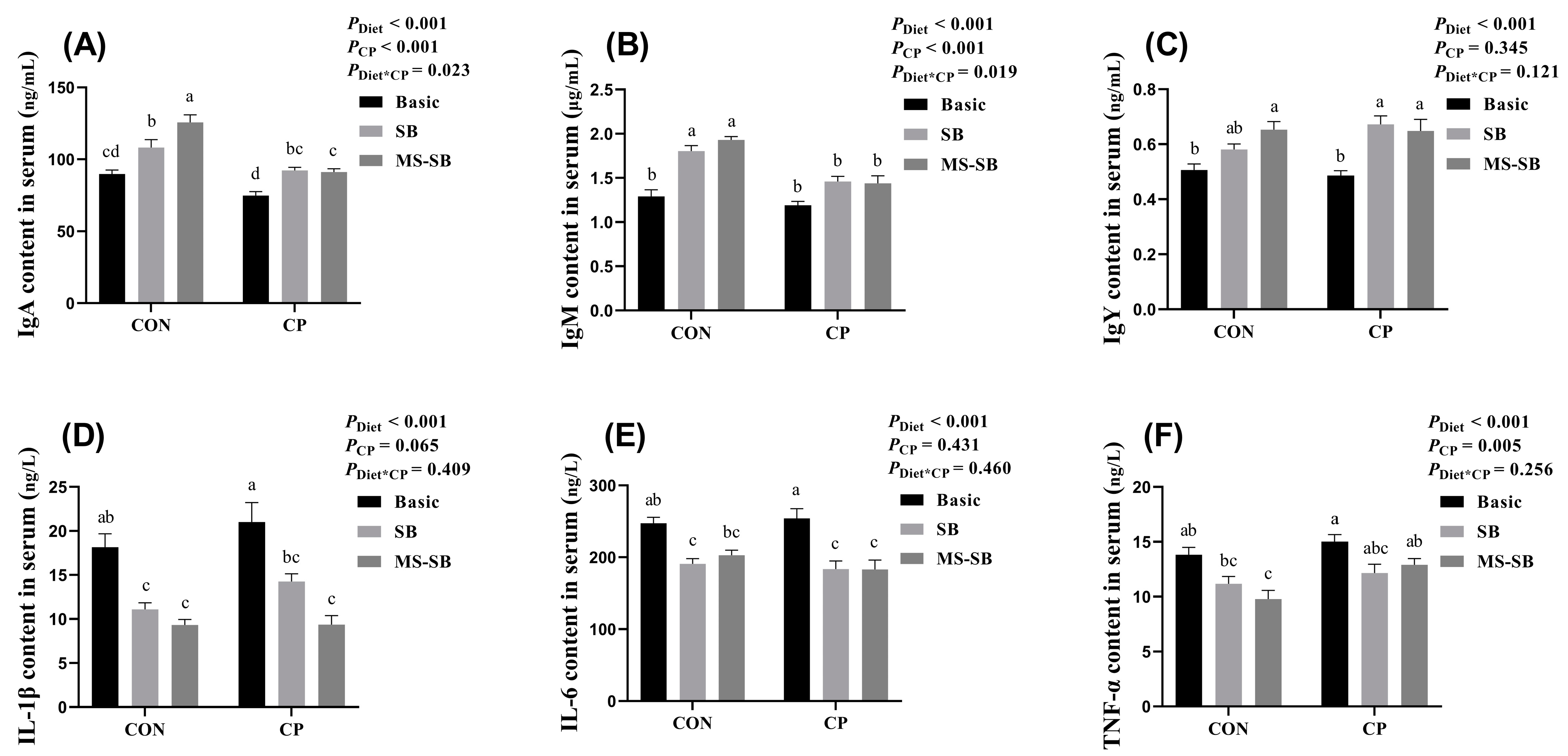
3.2. Microencapsulated Sodium Butyrate Alleviated Reduced Systemic Inflammation Caused by C. perfringens
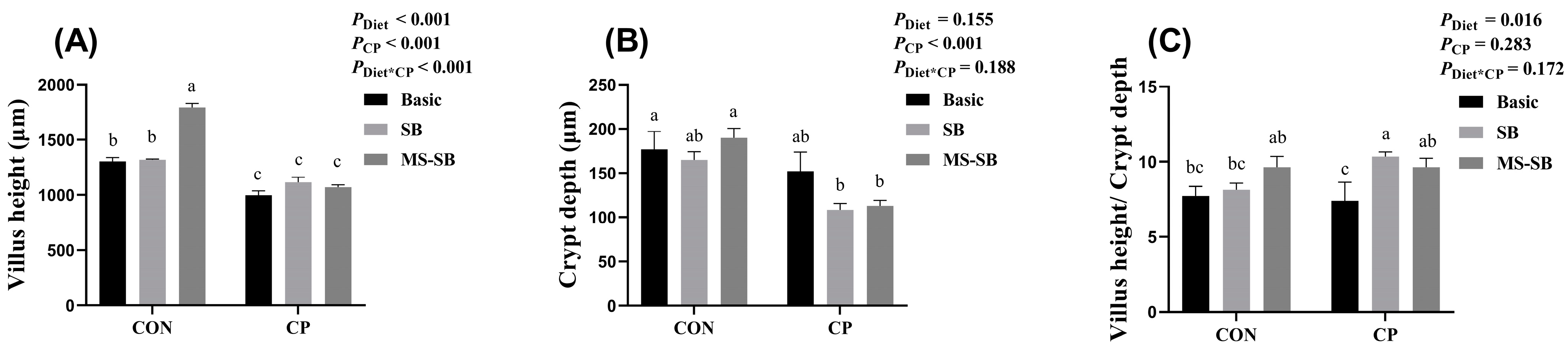
3.3. Microencapsulated Sodium Butyrate Repaired Intestinal Morphology Damaged by C. perfringens
3.4. Microencapsulated Sodium Butyrate Ameliorated VFAs under C. perfringens Challenge
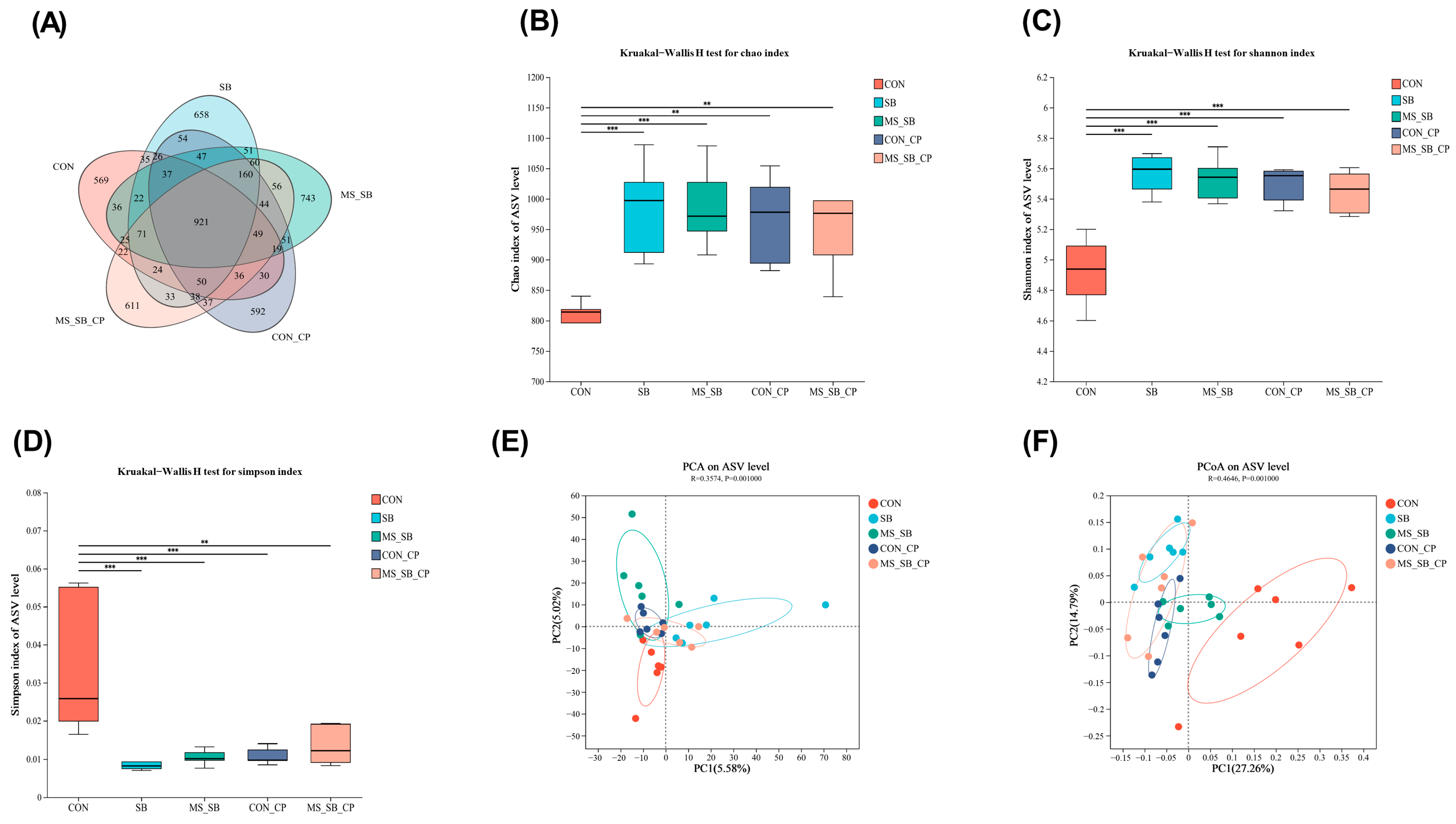
3.5. Microencapsulated Sodium Butyrate Modulated Gut Microbiota Community Variation Caused by C. perfringens
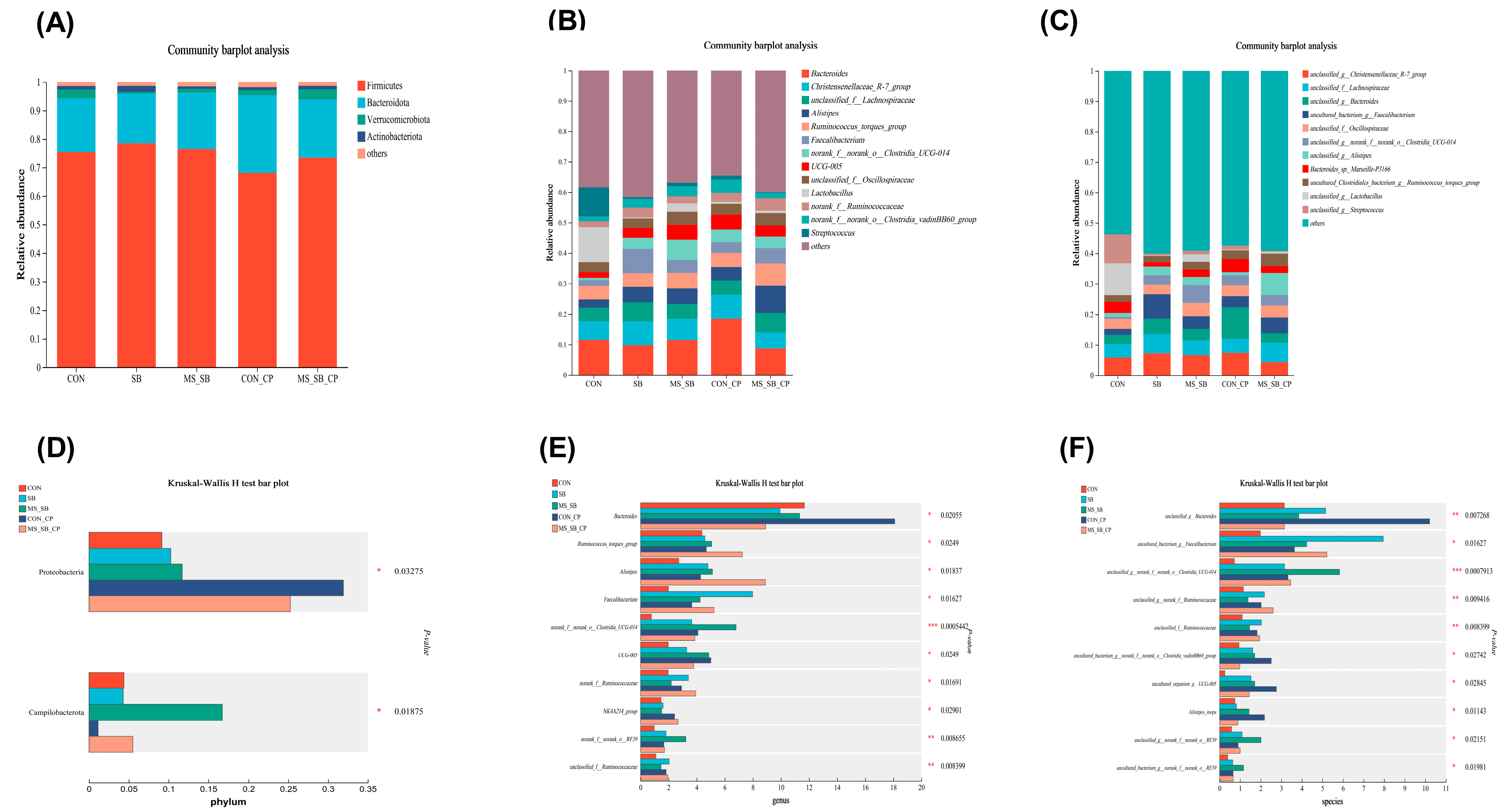
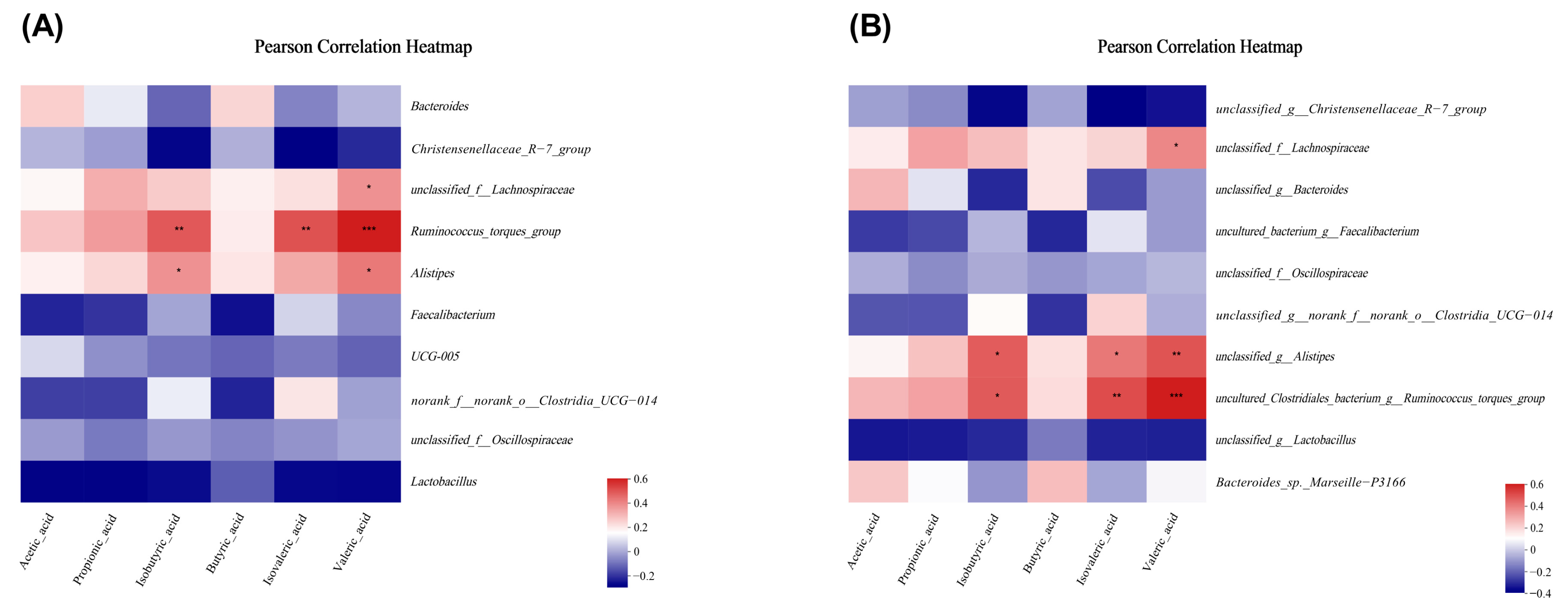
3.6. Microencapsulated Sodium Butyrate Modulated Gut Microbiota Community Composition Caused by C. perfringens
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Z.; Wang, W.; Liu, D.; Guo, Y. Effects of Lactobacillus acidophilus on the growth performance and intestinal health of broilers challenged with Clostridium perfringens. J. Anim. Sci. Biotechnol. 2018, 9, 25. [Google Scholar] [CrossRef]
- Salvi, P.S.; Cowles, R.A. Butyrate and the Intestinal Epithelium: Modulation of Proliferation and Inflammation in Homeostasis and Disease. Cells 2021, 10, 1775. [Google Scholar] [CrossRef]
- Sun, Q.; Ji, Y.-C.; Wang, Z.-L.; She, X.; He, Y.; Ai, Q.; Li, L.-Q. Sodium Butyrate Alleviates Intestinal Inflammation in Mice with Necrotizing Enterocolitis. Mediat. Inflamm. 2021, 2021, 6259381. [Google Scholar] [CrossRef]
- Manrique Vergara, D.; González Sánchez, M.E. Short chain fatty acids (butyric acid) and intestinal diseases. Nutr. Hosp. 2017, 34, 58–61. [Google Scholar] [CrossRef]
- Chen, G.; Ran, X.; Li, B.; Li, Y.; He, D.; Huang, B.; Fu, S.; Liu, J.; Wang, W. Sodium Butyrate Inhibits Inflammation and Maintains Epithelium Barrier Integrity in a TNBS-induced Inflammatory Bowel Disease Mice Model. EBioMedicine 2018, 30, 317–325. [Google Scholar] [CrossRef]
- Zhou, D.; Pan, Q.; Xin, F.Z.; Zhang, R.N.; He, C.X.; Chen, G.Y.; Liu, C.; Chen, Y.W.; Fan, J.G. Sodium butyrate attenuates high-fat diet-induced steatohepatitis in mice by improving gut microbiota and gastrointestinal barrier. World J. Gastroenterol. 2017, 23, 60–75. [Google Scholar] [CrossRef]
