A Comparative Analysis of Sparisoma cretense in Island Environments: Unraveling Metal Accumulation Differences in the Canary Islands (Spain, NW African Waters)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Sampling and Analysis
2.2. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Hawkes, S.J. What Is a “Heavy Metal”? J. Chem. Educ. 1997, 74, 1374. [Google Scholar] [CrossRef]
- Castro-González, M.I.; Méndez-Armenta, M. Heavy Metals: Implications Associated to Fish Consumption. Environ. Toxicol. Pharmacol. 2008, 26, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Khan, E. Bioaccumulation of Non-Essential Hazardous Heavy Metals and Metalloids in Freshwater Fish. Risk to Human Health. Environ. Chem. Lett. 2018, 16, 903–917. [Google Scholar] [CrossRef]
- Bánfalvi, G. Heavy Metals, Trace Elements and Their Cellular Effects. Cell. Eff. Heavy Met. 2011, 3–28. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Ilahi, I. Environmental Chemistry and Ecotoxicology of Hazardous Heavy Metals: Environmental Persistence, Toxicity, and Bioaccumulation. J. Chem. 2019, 2019, 6730305. [Google Scholar] [CrossRef]
- Khristoforova, N.K.; Emelyanov, A.A.; Efimov, A.V. Bioindication of Heavy-Metal Pollution in the Coastal Marine Waters of Russky Island (Peter the Great Bay, Sea of Japan). Russ. J. Mar. Biol. 2018, 44, 572–579. [Google Scholar] [CrossRef]
- Li, H.; Ji, H.; Shi, C.; Gao, Y.; Zhang, Y.; Xu, X.; Ding, H.; Tang, L.; Xing, Y. Distribution of Heavy Metals and Metalloids in Bulk and Particle Size Fractions of Soils from Coal-Mine Brownfield and Implications on Human Health. Chemosphere 2017, 172, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Mohan, S.V.; Nithila, P.; Reddy, S.J. Estimation of Heavy Metals in Drinking Water and Development of Heavy Metal Pollution Index. J. Environ. Sci. Health Part A 1996, 31, 283–289. [Google Scholar] [CrossRef]
- Kannan, K.; Yasunaga, Y.; Iwata, H.; Ichihashi, H.; Tanabe, S.; Tatsukawa, R. Concentrations of Heavy Metals, Organochlorines, and Organotins in Horseshoe Crab, Tachypleus Tridentatus, from Japanese Coastal Waters. Arch. Environ. Contam. Toxicol. 1995, 28, 40–47. [Google Scholar] [CrossRef]
- Thorne-Bazarra, T.; Lozano-Bilbao, E.; Hardisson, A.; González-Weller, D.; Rubio, C.; Paz, S.; Gutiérrez, Á.J. Seagrass Meadows Serve as Buffers for Metal Concentrations in the Fish Species Sparisoma Cretense in the Canary Islands (Atlantic EC, Spain). Reg. Stud. Mar. Sci. 2023, 67, 103192. [Google Scholar] [CrossRef]
- Burger, J. Bioindicators: A Review of Their Use in the Environmental Literature 1970–2005. Environ. Bioindic. 2006, 1, 136–144. [Google Scholar] [CrossRef]
- Markert, B.A.; Breure, A.M.; Zechmeister, H.G. Bioindicators and Biomonitors; Elsevier: Amsterdam, The Netherlands, 2003; ISBN 0080527973. [Google Scholar]
- Tokatli, C. Comparisons of Diatoms and Fishes as Toxic Metal Bioindicator: A Case Study of an A-Class Wetland in Northwest Turkey under Effect of an Intensive Paddy Cultivation Stress. Environ. Sci. Pollut. Res. 2022, 29, 87231–87244. [Google Scholar] [CrossRef] [PubMed]
- Plessl, C.; Otachi, E.O.; Körner, W.; Avenant-Oldewage, A.; Jirsa, F. Fish as Bioindicators for Trace Element Pollution from Two Contrasting Lakes in the Eastern Rift Valley, Kenya: Spatial and Temporal Aspects. Environ. Sci. Pollut. Res. 2017, 24, 19767–19776. [Google Scholar] [CrossRef] [PubMed]
- Peycheva, K.; Panayotova, V.; Stancheva, R.; Makedonski, L.; Merdzhanova, A.; Parrino, V.; Nava, V.; Cicero, N.; Fazio, F. Risk Assessment of Essential and Toxic Elements in Freshwater Fish Species from Lakes near Black Sea, Bulgaria. Toxics 2022, 10, 675. [Google Scholar] [CrossRef] [PubMed]
- Azaman, F.; Juahir, H.; Yunus, K.; Azid, A.; Kamarudin, M.K.A.; Toriman, M.E.; Mustafa, A.D.; Amran, M.A.; Hasnam, C.N.C.; Saudi, A.S.M. Heavy Metal in Fish: Analysis and Human Health-a Review. J. Teknol. 2015, 77, 61–69. [Google Scholar] [CrossRef]
