Diagnostic Approach to Enteric Disorders in Pigs
Abstract
:Simple Summary
Abstract
1. Introduction
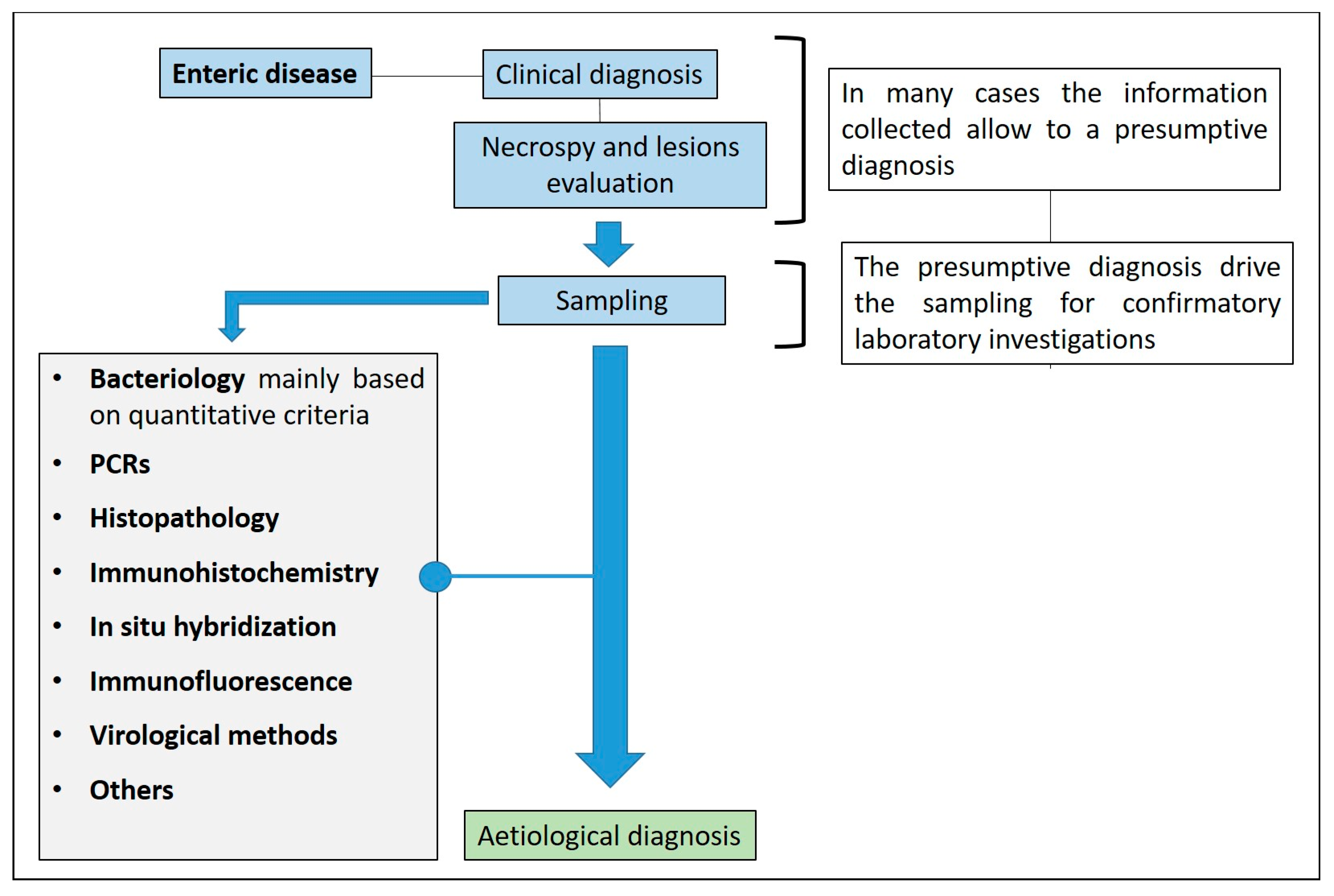
2. The Diagnostic Approach to Enteric Diseases
2.1. General Considerations
2.2. Clinical Examination of Enteric Diseases in Pigs
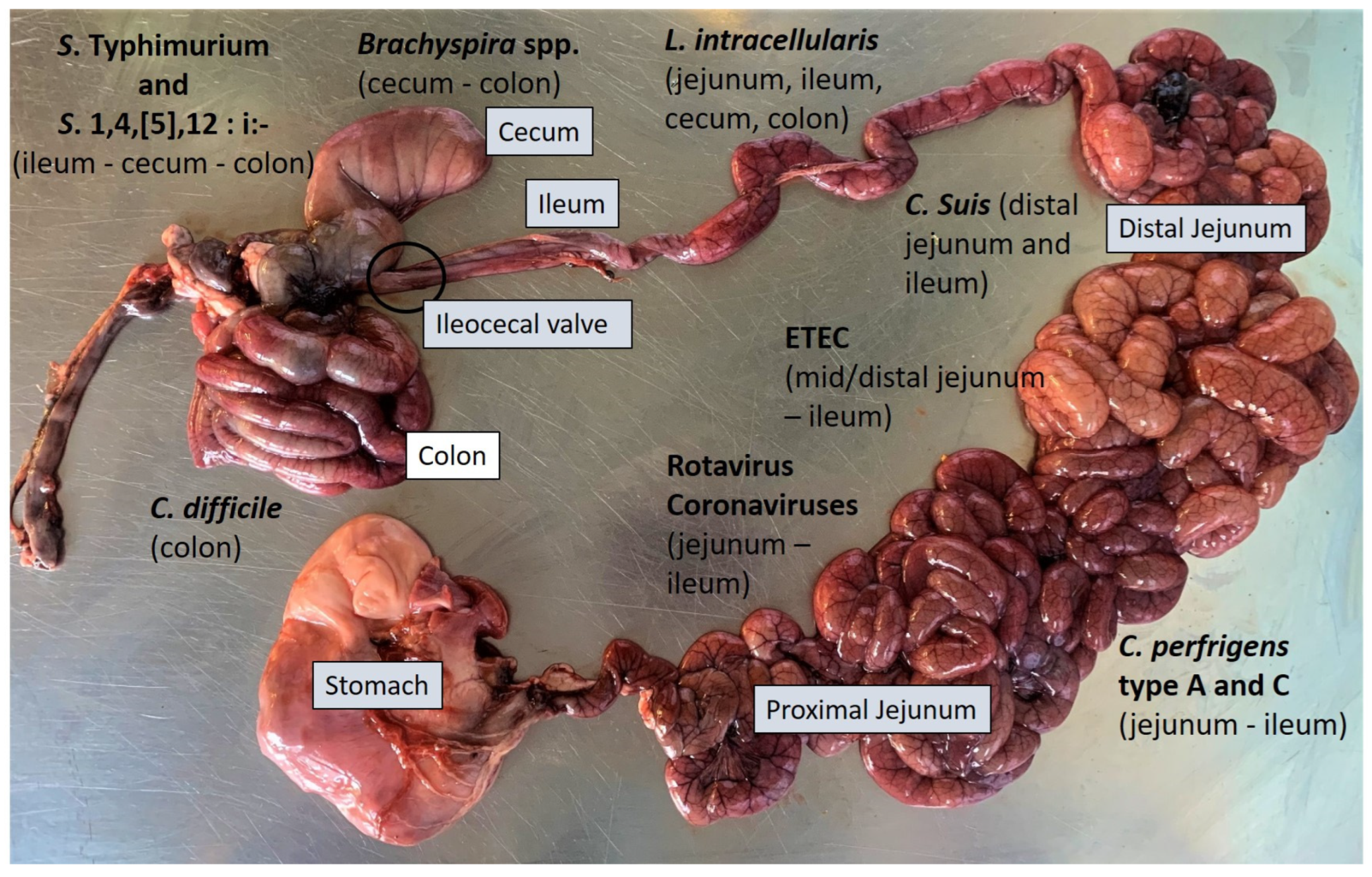