- Posthaus, H.; Kittl, S.; Tarek, B.; Bruggisser, J. Clostridium perfringens type C necrotic enteritis in pigs: Diagnosis, pathogenesis, and prevention. J. Vet. Diagn. Investig. Off. Publ. Am. Assoc. Vet. Lab. Diagn. Inc. 2020, 32, 203–212. [Google Scholar] [CrossRef]
- Wade, B.; Keyburn, A. The true cost of necrotic enteritis. World Poult. 2015, 31, 16–17. [Google Scholar]
- Hustá, M.; Tretiak, S.; Ducatelle, R.; Van Immerseel, F.; Goossens, E. Clostridium perfringens strains proliferate to high counts in the broiler small intestinal tract, in accordance with necrotic lesion severity, and sporulate in the distal intestine. Vet. Microbiol. 2023, 280, 109705. [Google Scholar] [CrossRef]
- Daneshmand, A.; Kermanshahi, H.; Mohammed, J.; Sekhavati, M.H.; Javadmanesh, A.; Ahmadian, M.; Alizadeh, M.; Razmyar, J.; Kulkarni, R.R. Intestinal changes and immune responses during Clostridium perfringens-induced necrotic enteritis in broiler chickens. Poult. Sci. 2022, 101, 101652. [Google Scholar] [CrossRef]
- Marcano, V.; Gamble, T.; Maschek, K.; Stabler, L.; Fletcher, O.; Davis, J.; Troan, B.V.; Villegas, A.M.; Tsai, Y.Y.; Barbieri, N.L.; et al. Necrotizing Hepatitis Associated with Clostridium perfringens in Broiler Chicks. Avian Dis. 2022, 66, 337–344. [Google Scholar] [CrossRef]
- Tian, M.; Li, L.; Tian, Z.; Zhao, H.; Chen, F.; Guan, W.; Zhang, S. Glyceryl butyrate attenuates enterotoxigenic Escherichia coli-induced intestinal inflammation in piglets by inhibiting the NF-κB/MAPK pathways and modulating the gut microbiota. Food Funct. 2022, 13, 6282–6292. [Google Scholar] [CrossRef]
- Pace, F.; Rudolph, S.E.; Chen, Y.; Bao, B.; Kaplan, D.L.; Watnick, P.I. The Short-Chain Fatty Acids Propionate and Butyrate Augment Adherent-Invasive Escherichia coli Virulence but Repress Inflammation in a Human Intestinal Enteroid Model of Infection. Microbiol. Spectr. 2021, 9, e0136921. [Google Scholar] [CrossRef]
- Kaczmarek, S.A.; Barri, A.; Hejdysz, M.; Rutkowski, A. Effect of different doses of coated butyric acid on growth performance and energy utilization in broilers. Poult. Sci. 2016, 95, 851–859. [Google Scholar] [CrossRef]