- Costa, F.; Coelho, J.P.; Baptista, J.; Martinho, F.; Pereira, M.E.; Pardal, M.A. Mercury Accumulation in Fish Species along the Portuguese Coast: Are There Potential Risks to Human Health? Mar. Pollut. Bull. 2020, 150, 110740. [Google Scholar] [CrossRef] [PubMed]
- Bencheikh, Z.; Refes, W.; Brito, P.M.; Prodocimo, M.M.; Gusso-Choueri, P.K.; Choueri, R.B.; de Oliveira Ribeiro, C.A. Chemical Pollution Impairs the Health of Fish Species and Fishery Activities along the Algeria Coastline, Mediterranean Sea. Environ. Monit. Assess. 2022, 194, 497. [Google Scholar] [CrossRef]
- Steinhausen, S.L.; Agyeman, N.; Turrero, P.; Ardura, A.; Garcia-Vazquez, E. Heavy Metals in Fish Nearby Electronic Waste May Threaten Consumer’s Health. Examples from Accra, Ghana. Mar. Pollut. Bull. 2022, 175, 113162. [Google Scholar] [CrossRef]
- Uche-Soria, M.; Rodríguez-Monroy, C. Solutions to Marine Pollution in Canary Islands’ Ports: Alternatives and Optimization of Energy Management. Resources 2019, 8, 59. [Google Scholar] [CrossRef]
- Domínguez, L.M.; Ferrer, F.O. Aquaculture and Marine Biodiversity Boost: Case Examples from the Canary Islands. Water Resour. Manag. 2009, 97, 97–102. [Google Scholar]
- Barton, E.D.; Arístegui, J.; Tett, P.; Cantón, M.; García-Braun, J.; Hernández-León, S.; Nykjaer, L.; Almeida, C.; Almunia, J.; Ballesteros, S.; et al. The Transition Zone of the Canary Current Upwelling Region. Prog. Oceanogr. 1998, 41, 455–504. [Google Scholar] [CrossRef]
- Jiménez, S.; Sotillo, B.; Acosta, C.; Santamaría, M.T.G. Deep-Sea Research Part II Seasonal Evolution of Small Pelagic Fi Sh Landings Index in Relation to Oceanographic Variables in the Canary Islands (Spain). Deep-Sea Res. Part II 2019, 159, 84–91. [Google Scholar] [CrossRef]
- Baztan, J.; Carrasco, A.; Chouinard, O.; Cleaud, M.; Gabaldon, J.E.; Huck, T.; Jaffrès, L.; Jorgensen, B.; Miguelez, A.; Paillard, C. Protected Areas in the Atlantic Facing the Hazards of Micro-Plastic Pollution: First Diagnosis of Three Islands in the Canary Current. Mar. Pollut. Bull. 2014, 80, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Bilbao, E.; Lozano, G.; Jiménez, S.; Jurado-Ruzafa, A.; Hardisson, A.; Rubio, C.; Weller, D.-G.; Paz, S.; Gutiérrez, Á.J. Seasonal and Ontogenic Variations of Metal Content in the European Pilchard (Sardina Pilchardus) in Northwestern African Waters. Environ. Pollut. 2020, 266, 115113. [Google Scholar] [CrossRef]
- Lozano-Bilbao, E.; Lozano, G.; Jiménez, S.; Jurado-Ruzafa, A.; Hardisson, A.; Rubio, C.; Weller, D.G.; Paz, S.; Gutiérrez, Á.J. Ontogenic and Seasonal Variations of Metal Content in a Small Pelagic Fish (Trachurus Picturatus) in Northwestern African Waters. Mar. Pollut. Bull. 2020, 156, 111251. [Google Scholar] [CrossRef]
- Lozano-Bilbao, E.; Lozano, G.; Jiménez, S.; Jurado-Ruzafa, A.; Hardisson, A.; Rubio, C.; Weller, D.G.; Paz, S.; Gutiérrez, Á.J. Influence of Biometric and Seasonal Parameters on the Metal Content of Scomber Colias in Northwestern African Waters. Biol. Trace. Elem. Res. 2021, 199, 3886–3897. [Google Scholar] [CrossRef]
- Lozano-Bilbao, E.; Delgado-Suárez, I.; Hardisson, A.; González-Weller, D.; Paz, S.; Gutiérrez, Á.J. Impact of the Lockdown Period during the COVID-19 Pandemic on the Metal Content of the Anemone Anemonia Sulcata in the Canary Islands (CE Atlantic, Spain). Chemosphere 2023, 345, 140499. [Google Scholar] [CrossRef]
- Froese, R.; Pauly, D. Comment on “Metabolic Scaling Is the Product of Life-History Optimization”. Science 2023, 380, eade6084. [Google Scholar] [CrossRef]
- Afonso, P.; Morato, T.; Santos, R.S. Spatial Patterns in Reproductive Traits of the Temperate Parrotfish Sparisoma cretense. Fish. Res. 2008, 90, 92–99. [Google Scholar] [CrossRef]
- Afonso, A.; Gutiérrez, Á.J.; Lozano, G.; González-Weller, D.; Lozano-Bilbao, E.; Rubio, C.; Caballero, J.M.; Revert, C.; Hardisson, A. Metals in Diplodus Sargus Cadenati and Sparisoma Cretense—A Risk Assessment for Consumers. Environ. Sci. Pollut. Res. 2018, 25, 2630–2642. [Google Scholar] [CrossRef]