2.3. Necropsy and Gross Evaluation
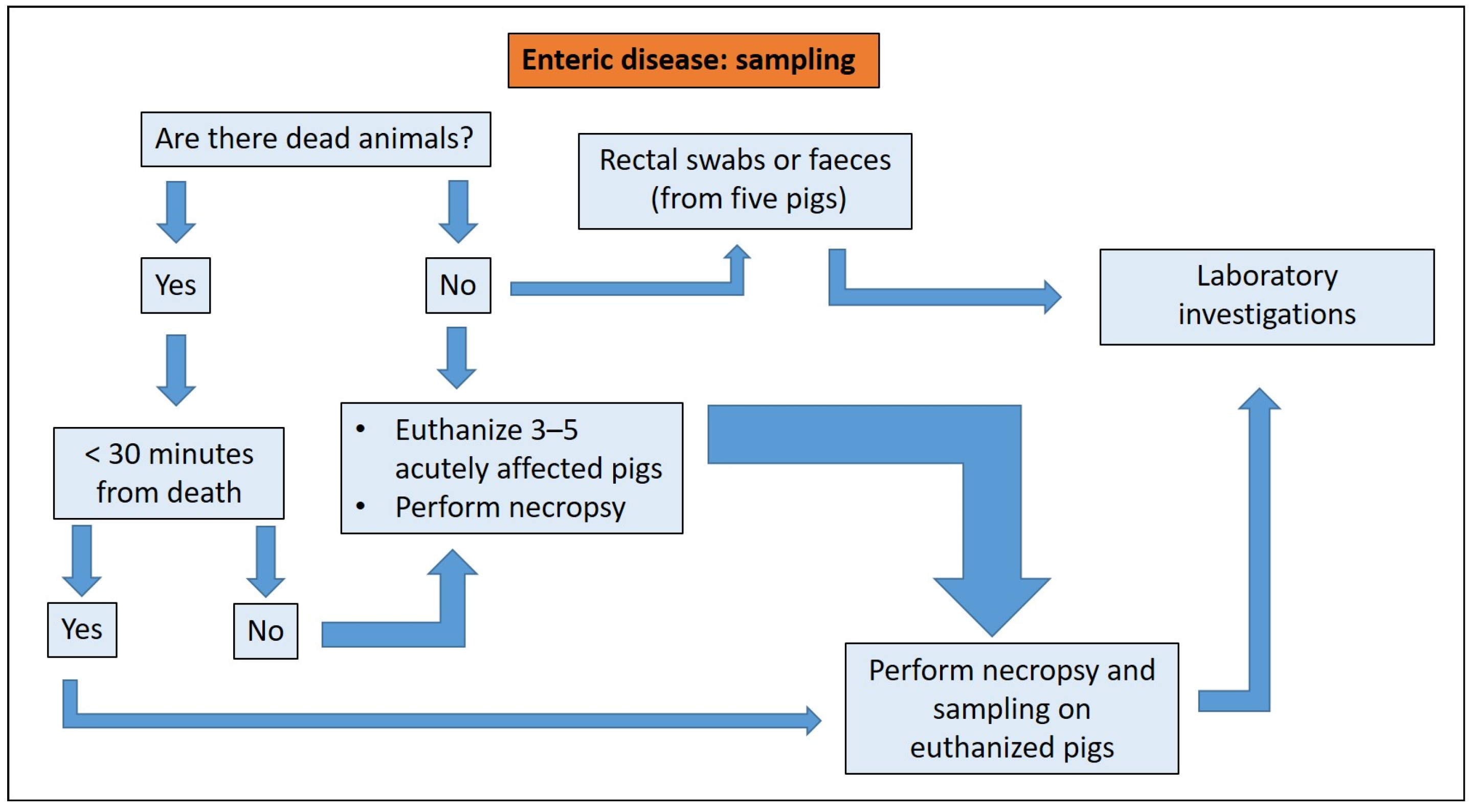
2.4. Sampling
2.5. Diagnostic Investigations
3. Diagnostic Approach to the Main Enteric Diseases of Pigs
3.1. Enteric Colibacillosis
3.1.1. Aetiology and Clinical Presentation
- (1)
- alkaline pH;
- (2)
- watery to creamy consistency;
- (3)
- a distinctive smell;