- Pourjafar, H.; Noori, N.; Gandomi, H.; Basti, A.A.; Ansari, F. Viability of microencapsulated and non-microencapsulated Lactobacilli in a commercial beverage. Biotechnol. Rep. 2020, 25, e00432. [Google Scholar] [CrossRef]
- Pesti, G.M. Nutrient Requirements of Poultry; National Academy Press: Washington, DC, USA, 1994. [Google Scholar]
- Sciences, G.A.o.A. Nutrient Requirements of Yellow Chickens; Ministry of Agriculture and Rural Affairs, PRC: Beijing, China, 2020. [Google Scholar]
- Yu, X.; Dai, Z.; Cao, G.; Cui, Z.; Zhang, R.; Xu, Y.; Wu, Y.; Yang, C. Protective effects of Bacillus licheniformis on growth performance, gut barrier functions, immunity and serum metabolome in lipopolysaccharide-challenged weaned piglets. Front. Immunol. 2023, 14, 1140564. [Google Scholar] [CrossRef]
- Cong, J.; Zhou, P.; Zhang, R. Intestinal Microbiota-Derived Short Chain Fatty Acids in Host Health and Disease. Nutrients 2022, 14, 1977. [Google Scholar] [CrossRef]
- He, W.; Goes, E.C.; Wakaruk, J.; Barreda, D.R.; Korver, D.R. A Poultry Subclinical Necrotic Enteritis Disease Model Based on Natural Clostridium perfringens Uptake. Front. Physiol. 2022, 13, 788592. [Google Scholar] [CrossRef]
- Villagrán-de la Mora, Z.; Vázquez-Paulino, O.; Avalos, H.; Ascencio, F.; Nuño, K.; Villarruel-López, A. Effect of a Synbiotic Mix on Lymphoid Organs of Broilers Infected with Salmonella typhimurium and Clostridium perfringens. Animals 2020, 10, 886. [Google Scholar] [CrossRef]
- Sikandar, A.; Zaneb, H.; Younus, M.; Masood, S.; Aslam, A.; Khattak, F.; Ashraf, S.; Yousaf, M.S.; Rehman, H. Effect of sodium butyrate on performance, immune status, microarchitecture of small intestinal mucosa and lymphoid organs in broiler chickens. Asian-Australas. J. Anim. Sci. 2017, 30, 690–699. [Google Scholar] [CrossRef]
- Elnesr, S.; Ropy, A.; Abdel-Razik, A. Effect of dietary sodium butyrate supplementation on growth, blood biochemistry, haematology and histomorphometry of intestine and immune organs of Japanese quail. Anim. Int. J. Anim. Biosci. 2019, 13, 1234–1244. [Google Scholar] [CrossRef]