- Petrakis, G.; Papaconstantinou, C. Biology of Sparisoma Cretense in the Dodecanese (Greece). J. Appl. Ichthyol. 1990, 6, 14–23. [Google Scholar] [CrossRef]
- Beyahe, M.H.; Khallahi, B.; García-Isarch, E.; Fernández-Peralta, L. Report of the FAO/CECAF Working Group on the Assessment of Demersal Resources–Subgroup North Nouakchott, Mauritania, 2–10 December 2019; FAO: Rome, Italy, 2020. [Google Scholar]
- Corral, S.; Manrique de Lara, D.R. Participatory Artisanal Fisheries Management in Islands: Application to the Canary Islands (Spain). Mar. Policy 2017, 81, 45–52. [Google Scholar] [CrossRef]
- García-Romero, L.; Carreira-Galbán, T.; Rodríguez-Báez, J.Á.; Máyer-Suárez, P.; Hernández-Calvento, L.; Yánes-Luque, A. Mapping Environmental Impacts on Coastal Tourist Areas of Oceanic Islands (Gran Canaria, Canary Islands): A Current and Future Scenarios Assessment. Remote Sens. 2023, 15, 1586. [Google Scholar] [CrossRef]
- Jennings, S.; Reynolds, J.D.; Mills, S.C. Life History Correlates of Responses to Fisheries Exploitation. Proc. R. Soc. Lond. B. Biol. Sci. 1998, 265, 333–339. [Google Scholar] [CrossRef]
- Lam, V.W.Y.; Allison, E.H.; Bell, J.D.; Blythe, J.; Cheung, W.W.L.; Frölicher, T.L.; Gasalla, M.A.; Sumaila, U.R. Climate Change, Tropical Fisheries and Prospects for Sustainable Development. Nat. Rev. Earth. Environ. 2020, 1, 440–454. [Google Scholar] [CrossRef]
- Clavelle, T.; Lester, S.E.; Gentry, R.; Froehlich, H.E. Interactions and Management for the Future of Marine Aquaculture and Capture Fisheries. Fish Fish. 2019, 20, 368–388. [Google Scholar] [CrossRef]
- Gutiérrez, A.; Lozano-Bilbao, E.; Gutiérrez-Fernández, Á.J.; Paz-Montelongo, S.; González-Weller, D.; Rubio-Armendáriz, C.; Niebla-Canelo, D.; Alejandro-Vega, S.; Hardisson, A. Metal Levels in Serranus Atricauda and Sparisoma Cretense from the North-Eastern Atlantic Ocean—Contribution to Risk Assessment. Appl. Sci. 2023, 13, 5213. [Google Scholar] [CrossRef]
- López, E.P.; García, F.C. Agrotourism, Sustainable Tourism and Ultraperipheral Areas: The Case of Canary Islands. PASOS Rev. De Tur. Y Patrim. Cult. 2006, 4, 85–97. [Google Scholar]
- Baute Díaz, N.; Simancas Cruz, M.R.; Padrón Fumero, N.; Herrera Priano, F.Á.; Rodríguez González, P.; Gutiérrez Taño, D.; Santana Turégano, M.A.; Guerra Lombardi, V.; García Altmann, S.; García González, S. Tourism Observatory of the Canary Islands. Canary Islands Tourism Sustainability; Progress Report; 2022. [Google Scholar]
- Yuan, Z.; Luo, T.; Liu, X.; Hua, H.; Zhuang, Y.; Zhang, X.; Zhang, L.; Zhang, Y.; Xu, W.; Ren, J. Tracing Anthropogenic Cadmium Emissions: From Sources to Pollution. Sci. Total Environ. 2019, 676, 87–96. [Google Scholar] [CrossRef]
- Irabien, M.J.; Velasco, F. Heavy Metals in Oka River Sediments (Urdaibai National Biosphere Reserve, Northern Spain): Lithogenic and Anthropogenic Effects. Environ. Geol. 1999, 37, 54–63. [Google Scholar] [CrossRef]
- Raimundo, J.; Pereira, P.; Caetano, M.; Cabrita, M.T.; Vale, C. Decrease of Zn, Cd and Pb Concentrations in Marine Fish Species over a Decade as Response to Reduction of Anthropogenic Inputs: The Example of Tagus Estuary. Mar. Pollut. Bull. 2011, 62, 2854–2858. [Google Scholar] [CrossRef] [PubMed]
- Tyler, G.; Yvon, J. ICP-OES, ICP-MS and AAS Techniques Compared. ICP Opt. Emiss. Spectrosc. Tech. Note 1995, 5, 1–11. [Google Scholar]
- Elzey, S.; Tsai, D.H.; Rabb, S.A.; Yu, L.L.; Winchester, M.R.; Hackley, V.A. Quantification of Ligand Packing Density on Gold Nanoparticles Using ICP-OES. Anal. Bioanal. Chem. 2012, 403, 145–149. [Google Scholar] [CrossRef]
- Bakircioglu, D.; Kurtulus, Y.B.; Yurtsever, S. Comparison of Extraction Induced by Emulsion Breaking, Ultrasonic Extraction and Wet Digestion Procedures for Determination of Metals in Edible Oil Samples in Turkey Using ICP-OES. Food Chem. 2013, 138, 770–775. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.R. The Resource for the Power Industry Professional. Proc. ASME Power 2004, 32, 35–40. [Google Scholar]