- (4)
- white to yellow colour;
- (5)
- possible various shades of brown.
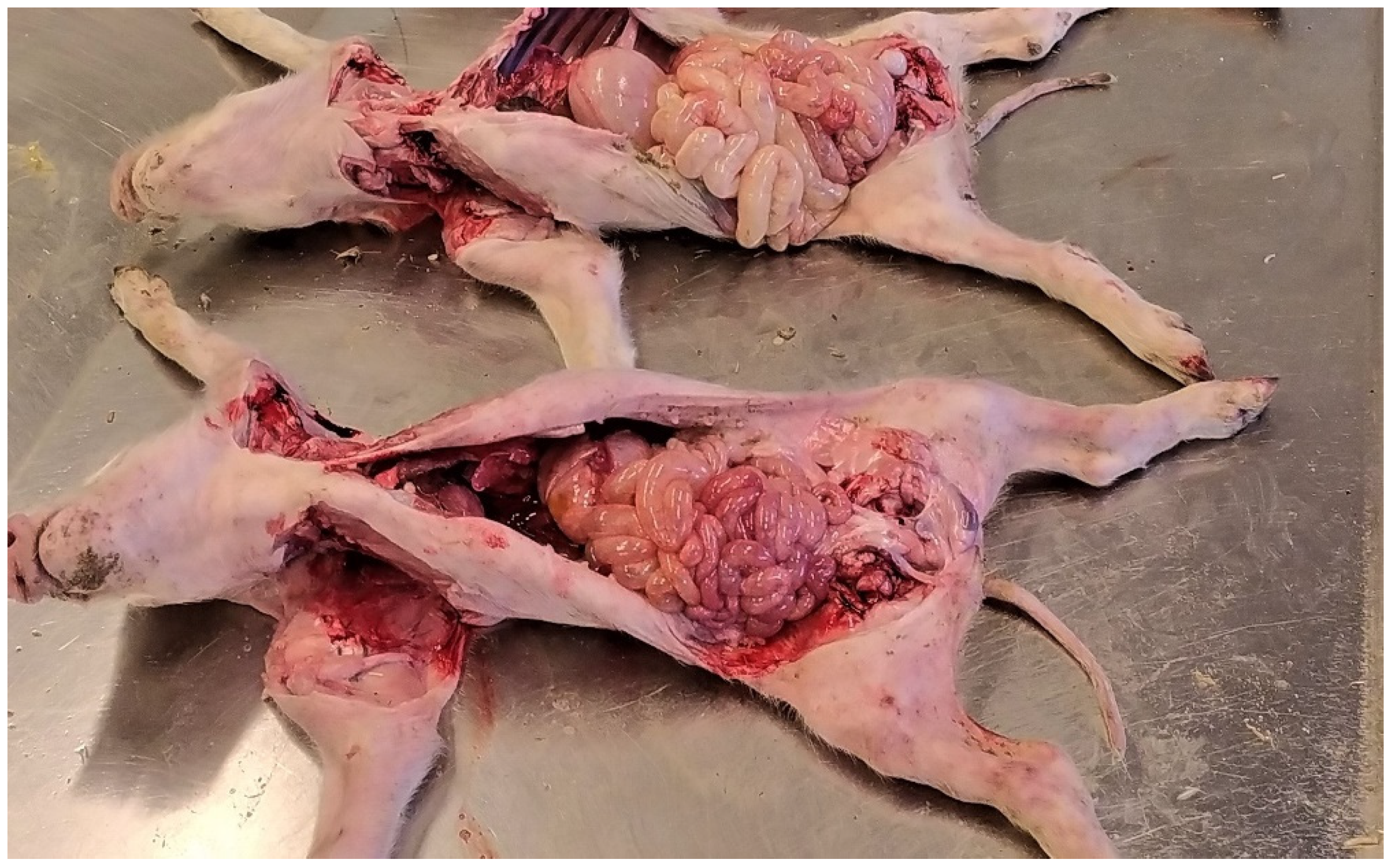
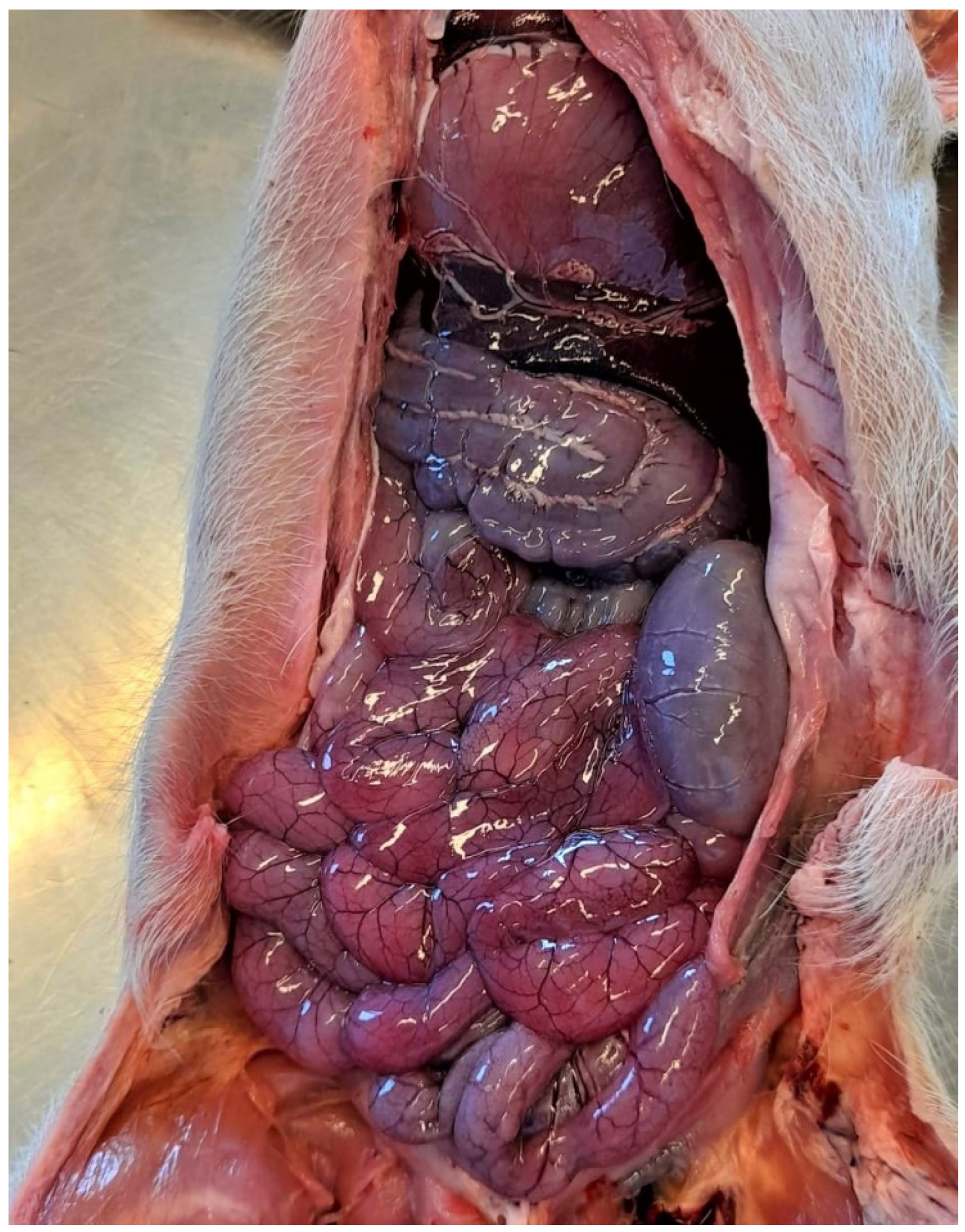
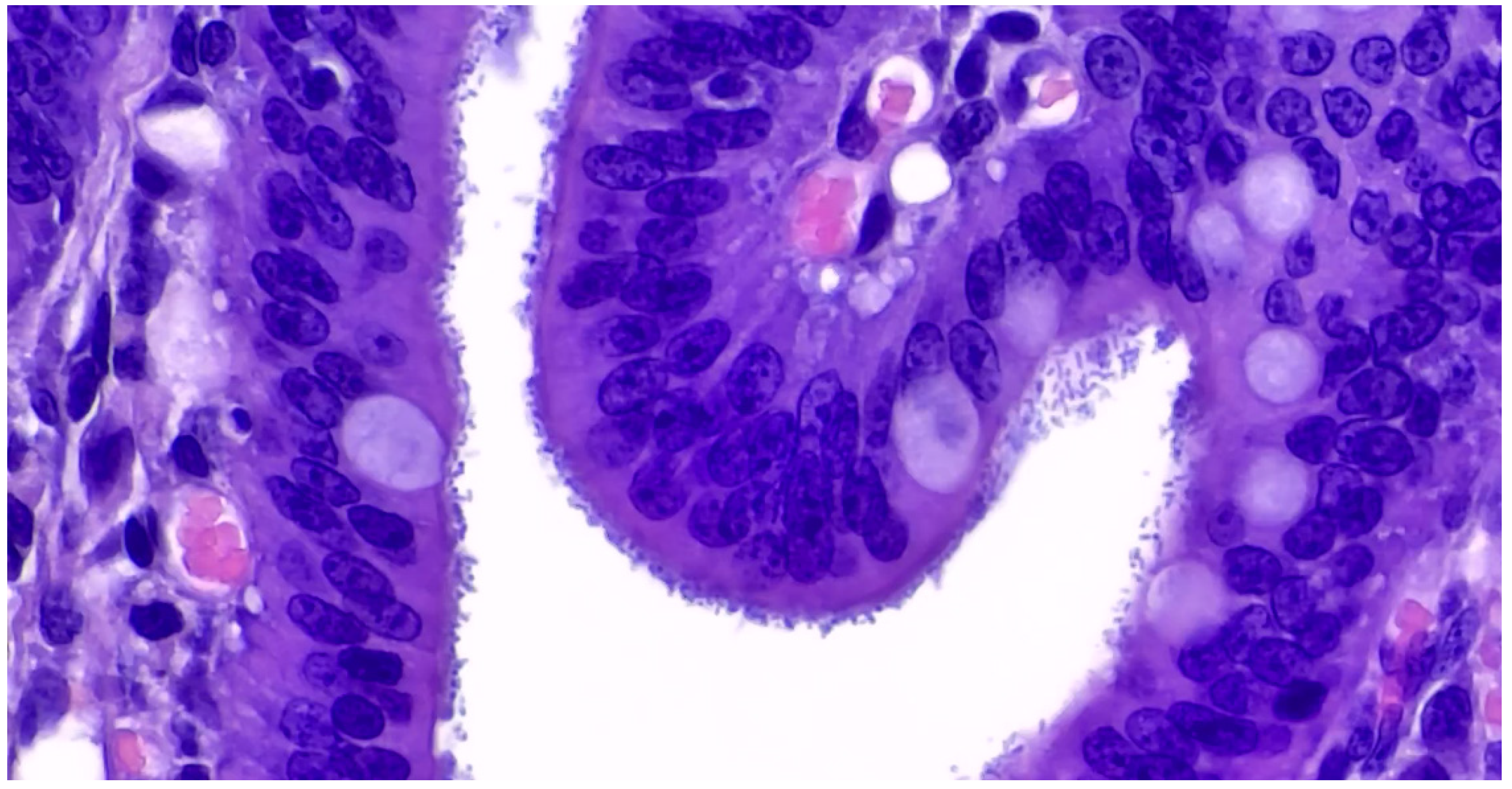
3.1.2. Pathological Changes
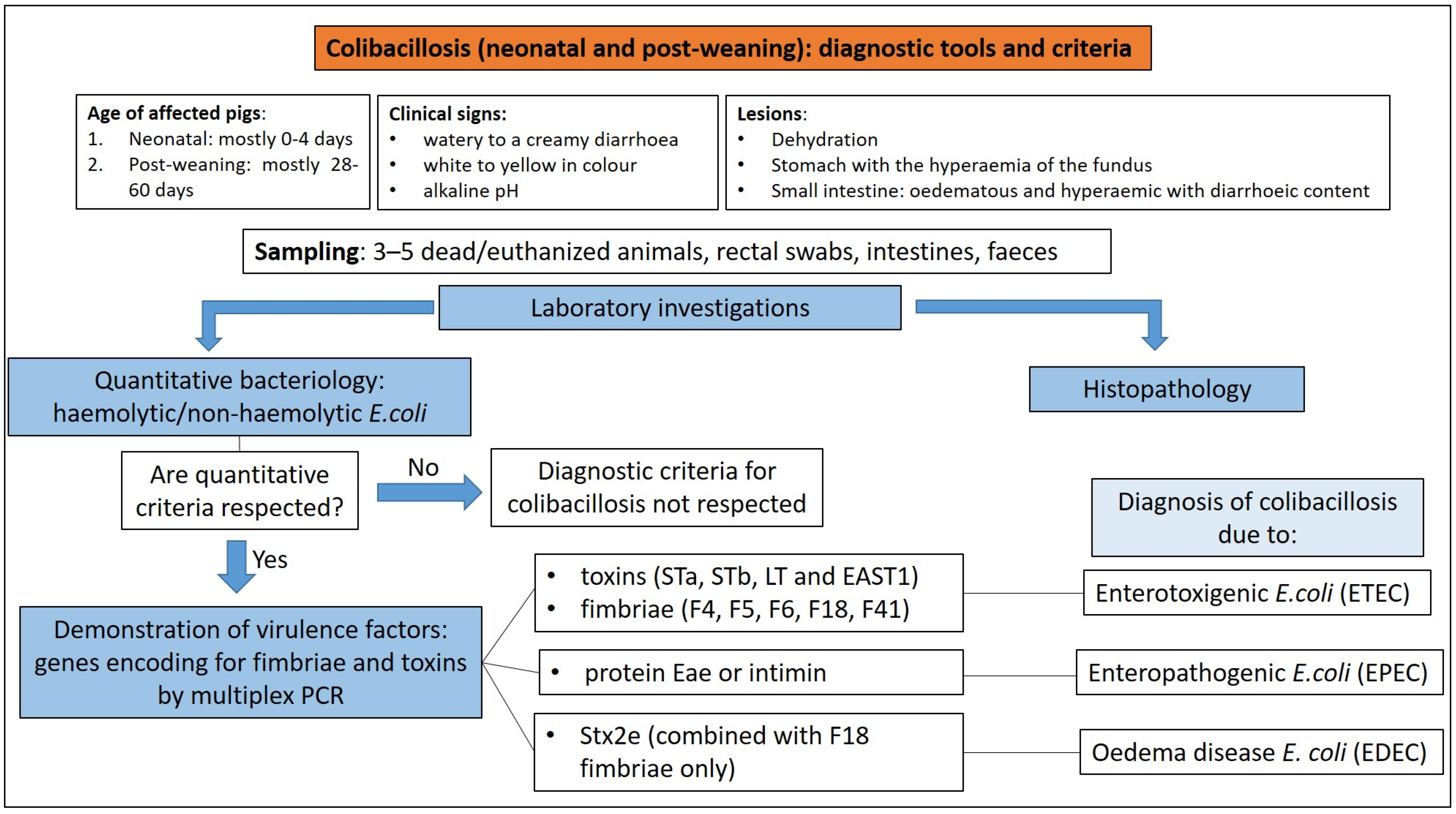
3.1.3. Diagnostic Tools and Criteria