- Ferreira, N.S.; Tostes, R.C.; Paradis, P.; Schiffrin, E.L. Aldosterone, inflammation, immune system, and hypertension. Am. J. Hypertens. 2021, 34, 15–27. [Google Scholar] [CrossRef]
- Hand, T.W.; Reboldi, A. Production and function of immunoglobulin A. Annu. Rev. Immunol. 2021, 39, 695–718. [Google Scholar] [CrossRef]
- Zhang, R.; Qin, S.; Yang, C.; Niu, Y.; Feng, J. The protective effects of Bacillus licheniformis against inflammatory responses and intestinal barrier damage in broilers with necrotic enteritis induced by Clostridium perfringens. J. Sci. Food Agric. 2023, 13, 6958–6965. [Google Scholar] [CrossRef]
- Azad, M.A.K.; Sarker, M.; Wan, D. Immunomodulatory effects of probiotics on cytokine profiles. BioMed Res. Int. 2018, 2018, 8063647. [Google Scholar] [CrossRef]
- Crawford, C.K.; Lopez Cervantes, V.; Quilici, M.L.; Armién, A.G.; Questa, M.; Matloob, M.S.; Huynh, L.D.; Beltran, A.; Karchemskiy, S.J.; Crakes, K.R. Inflammatory cytokines directly disrupt the bovine intestinal epithelial barrier. Sci. Rep. 2022, 12, 14578. [Google Scholar] [CrossRef]
- Yu, R.; Jiang, S.; Tao, Y.; Li, P.; Yin, J.; Zhou, Q. Inhibition of HMGB1 improves necrotizing enterocolitis by inhibiting NLRP3 via TLR4 and NF-κB signaling pathways. J. Cell. Physiol. 2019, 234, 13431–13438. [Google Scholar] [CrossRef]
- Di Tommaso, N.; Gasbarrini, A.; Ponziani, F.R. Intestinal barrier in human health and disease. Int. J. Environ. Res. Public Health 2021, 18, 12836. [Google Scholar] [CrossRef]
- Bischoff, S.C.; Barbara, G.; Buurman, W.; Ockhuizen, T.; Schulzke, J.-D.; Serino, M.; Tilg, H.; Watson, A.; Wells, J.M. Intestinal permeability–a new target for disease prevention and therapy. BMC Gastroenterol. 2014, 14, 189. [Google Scholar] [CrossRef]
- Pérez-Reytor, D.; Puebla, C.; Karahanian, E.; García, K. Use of short-chain fatty acids for the recovery of the intestinal epithelial barrier affected by bacterial toxins. Front. Physiol. 2021, 12, 650313. [Google Scholar] [CrossRef]
- Fasina, Y.O.; Lillehoj, H.S. Characterization of intestinal immune response to Clostridium perfringens infection in broiler chickens. Poult. Sci. 2019, 98, 188–198. [Google Scholar] [CrossRef]