- Anderson, M.; Braak, C. Ter Permutation Tests for Multi-Factorial Analysis of Variance. J. Stat. Comput. Simul. 2003, 73, 85–113. [Google Scholar] [CrossRef]
- Aguilar, A.; Borrell, A.; Pastor, T. Biological Factors Affecting Variability of Persistent Pollutant Levels in Cetaceans. J. Cetacean Res. Manag. 1999, 1, 83–116. [Google Scholar] [CrossRef]
- Renwick, A.G. Safety Factors and Establishment of Acceptable Daily Intakes. Food Addit. Contam. 1991, 8, 135–149. [Google Scholar] [CrossRef]
- Mostofa, K.M.G.; Liu, C.-Q.; Vione, D.; Gao, K.; Ogawa, H. Sources, Factors, Mechanisms and Possible Solutions to Pollutants in Marine Ecosystems. Environ. Pollut. 2013, 182, 461–478. [Google Scholar] [CrossRef]
- Yang, T.; Meng, J.; Jeyakumar, P.; Cao, T.; Liu, Z.; He, T.; Cao, X.; Chen, W.; Wang, H. Effect of Pyrolysis Temperature on the Bioavailability of Heavy Metals in Rice Straw-Derived Biochar. Environ. Sci. Pollut. Res. 2021, 28, 2198–2208. [Google Scholar] [CrossRef] [PubMed]
- Luoma, S.N. Bioavailability of Trace Metals to Aquatic Organisms—A Review. Sci. Total Environ. 1983, 28, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Hooda, P.S.; Alloway, B.J. Effects of Time and Temperature on the Bioavailability of Cd and Pb from Sludge-amended Soils. J. Soil Sci. 1993, 44, 97–110. [Google Scholar] [CrossRef]
- Mebane, C.A.; Chowdhury, M.J.; De Schamphelaere, K.A.C.; Lofts, S.; Paquin, P.R.; Santore, R.C.; Wood, C.M. Metal Bioavailability Models: Current Status, Lessons Learned, Considerations for Regulatory Use, and the Path Forward. Environ. Toxicol. Chem. 2020, 39, 60–84. [Google Scholar] [CrossRef] [PubMed]
- Clearwater, S.J.; Farag, A.M.; Meyer, J.S. Bioavailability and Toxicity of Dietborne Copper and Zinc to Fish. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2002, 132, 269–313. [Google Scholar] [CrossRef] [PubMed]
- Watzke, H.J. Impact of Processing on Bioavailability Examples of Minerals in Foods. Trends Food Sci. Technol. 1998, 9, 320–327. [Google Scholar] [CrossRef]
- Guinot, D.; Ureña, R.; Pastor, A.; Varó, I.; Del Ramo, J.; Torreblanca, A. Long-Term Effect of Temperature on Bioaccumulation of Dietary Metals and Metallothionein Induction in Sparus Aurata. Chemosphere 2012, 87, 1215–1221. [Google Scholar] [CrossRef]
- Sharpley, R. Tourism, Sustainable Development and the Theoretical Divide: 20 Years On. J. Sustain. Tour. 2020, 28, 1932–1946. [Google Scholar] [CrossRef]
- Ko, J.T.G. Assessing Progress of Tourism Sustainability. Ann. Tour. Res. 2001, 28, 817–820. [Google Scholar] [CrossRef]
- Fernández, J.I.P.; Rivero, M.S. Measuring Tourism Sustainability: Proposal for a Composite Index. Tour. Econ. 2009, 15, 277–296. [Google Scholar] [CrossRef]
- Hernández Sánchez, N.; Oskam, J. A “New Tourism Cycle” on the Canary Islands: Scenarios for Digital Transformation and Resilience of Small and Medium Tourism Enterprises. J. Tour. Futures 2022. [Google Scholar] [CrossRef]
- Casagrandi, R.; Rinaldi, S. A Theoretical Approach to Tourism Sustainability. Conserv. Ecol. 2002, 6, 13. [Google Scholar] [CrossRef]
- Garín-Mun, T. Inbound International Tourism to Canary Islands: A Dynamic Panel Data Model. Tour. Manag. 2006, 27, 281–291. [Google Scholar] [CrossRef]
- Mikhailenko, A.V.; Ruban, D.A.; Ermolaev, V.A.; van Loon, A.J. Cadmium Pollution in the Tourism Environment: A Literature Review. Geosciences 2020, 10, 242. [Google Scholar] [CrossRef]
- Bianchi, R. V Tourism Restructuring and the Politics of Sustainability: A Critical View from the European Periphery (The Canary Islands). J. Sustain. Tour. 2004, 12, 495–529. [Google Scholar] [CrossRef]
- Russell, R.D. Evolutionary Model for Lead Isotopes in Conformable Ores and in Ocean Volcanics. Rev. Geophys. 1972, 10, 529–549. [Google Scholar] [CrossRef]
- Watanabe, M.; Hokazono, A.; Handa, T.; Ichino, T.; Kuwaki, N. Corrosion of Copper and Silver Plates by Volcanic Gases. Corros. Sci. 2006, 48, 3759–3766. [Google Scholar] [CrossRef]
- Matus, F.; Amigo, X.; Kristiansen, S.M. Aluminium Stabilization Controls Organic Carbon Levels in Chilean Volcanic Soils. Geoderma 2006, 132, 158–168. [Google Scholar] [CrossRef]