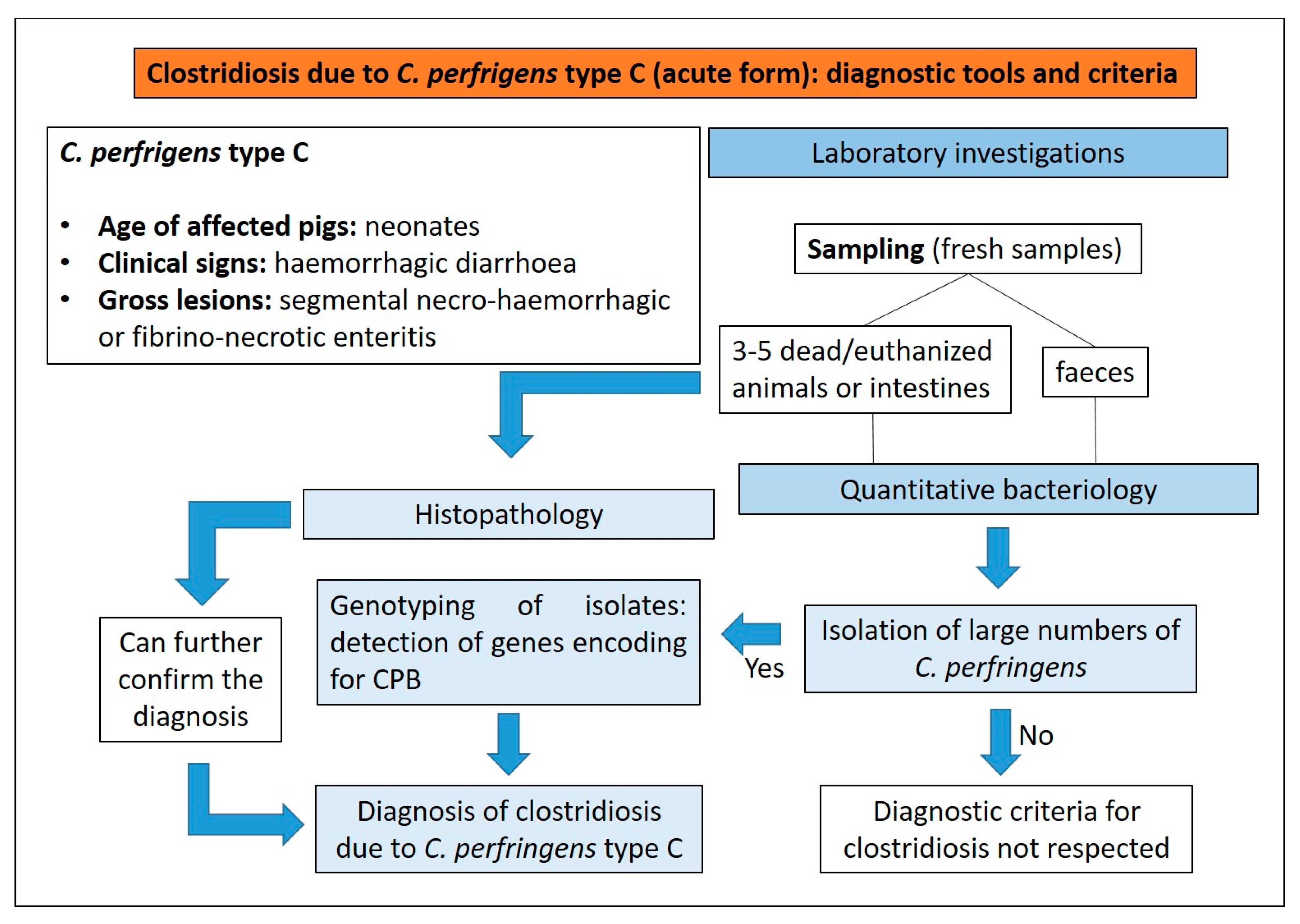
3.2. Clostridiosis
3.2.1. Clostridium perfringens Type C
Aetiology and Clinical Presentation
- (1)
- piglet’s immune status;
- (2)
- age of affected piglets (peracute and acute disease affect piglets mainly within the first 3 days of life);
- (3)
- virulence of the C. perfringens strain involved.