- Ibrahim, D.; Ismail, T.A.; Khalifa, E.; Abd El-Kader, S.A.; Mohamed, D.I.; Mohamed, D.T.; Shahin, S.E.; Abd El-Hamid, M.I. Supplementing Garlic Nanohydrogel Optimized Growth, Gastrointestinal Integrity and Economics and Ameliorated Necrotic Enteritis in Broiler Chickens Using a Clostridium perfringens Challenge Model. Animal 2021, 11, 2027. [Google Scholar] [CrossRef]
- Navarro, M.A.; Li, J.; McClane, B.A.; Morrell, E.; Beingesser, J.; Uzal, F.A. NanI sialidase is an important contributor to Clostridium perfringens type F strain F4969 intestinal colonization in mice. Infect. Immun. 2018, 86, 10–1128. [Google Scholar] [CrossRef]
- Dąbek-Drobny, A.; Kaczmarczyk, O.; Woźniakiewicz, M.; Paśko, P.; Dobrowolska-Iwanek, J.; Woźniakiewicz, A.; Piątek-Guziewicz, A.; Zagrodzki, P.; Zwolińska-Wcisło, M. Association between Fecal Short-Chain Fatty Acid Levels, Diet, and Body Mass Index in Patients with Inflammatory Bowel Disease. Biology 2022, 11, 108. [Google Scholar] [CrossRef]
- Fukuda, S.; Toh, H.; Hase, K.; Oshima, K.; Nakanishi, Y.; Yoshimura, K.; Tobe, T.; Clarke, J.M.; Topping, D.L.; Suzuki, T.; et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 2011, 469, 543–547. [Google Scholar] [CrossRef]
- Yang, J.; Wei, H.; Zhou, Y.; Szeto, C.H.; Li, C.; Lin, Y.; Coker, O.O.; Lau, H.C.H.; Chan, A.W.H.; Sung, J.J.Y.; et al. High-Fat Diet Promotes Colorectal Tumorigenesis Through Modulating Gut Microbiota and Metabolites. Gastroenterology 2022, 162, 135–149.e132. [Google Scholar] [CrossRef]
- McDonald, J.A.; Mullish, B.H.; Pechlivanis, A.; Liu, Z.; Brignardello, J.; Kao, D.; Holmes, E.; Li, J.V.; Clarke, T.B.; Thursz, M.R. Inhibiting growth of Clostridioides difficile by restoring valerate, produced by the intestinal microbiota. Gastroenterology 2018, 155, 1495–1507.e1415. [Google Scholar] [CrossRef]
- Pietrzak, A.; Banasiuk, M.; Szczepanik, M.; Borys-Iwanicka, A.; Pytrus, T.; Walkowiak, J.; Banaszkiewicz, A. Sodium Butyrate Effectiveness in Children and Adolescents with Newly Diagnosed Inflammatory Bowel Diseases-Randomized Placebo-Controlled Multicenter Trial. Nutrients 2022, 14, 3283. [Google Scholar] [CrossRef]