- Lozano-Bilbao, E.; Lozano, G.; Gutiérrez, Á.J.; Hardisson, A.; Rubio, C.; Paz, S.; Weller, D.G. The Influence of the Degassing Phase of the Tagoro Submarine Volcano (Canary Islands) on the Metal Content of Three Species of Cephalopods. Mar. Pollut. Bull. 2022, 182, 113964. [Google Scholar] [CrossRef]
- Drews, A.; Greatbatch, R.J. Atlantic Multidecadal Variability in a Model with an Improved North Atlantic Current. Geophys. Res. Lett. 2016, 43, 8199–8206. [Google Scholar] [CrossRef]
- Pacyna, E.G.; Pacyna, J.M.; Steenhuisen, F.; Wilson, S. Global Anthropogenic Mercury Emission Inventory for 2000. Atmos. Environ. 2006, 40, 4048–4063. [Google Scholar] [CrossRef]
- Durrieu de Madron, X.; Guieu, C.; Sempéré, R.; Conan, P.; Cossa, D.; D’Ortenzio, F.; Estournel, C.; Gazeau, F.; Rabouille, C.; Stemmann, L.; et al. Marine Ecosystems’ Responses to Climatic and Anthropogenic Forcings in the Mediterranean. Prog. Oceanogr. 2011, 91, 97–166. [Google Scholar] [CrossRef]
- Meskhidze, N.; Chameides, W.L.; Nenes, A.; Chen, G. Iron Mobilization in Mineral Dust: Can Anthropogenic SO2 Emissions Affect Ocean Productivity? Geophys. Res. Lett. 2003, 30. [Google Scholar] [CrossRef]
- Glover, A.G.; Smith, C.R. The Deep-Sea Floor Ecosystem: Current Status and Prospects of Anthropogenic Change by the Year 2025. Environ. Conserv. 2003, 30, S0376892903000225. [Google Scholar] [CrossRef]
- Murphy, C.B.; Spiegel, S.J. Bioaccumulation and Toxicity of Heavy Metals and Related Trace Elements. J. Water Pollut. Control Fed. 1983, 55, 816–822. [Google Scholar]
- Murphy, C.B. Bioaccumulation and Toxicity of Heavy Metals and Related Trace Elements. J. Water Pollut. Control Fed. 1981, 53, 993–999. [Google Scholar]
- Espino, F.; González, J.A.; Haroun, R.; Tuya, F. Abundance and Biomass of the Parrotfish Sparisoma Cretense in Seagrass Meadows: Temporal and Spatial Differences between Seagrass Interiors and Seagrass Adjacent to Reefs. Environ. Biol. Fishes 2015, 98, 121–133. [Google Scholar] [CrossRef]
- Domingues, V.S.; Alexandrou, M.; Almada, V.C.; Robertson, D.R.; Brito, A.; Santos, R.S.; Bernardi, G. Tropical Fishes in a Temperate Sea: Evolution of the Wrasse Thalassoma Pavo and the Parrotfish Sparisoma Cretense in the Mediterranean and the Adjacent Macaronesian and Cape Verde Archipelagos. Mar. Biol. 2008, 154, 465–474. [Google Scholar] [CrossRef]
- Ramos-Miras, J.J.; Sanchez-Muros, M.J.; Morote, E.; Torrijos, M.; Gil, C.; Zamani-Ahmadmahmoodi, R.; Martin, J.A.R. Potentially Toxic Elements in Commonly Consumed Fish Species from the Western Mediterranean Sea (Almería Bay): Bioaccumulation in Liver and Muscle Tissues in Relation to Biometric Parameters. Sci. Total Environ. 2019, 671, 280–287. [Google Scholar] [CrossRef]
- Bordbar, L.; Dassenakis, M.; Catsiki, V.A.; Megalofonou, P. Influence of a Ferronickel Smelting Plant Activity on the Coastal Zone through Investigation of Metal Bioaccumulation on Two Gastropod Species (Patella Caerulea and Phorcus Turbinatus). J. Environ. Anal. Toxicol. 2015, 1, s7. [Google Scholar]
- Escobar-Chicho, M.; Soto, L.A.; Vanegas-Pérez, C.; Estradas-Romero, A. Heavy Metal Bioaccumulation in the Anemone Paraphelliactis Pabista Dunn, 1982 (Actiniaria: Hormathiidae) from the Hydrothermal System of Guaymas Basin, Gulf of California. Bull. Environ. Contam. Toxicol. 2019, 102, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Gaudry, A.; Zeroual, S.; Gaie-Levrel, F.; Moskura, M.; Boujrhal, F.Z.; El Moursli, R.C.; Guessous, A.; Mouradi, A.; Givernaud, T.; Delmas, R. Heavy Metals Pollution of the Atlantic Marine Environment by the Moroccan Phosphate Industry, as Observed through Their Bioaccumulation in Ulva Lactuca. Water Air Soil Pollut. 2007, 178, 267–285. [Google Scholar] [CrossRef]
- Reglamento (UE) No 488/2014; Reglamento (UE) No 488/2014 DE LA COMISIÓN de 12 de Mayo de 2014 Que Modifica El Reglamento (CE) No. 1881/2006 Por Lo Que Respecta al Contenido Máximo de Cadmio En Los Productos Alimenticios. 2014.