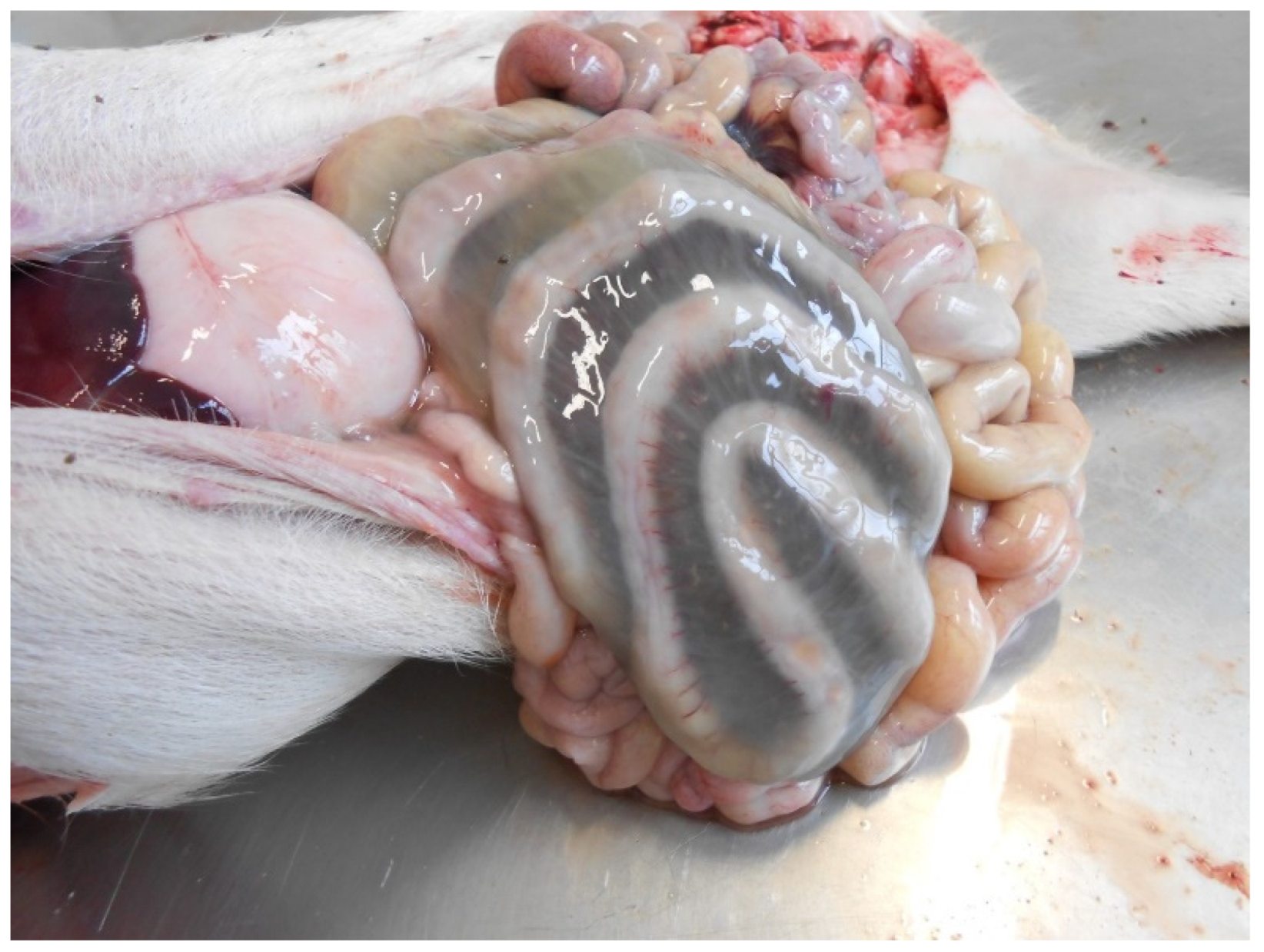
Pathological Changes
Diagnostic Tools and Criteria
- (1)
- presence of haemorrhagic diarrhoea;
- (2)
- rapid death;
- (3)
- segmental necro-haemorrhagic or fibrino-necrotic enteritis.
3.2.2. Clostridium perfringens Type A
Aetiology and Clinical Presentation
Pathological Changes
Diagnostic Tools and Criteria
- (1)
- non-haemorrhagic diarrhoea of otherwise unexplained origin;
- (2)
- isolation of large numbers of C. perfringens type A from the small intestine using quantitative bacteriology;
- (3)
- detection of genes for toxins.

3.2.3. Clostridioides difficile
Aetiology and Clinical Presentation
Pathological Changes
Diagnostic Tools and Criteria
- (1)
- compatible clinical history;
- (2)
- clinical signs;
- (3)
- gross and microscopic findings;
- (4)
- detection of TcdA and/or TcdB in faeces or colonic contents;
- (5)
- isolation by bacteriology and detection by PCR of genes encoding for TcdA and/or TcdB toxins is important for confirmation, considering that some isolates produce only TcdB or no toxins at all.

3.3. Intestinal Enterococcosis
3.3.1. Aetiology and Clinical Presentation
3.3.2. Pathological Changes
- (1)
- detachment of enterocytes with a rounded shape and eosinophilic cytoplasm;
- (2)
- foci of shallow mucosal erosions with exudation of fibrin and neutrophils;
- (3)
- capillary microthrombi in the lamina propria of villous tips;
- (4)
- altered villous:crypt ratio in the proximal or distal jejunum.
3.3.3. Diagnostic Tools and Criteria
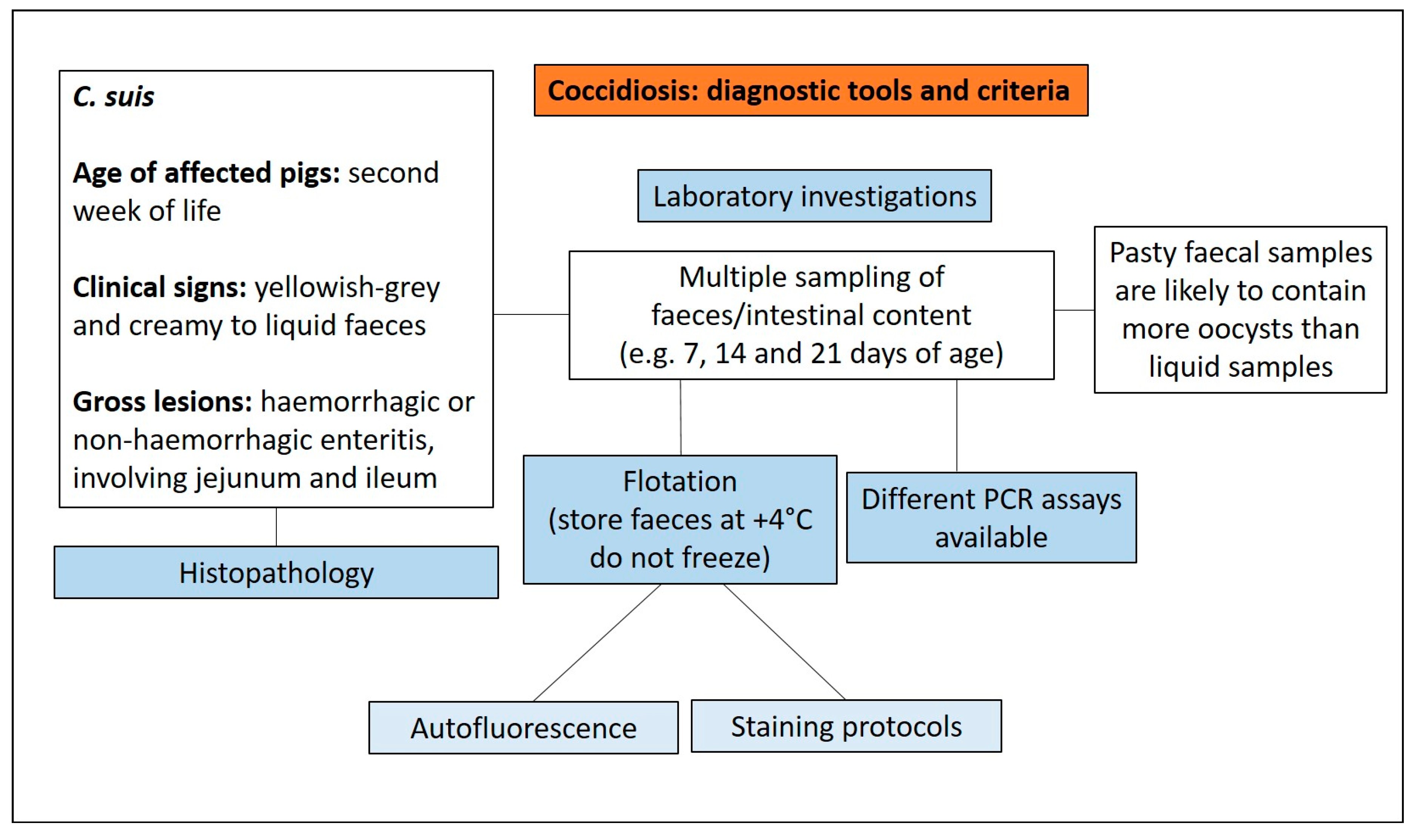
3.4. Coccidiosis
3.4.1. Aetiology and Clinical Presentation
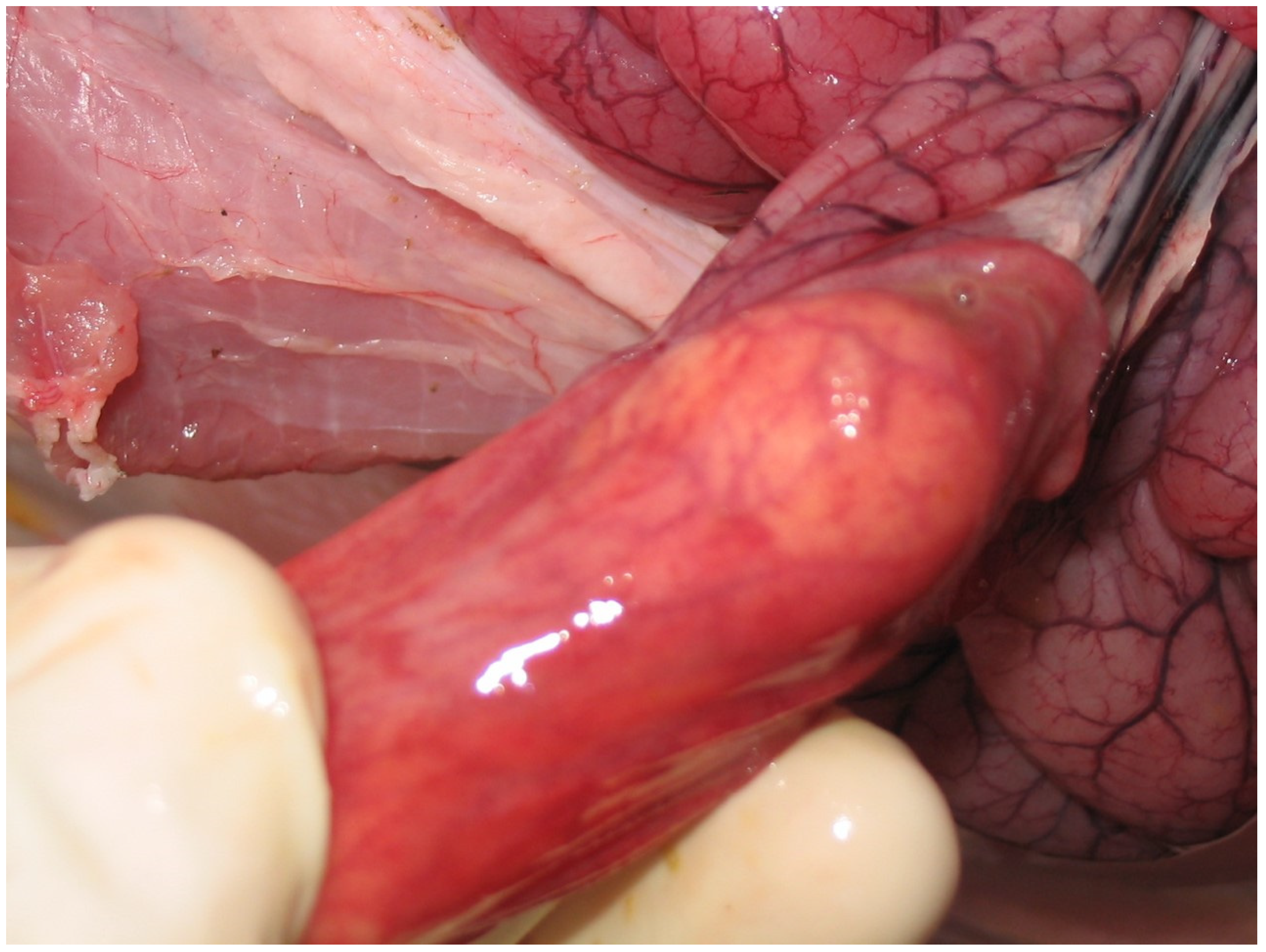
3.4.2. Pathological Changes
3.4.3. Diagnostic Tools and Criteria
- (1)
- Enterotoxigenic E. coli;
- (2)
- Coronaviruses;
- (3)
- Rotavirus;
- (4)
- C. perfringens type C;
- (5)
- Strongyloides ransomi.
3.5. Rotavirosis