- Jung, T.-H.; Park, J.H.; Jeon, W.-M.; Han, K.-S. Butyrate modulates bacterial adherence on LS174T human colorectal cells by stimulating mucin secretion and MAPK signaling pathway. Nutr. Res. Pract. 2015, 9, 343–349. [Google Scholar] [CrossRef]
- Liu, P.; Wang, Y.; Yang, G.; Zhang, Q.; Meng, L.; Xin, Y.; Jiang, X. The role of short-chain fatty acids in intestinal barrier function, inflammation, oxidative stress, and colonic carcinogenesis. Pharmacol. Res. 2021, 165, 105420. [Google Scholar] [CrossRef]
- Mallo, J.J.; Sol, C.; Puyalto, M.; Bortoluzzi, C.; Applegate, T.J.; Villamide, M.J. Evaluation of sodium butyrate and nutrient concentration for broiler chickens. Poult. Sci. 2021, 100, 101456. [Google Scholar] [CrossRef]
- Krix-Jachym, K.; Onyszkiewicz, M.; Swiatkiewicz, M.; Fiedorowicz, M.; Sapierzynski, R.; Grieb, P.; Rekas, M. Evaluation of butyric acid as a potential supportive treatment in anterior uveitis. Ophthalmol. J. 2022, 7, 117–126. [Google Scholar] [CrossRef]
- Pham, V.T.; Fehlbaum, S.; Seifert, N.; Richard, N.; Bruins, M.J.; Sybesma, W.; Rehman, A.; Steinert, R.E. Effects of colon-targeted vitamins on the composition and metabolic activity of the human gut microbiome—A pilot study. Gut Microbes 2021, 13, 1875774. [Google Scholar] [CrossRef]
- Makowski, Z.; Lipiński, K.; Mazur-Kuśnirek, M. The Effects of sodium butyrate, coated sodium butyrate, and butyric acid glycerides on nutrient digestibility, gastrointestinal function, and fecal microbiota in turkeys. Animals 2022, 12, 1836. [Google Scholar] [CrossRef]
- Xu, Y.; Yu, Y.; Shen, Y.; Li, Q.; Lan, J.; Wu, Y.; Zhang, R.; Cao, G.; Yang, C. Effects of Bacillus subtilis and Bacillus licheniformis on growth performance, immunity, short chain fatty acid production, antioxidant capacity, and cecal microflora in broilers. Poult. Sci. 2021, 100, 101358. [Google Scholar] [CrossRef]
- Yao, H.; Zhang, D.; Yu, H.; Yuan, H.; Shen, H.; Lan, X.; Liu, H.; Chen, X.; Meng, F.; Wu, X. Gut microbiota regulates chronic ethanol exposure-induced depressive-like behavior through hippocampal NLRP3-mediated neuroinflammation. Mol. Psychiatry 2023, 28, 919–930. [Google Scholar] [CrossRef]