- Reglamento (CE) No 420/2011; Reglamento (CE) No 420/2011 de La Comisión de 29 de Abril de 2011 Que Modifica El Reglamento (CE) No 1881/2006, Por El Que Se Fija El Contenido Máximo de Determinados Contaminantes En Los Productos Alimenticios. 2011; Volume L 111/3. 3.
- Reglamento (CE) No 1881/2006; Reglamento (CE) No 1881/2006 DE LA COMISIÓN de 19 de Diciembre de 2006 Por El Que Se Fija El Contenido Máximo de Determinados Contaminantes En Los Productos Alimenticios. 2006.
- Reglamento (UE) 2015/1005; Reglamento (UE) 2015/1005 DE LA COMISIÓN de 25 de Junio de 2015 Que Modifica El Reglamento (CE) No. 1881/2006 Por Lo Que Respecta al Contenido Máximo de Plomo En Determinados Productos Alimenticios. 2015.
- IUPAC (International Union of Pure and Applied Chemistry). Nomenclature in Evaluation of Analytical Methods including Detection and Quantificaction Capabilities. Pure Appl. Chem. 1995, 67, 1699–1723. [Google Scholar] [CrossRef]

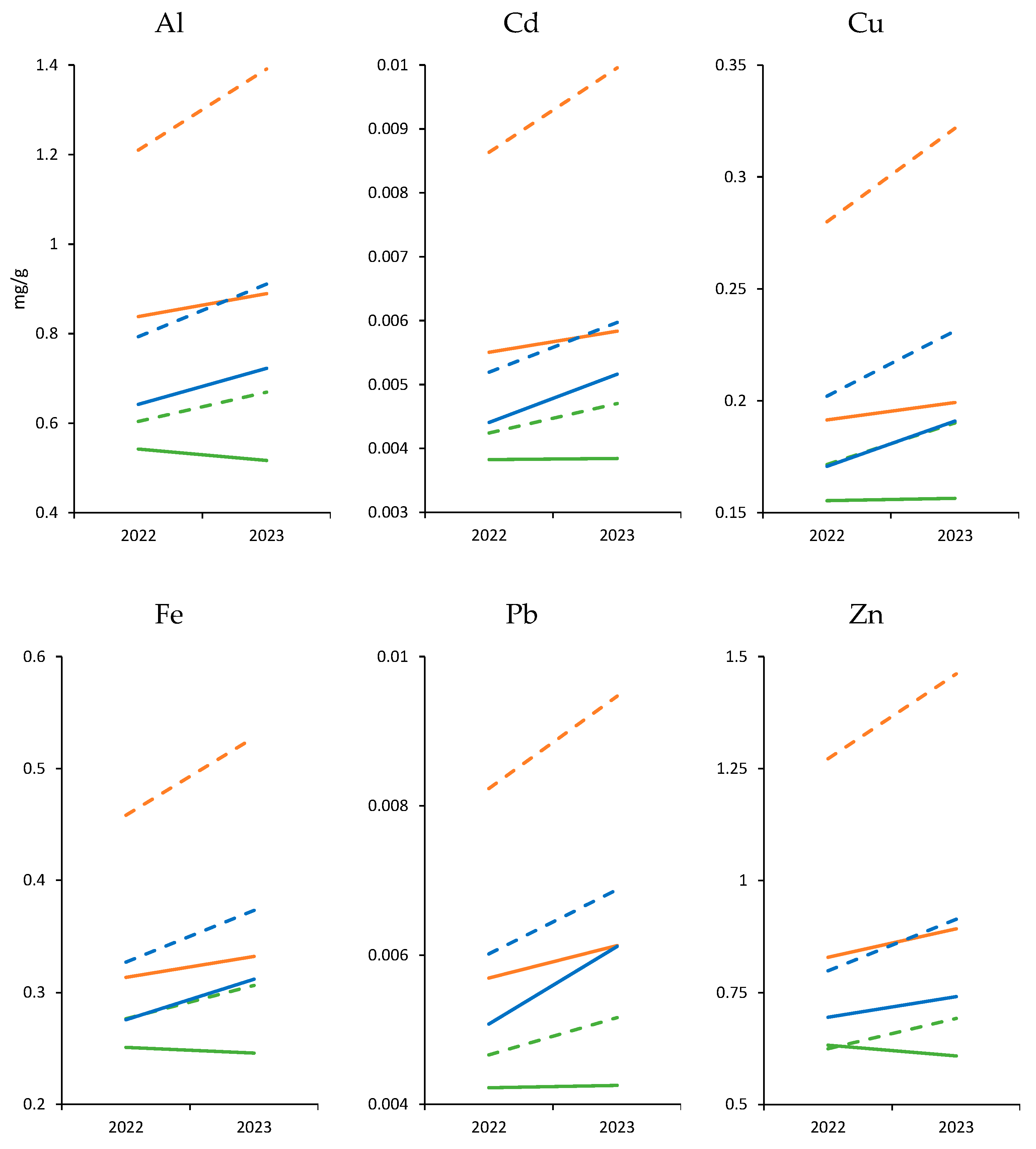
| El Hierro | Gran Canaria | Lanzarote | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2022 | 2023 | 2022 | 2023 | 2022 | 2023 | |||||||
| Cold | Warm | Cold | Warm | Cold | Warm | Cold | Warm | Cold | Warm | Cold | Warm | |
| Al | 0.542 ± 0.114 (0.372–0.688) | 0.604 ± 0.153 (0.391–0.825) | 0.516 ± 0.102 (0.38–0.682) | 0.669 ± 0.169 (0.434–0.914) | 0.838 ± 0.147 (0.668–1.135) | 1.210 ± 0.112 (1.029–1.398) | 0.889 ± 0.145 (0.744–1.192) | 1.391 ± 0.153 (1.131–1.676) | 0.642 ± 0.108 (0.409–0.756) | 0.793 ± 0.06 (0.674–0.86) | 0.722 ± 0.072 (0.634–0.832) | 0.911 ± 0.074 (0.808–1.026) |
| Zn | 0.632 ± 0.14 (0.458–0.9) | 0.625 ± 0.166 (0.346–0.87) | 0.609 ± 0.097 (0.454–0.719) | 0.692 ± 0.184 (0.383–0.964) | 0.829 ± 0.159 (0.645–1.109) | 1.272 ± 0.153 (1.01–1.485) | 0.893 ± 0.151 (0.678–1.164) | 1.462 ± 0.187 (1.11–1.636) | 0.695 ± 0.154 (0.503–0.989) | 0.798 ± 0.151 (0.569–1.017) | 0.741 ± 0.139 (0.554–0.948) | 0.914 ± 0.157 (0.683–1.118) |
| Cd | 0.004 ± 0.001 (0.003–0.005) | 0.004 ± 0.001 (0.003–0.005) | 0.004 ± 0.001 (0.003–0.005) | 0.005 ± 0.001 (0.003–0.006) | 0.006 ± 0.002 (0.004–0.009) | 0.009 ± 0.001 (0.007–0.011) | 0.006 ± 0.002 (0.004–0.009) | 0.01 ± 0.002 (0.008–0.013) | 0.004 ± 0.001 (0.003–0.006) | 0.005 ± 0.001 (0.004–0.007) | 0.005 ± 0.001 (0.004–0.006) | 0.006 ± 0.001 (0.004–0.008) |