3.5.1. Aetiology and Clinical Presentation
- (1)
- RV strain;
- (2)
- age of pigs;
- (3)
- immune status;
- (4)
- overall herd health;
- (5)
- presence of secondary bacterial or viral infections.
3.5.2. Pathological Changes
3.5.3. Diagnostic Tools and Criteria
3.6. Porcine Enteric Coronaviruses (PECs)
3.6.1. Aetiology and Clinical Presentation
- (1)
- less acidic pH in the stomach compared to older pigs;
- (2)
- renewal of enterocytes lining the intestinal villi from progenitor cells in the intestinal crypts is less rapid than in older pigs;
- (3)
- the neonatal immune system is not fully mature;
- (4)
- higher vulnerability to the electrolyte and fluid imbalances that result from poor digestion and severe malabsorption diarrhoea.
3.6.2. Pathological Changes
3.6.3. Diagnostic Tools and Criteria
3.7. Enteric Salmonellosis
3.7.1. Aetiology and Clinical Presentation
- (1)
- fever;
- (2)
- inanition;
- (3)
- decreased feed intake;
- (4)
- yellow watery diarrhoea that may contain blood and mucous (especially in the later stages);
- (5)
- dehydration.
- (1)
- tonsils;
- (2)
- lower intestinal tract;
- (3)
- submandibular and ileocolic lymph nodes.
3.7.2. Pathological Changes
- (1)
- thickened oedematous wall;
- (2)
- red mucosa with a granular appearance;
- (3)
- multifocal or coalescing mucosal erosions;
- (4)
- presence of adherent fibrino-necrotic debris on the mucosa;
- (5)
- enlarged and congested mesenteric lymph nodes.
- (1)
- focal to diffuse necrosis of crypt and enterocytes;
- (2)
- infiltration of neutrophils, macrophages and lymphocytes in the lamina propria and submucosa;
- (3)
- presence of fibrin thrombi in capillaries of the lamina propria;
- (4)
- less frequently, fibrin thrombi can be detected in larger vessels in the submucosa;
- (5)
- necrosis, with ulcerative lesions involving the mucosa, the submucosa and lymphoid patches.