- Li, C.; Wang, S.; Chen, S.; Wang, X.; Deng, X.; Liu, G.; Chang, W.; Beckers, Y.; Cai, H. Screening and characterization of Pediococcus acidilactici LC-9-1 toward selection as a potential probiotic for poultry with antibacterial and antioxidative properties. Antioxidants 2023, 12, 215. [Google Scholar] [CrossRef]
- Pammi, M.; Cope, J.; Tarr, P.I.; Warner, B.B.; Morrow, A.L.; Mai, V.; Gregory, K.E.; Kroll, J.S.; McMurtry, V.; Ferris, M.J. Intestinal dysbiosis in preterm infants preceding necrotizing enterocolitis: A systematic review and meta-analysis. Microbiome 2017, 5, 31. [Google Scholar] [CrossRef]
- Zhang, B.; Lv, Z.; Li, Z.; Wang, W.; Li, G.; Guo, Y. Dietary l-arginine Supplementation Alleviates the Intestinal Injury and Modulates the Gut Microbiota in Broiler Chickens Challenged by Clostridium perfringens. Front. Microbiol. 2018, 9, 1716. [Google Scholar] [CrossRef]
- Gong, L.; Wang, B.; Zhou, Y.; Tang, L.; Zeng, Z.; Zhang, H.; Li, W. Protective effects of Lactobacillus plantarum 16 and Paenibacillus polymyxa 10 against Clostridium perfringens infection in broilers. Front. Immunol. 2021, 11, 628374. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, S.; Luo, Z.; Liu, D. Supplemental Bacillus subtilis PB6 improves growth performance and gut health in broilers challenged with Clostridium perfringens. J. Immunol. Res. 2021, 2021, 2549541. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Cao, W.; Chen, X.; She, J. Intelligent Optimization and Control for Reheating Furnaces. In Intelligent Optimization and Control of Complex Metallurgical Processes; Springer: Singapore, 2020; pp. 223–271. [Google Scholar] [CrossRef]

| Ingredients (%) | Starter (Days 1–28) | Grower (Days 29–56) | Nutritional Level | Starter (Days 1–28) | Grower (Days 29–56) |
|---|---|---|---|---|---|
| Corn | 54.4 | 53 | Me (kcal/kg) | 2983 | 3090 |
| Soybean meal | 23.6 | 16 | CP (%) | 20.4 | 17.2 |
| Expanded soybean | 5 | 3 | Lysine (%) | 1.18 | 0.96 |
| Rice DDGS | 5 | 8 | Methionine (%) | 0.55 | 0.44 |
| Rice bran | / | 8 | Met + Cys (%) | 0.90 | 0.74 |
| Corn bran | / | 2 | Tryptophan (%) | 0.22 | 0.20 |
| Soybean oil | 2.2 | 4.5 | Threonine (%) | 0.88 | 0.78 |