| Pb | 0.004 ± 0.001 (0.004–0.005) | 0.005 ± 0.001 (0.004–0.005) | 0.004 ± 0.001 (0.004–0.005) | 0.005 ± 0.001 (0.004–0.006) | 0.006 ± 0.001 (0.005–0.009) | 0.008 ± 0.002 (0.006–0.011) | 0.006 ± 0.001 (0.005–0.009) | 0.009 ± 0.002 (0.007–0.013) | 0.005 ± 0.001 (0.004–0.008) | 0.006 ± 0.002 (0.005–0.01) | 0.006 ± 0.002 (0.005–0.009) | 0.007 ± 0.002 (0.006–0.011) |
| Fe | 0.251 ± 0.043 (0.198–0.326) | 0.277 ± 0.042 (0.224–0.343) | 0.246 ± 0.045 (0.196–0.333) | 0.306 ± 0.047 (0.248–0.38) | 0.314 ± 0.05 (0.244–0.402) | 0.458 ± 0.085 (0.365–0.619) | 0.332 ± 0.041 (0.295–0.41) | 0.528 ± 0.11 (0.413–0.742) | 0.276 ± 0.047 (0.217–0.358) | 0.327 ± 0.066 (0.246–0.441) | 0.312 ± 0.039 (0.264–0.394) | 0.374 ± 0.063 (0.295–0.485) |
| Cu | 0.155 ± 0.027 (0.126–0.199) | 0.172 ± 0.03 (0.138–0.215) | 0.156 ± 0.028 (0.125–0.203) | 0.19 ± 0.033 (0.152–0.238) | 0.191 ± 0.033 (0.155–0.245) | 0.28 ± 0.056 (0.199–0.377) | 0.199 ± 0.032 (0.166–0.25) | 0.322 ± 0.067 (0.238–0.452) | 0.171 ± 0.029 (0.139–0.218) | 0.202 ± 0.039 (0.157–0.269) | 0.191 ± 0.027 (0.169–0.24) | 0.231 ± 0.04 (0.188–0.295) |
| 2022 | 2023 | ||||
|---|---|---|---|---|---|
| Cold | Warm | Cold | Warm | ||
| Al | Lanzarote vs. Gran Canaria | 0.003 * | 0.001 * | 0.004 * | 0.001 * |
| Lanzarote vs. El Hierro | 0.120 * | 0.004 * | 0.001 * | 0.001 * | |
| Gran Canaria vs. El Hierro | 0.002 * | 0.001 * | 0.001 * | 0.001 * | |
| Zn | Lanzarote vs. Gran Canaria | 0.097 | 0.007 * | 0.067 | 0.001 * |
| Lanzarote vs. El Hierro | 0.390 | 0.051 | 0.052 | 0.001 * | |
| Gran Canaria vs. El Hierro | 0.020 * | 0.001 * | 0.001 * | 0.001 * | |
| Cd | Lanzarote vs. Gran Canaria | 0.080 | 0.001 * | 0.371 | 0.001 * |
| Lanzarote vs. El Hierro | 0.123 | 0.052 | 0.001 * | 0.021 * | |
| Gran Canaria vs. El Hierro | 0.001 * | 0.001 * | 0.001 * | 0.001 * | |
| Pb | Lanzarote vs. Gran Canaria | 0.356 | 0.001 * | 0.971 | 0.001 * |
| Lanzarote vs. El Hierro | 0.654 | 0.065 | 0.001 * | 0.001 * | |
| Gran Canaria vs. El Hierro | 0.002 * | 0.001 * | 0.001 * | 0.001 * | |
| Fe | Lanzarote vs. Gran Canaria | 0.135 | 0.001 * | 0.327 | 0.001 * |
| Lanzarote vs. El Hierro | 0.278 | 0.080 | 0.001 * | 0.038 * | |
| Gran Canaria vs. El Hierro | 0.015 * | 0.001 * | 0.001 * | 0.001 * | |
| Cu | Lanzarote vs. Gran Canaria | 0.119 | 0.001 * | 0.584 | 0.001 * |