3.7.3. Diagnostic Tools and Criteria
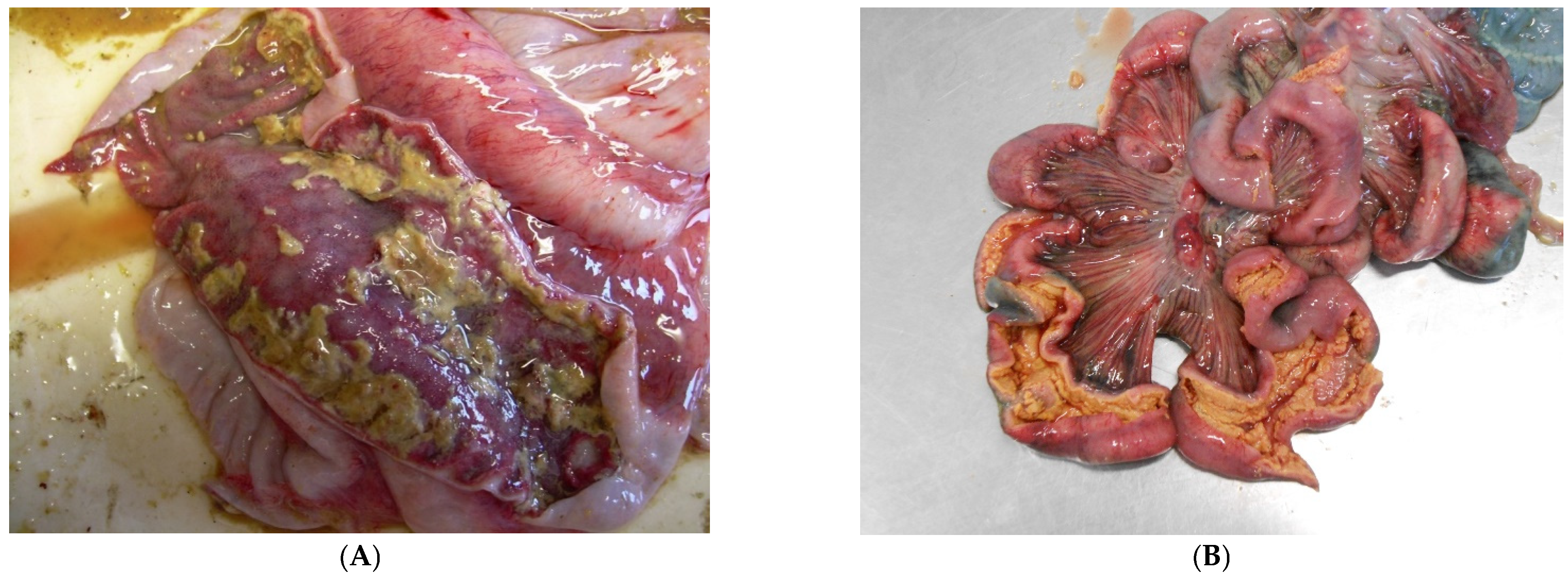
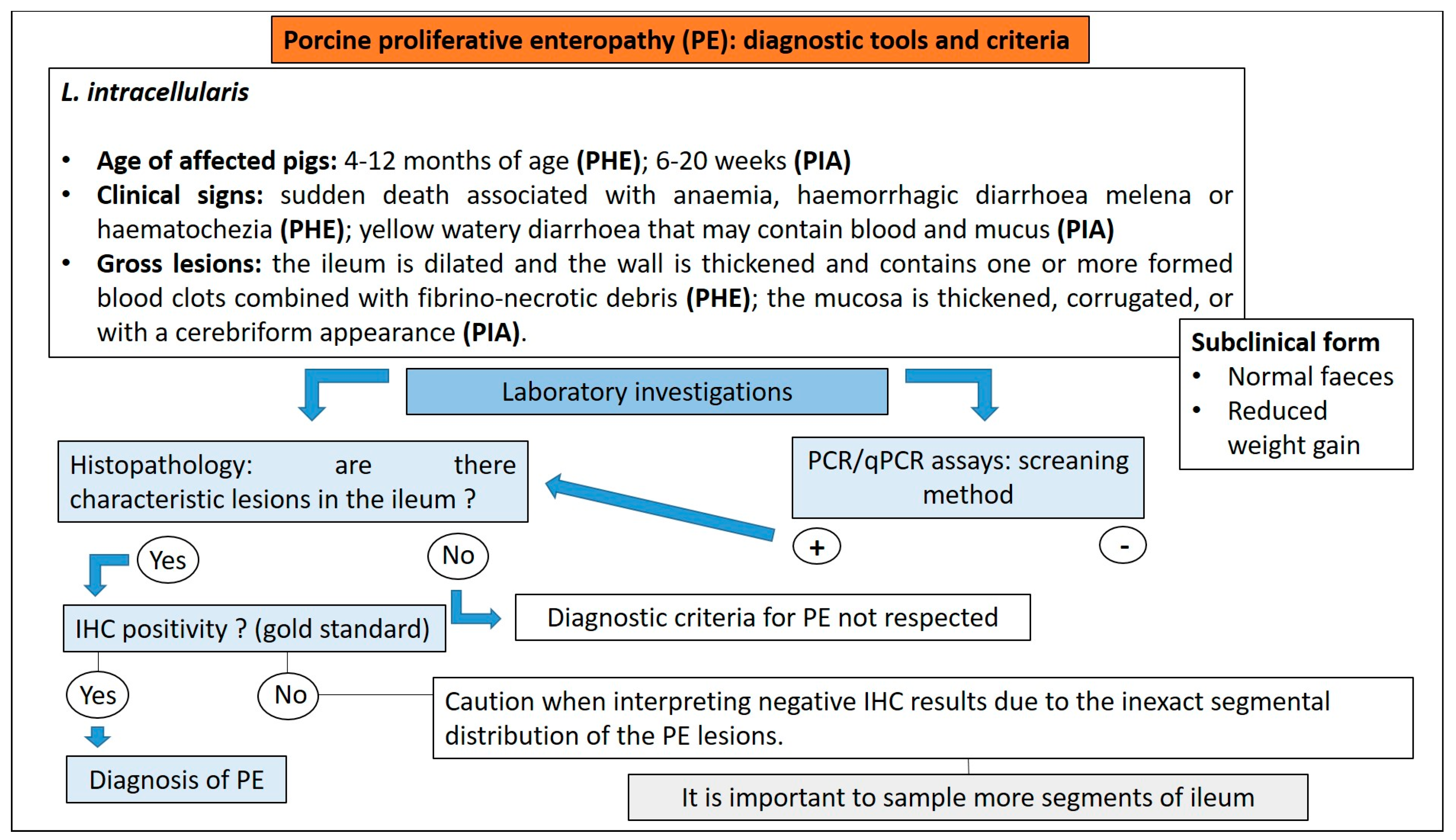
3.8. Proliferative Enteropathy
3.8.1. Aetiology and Clinical Presentation
- (1)
- The acute form, or proliferative haemorrhagic enteropathy (PHE), can be usually observed in young adult pigs 4–12 months of age. Clinical presentation is mainly characterised by sudden death associated with anaemia and haemorrhagic diarrhoea. In severe cases pigs may exsanguinate prior to the development of diarrhoea, and the only other clinical sign may be pallor [80]. However, in more prolonged cases, melena or haematochezia can be observed [79].
- (2)
- The chronic form, generally affecting 6–20 week old pigs, is the most common manifestation of PE, also known as porcine intestinal adenomatosis (PIA). Clinical signs, usually from mild to moderate, are characterised by non-haemorrhagic, grey or green, loose-to-watery diarrhoeic faeces associated with anorexia. Growth retardation may be observed despite normal feed intake. Infections by opportunistic bacteria can complicate chronic cases, resulting in necrotic enteritis or persistent diarrhoea, sometimes with liquid faeces containing fibrino-necrotic casts. In uncomplicated PE pigs usually recover about 5 weeks after the onset of clinical signs. In these cases, however, a reduction of pigs’ average daily weight gain and feed efficiency has been described [79].
- (3)
- The sub-clinical form is characterised by infected pigs with normal faeces, reduced weight gain, and less severe intestinal proliferative histological lesions, compared with the PIA.
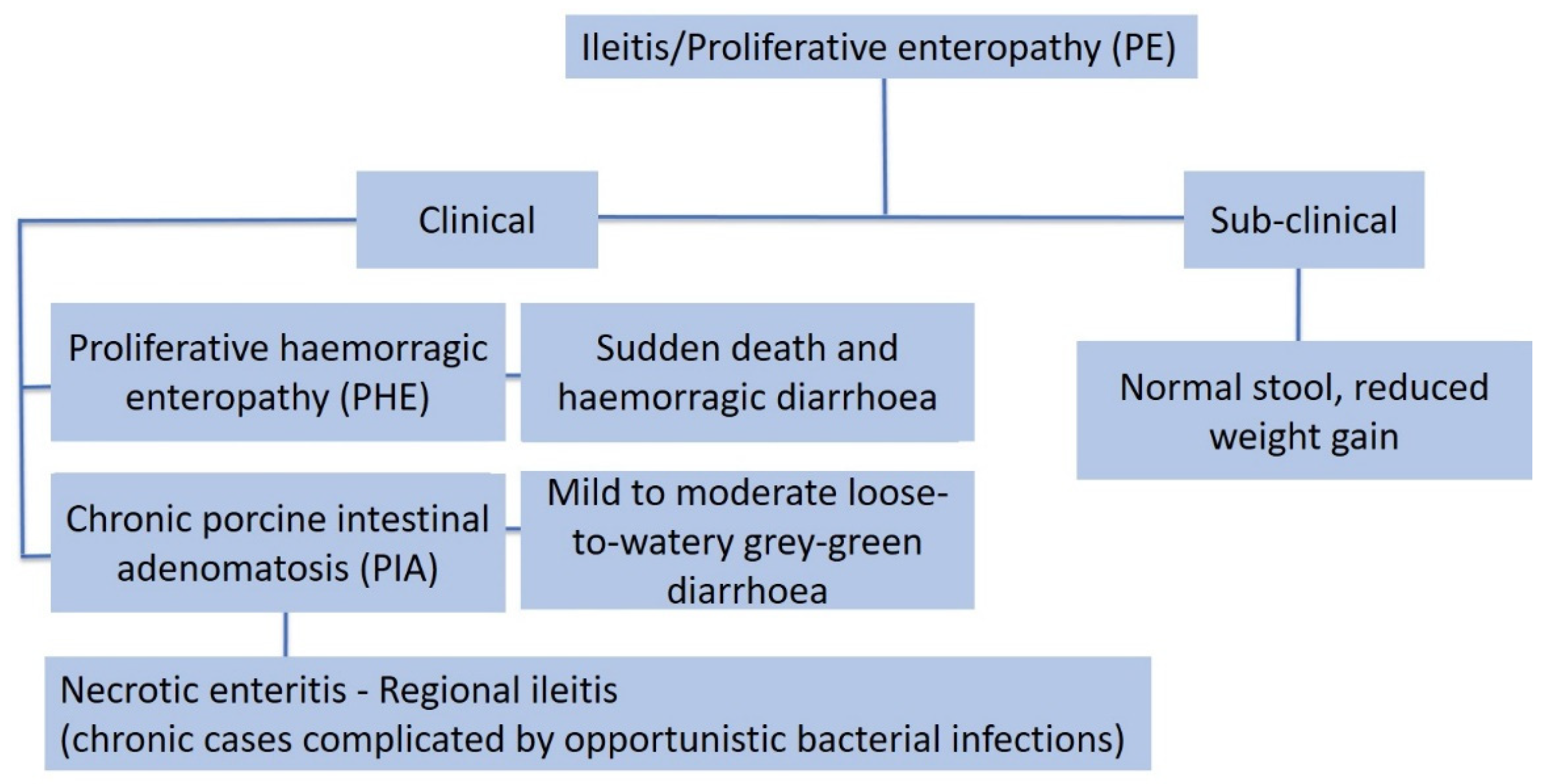
3.8.2. Pathological Changes
- (1)
- hyperplasia of crypt enterocytes with the formation of elongated, dilated and dysplastic crypts;
- (2)
- increased mitoses;
- (3)
- reduction in, or complete loss of, goblet cells.