| Limestone | 1.5 | 1.9 | Calcium (%) | 0.86 | 0.73 |
| Fermented soybean meal | 2.5 | / | Total P (%) | 0.70 | 0.71 |
| Corn gluten meal | 2.0 | / | Available P (%) | 0.43 | 0.44 |
| CaHPO4 (2H2O) | 2.0 | 1.8 | |||
| NaCl | 0.3 | 0.3 | |||
| Premix a | 1.5 | 1.5 | |||
| Total | 100.00 | 100.00 |
| Diet | Challenge | Liver Index | Spleen Index | Bursa of Fabricius Index | Thymus Index |
|---|---|---|---|---|---|
| Control | CON | 16.99 | 1.37 | 1.14 | 2.34 a |
| SB | 18.04 | 1.49 | 1.23 | 1.84 ab | |
| MS-SB | 17.67 | 1.77 | 0.76 | 2.43 a | |
| Control | CP | 17.77 | 1.84 | 0.53 | 1.46 bc |
| SB | 19.74 | 1.89 | 0.75 | 0.98 c | |
| MS-SB | 17.25 | 1.62 | 0.92 | 0.97 c | |
| SEM | 0.65 | 0.11 | 0.19 | 0.14 | |
| Main effect | |||||
| Diet | Control | 17.38 | 1.60 | 0.83 | 1.90 a |
| SB | 18.86 | 1.70 | 0.99 | 1.41 b | |
| MS-SB | 17.46 | 1.69 | 0.84 | 1.63 b | |
| Challenge | CON | 17.56 | 1.54 b | 0.73 | 2.24 a |
| CP | 18.25 | 1.78 a | 1.04 | 1.37 b | |
| p-value | |||||
| Diet | 0.057 | 0.743 | 0.694 | 0.005 | |
| Challenge | 0.221 | 0.034 | 0.063 | <0.001 | |
| Diet × Challenge | 0.304 | 0.051 | 0.128 | 0.023 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, T.; Sun, Y.; Dai, Z.; Liu, J.; Xiao, S.; Liu, Y.; Wang, X.; Yang, S.; Zhang, R.; Yang, C.; et al. Microencapsulated Sodium Butyrate Alleviates Immune Injury and Intestinal Problems Caused by Clostridium Perfringens through Gut Microbiota. Animals 2023, 13, 3784. https://doi.org/10.3390/ani13243784
Yang T, Sun Y, Dai Z, Liu J, Xiao S, Liu Y, Wang X, Yang S, Zhang R, Yang C, et al. Microencapsulated Sodium Butyrate Alleviates Immune Injury and Intestinal Problems Caused by Clostridium Perfringens through Gut Microbiota. Animals. 2023; 13(24):3784. https://doi.org/10.3390/ani13243784
Chicago/Turabian StyleYang, Ting, Yaowei Sun, Zhenglie Dai, Jinsong Liu, Shiping Xiao, Yulan Liu, Xiuxi Wang, Shenglan Yang, Ruiqiang Zhang, Caimei Yang, and et al. 2023. "Microencapsulated Sodium Butyrate Alleviates Immune Injury and Intestinal Problems Caused by Clostridium Perfringens through Gut Microbiota" Animals 13, no. 24: 3784. https://doi.org/10.3390/ani13243784