| Lanzarote vs. El Hierro | 0.278 | 0.091 | 0.029 * | 0.040 * | |
| Gran Canaria vs. El Hierro | 0.031 * | 0.001 * | 0.011 * | 0.001 * | |
| 2022 vs. 2023 | Lanzarote | Gran Canaria | El Hierro | |||
|---|---|---|---|---|---|---|
| Cold | Warm | Cold | Warm | Cold | Warm | |
| Al | 0.098 | 0.004 * | 0.467 | 0.070 | 0.673 | 0.444 |
| Zn | 0.509 | 0.150 | 0.654 | 0.063 | 0.543 | 0.432 |
| Cd | 0.213 | 0.651 | 0.551 | 0.099 | 0.761 | 0.129 |
| Pb | 0.195 | 0.343 | 0.172 | 0.981 | 0.431 | 0.421 |
| Fe | 0.121 | 0.152 | 0.451 | 0.170 | 0.801 | 0.206 |
| Cu | 0.163 | 0.150 | 0.611 | 0.187 | 0.921 | 0.245 |
| Cold vs. Warm | Lanzarote | Gran Canaria | El Hierro | |||
|---|---|---|---|---|---|---|
| 2022 | 2023 | 2022 | 2023 | 2022 | 2023 | |
| Al | 0.001 * | 0.001 * | 0.001 * | 0.001 * | 0.394 | 0.051 |
| Zn | 0.181 | 0.031 * | 0.001 * | 0.001 * | 0.889 | 0.542 |
| Cd | 0.098 | 0.078 | 0.001 * | 0.001 * | 0.338 | 0.077 |
| Pb | 0.211 | 0.654 | 0.001 * | 0.001 * | 0.543 | 0.001 * |
| Fe | 0.087 | 0.032 * | 0.001 * | 0.001 * | 0.239 | 0.019 * |
| Cu | 0.081 | 0.030 * | 0.002 * | 0.001 * | 0.245 | 0.045 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lozano-Bilbao, E.; Jurado-Ruzafa, A.; Lorenzo, J.M.; González, J.A.; Hardisson, A.; González-Weller, D.; Paz, S.; Rubio, C.; Gutiérrez, Á.J. A Comparative Analysis of Sparisoma cretense in Island Environments: Unraveling Metal Accumulation Differences in the Canary Islands (Spain, NW African Waters). Animals 2023, 13, 3787. https://doi.org/10.3390/ani13243787
Lozano-Bilbao E, Jurado-Ruzafa A, Lorenzo JM, González JA, Hardisson A, González-Weller D, Paz S, Rubio C, Gutiérrez ÁJ. A Comparative Analysis of Sparisoma cretense in Island Environments: Unraveling Metal Accumulation Differences in the Canary Islands (Spain, NW African Waters). Animals. 2023; 13(24):3787. https://doi.org/10.3390/ani13243787
Chicago/Turabian StyleLozano-Bilbao, Enrique, Alba Jurado-Ruzafa, José M. Lorenzo, José A. González, Arturo Hardisson, Dailos González-Weller, Soraya Paz, Carmen Rubio, and Ángel J. Gutiérrez. 2023. "A Comparative Analysis of Sparisoma cretense in Island Environments: Unraveling Metal Accumulation Differences in the Canary Islands (Spain, NW African Waters)" Animals 13, no. 24: 3787. https://doi.org/10.3390/ani13243787