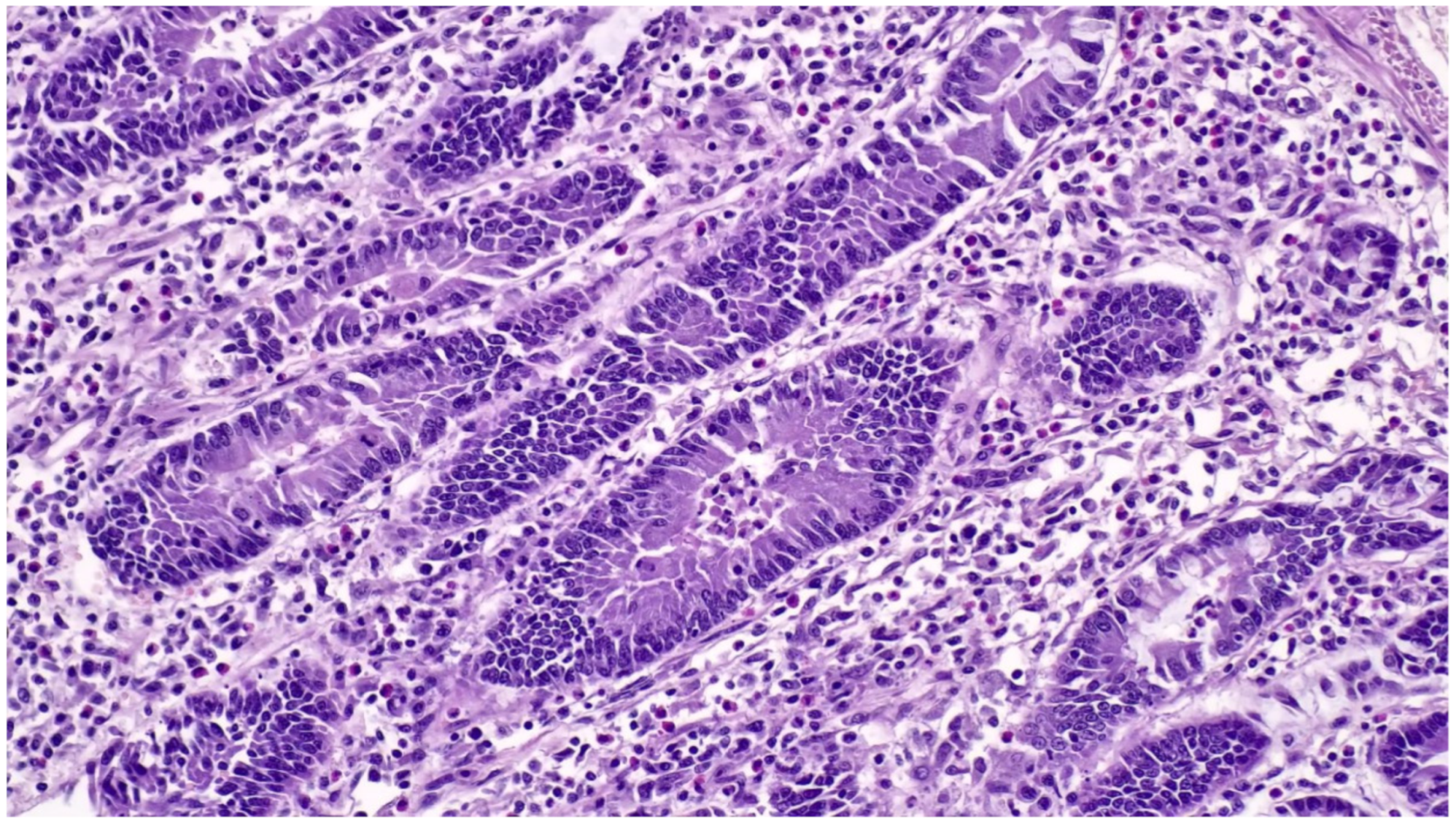
3.8.3. Diagnostic Tools and Criteria
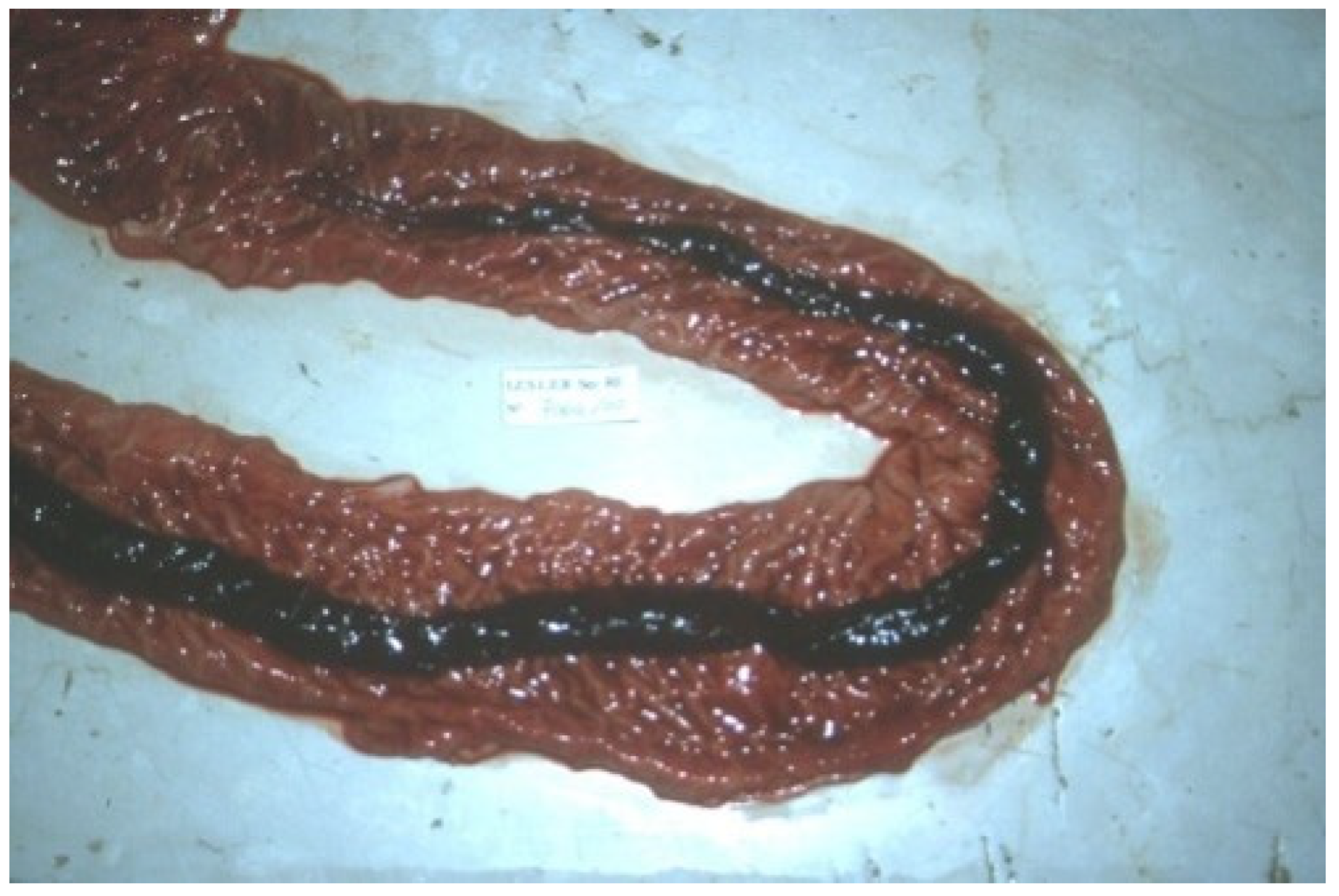
3.9. Swine Dysentery and Porcine Colonic Spirochetosis
3.9.1. Aetiology and Clinical Presentation
- (1)
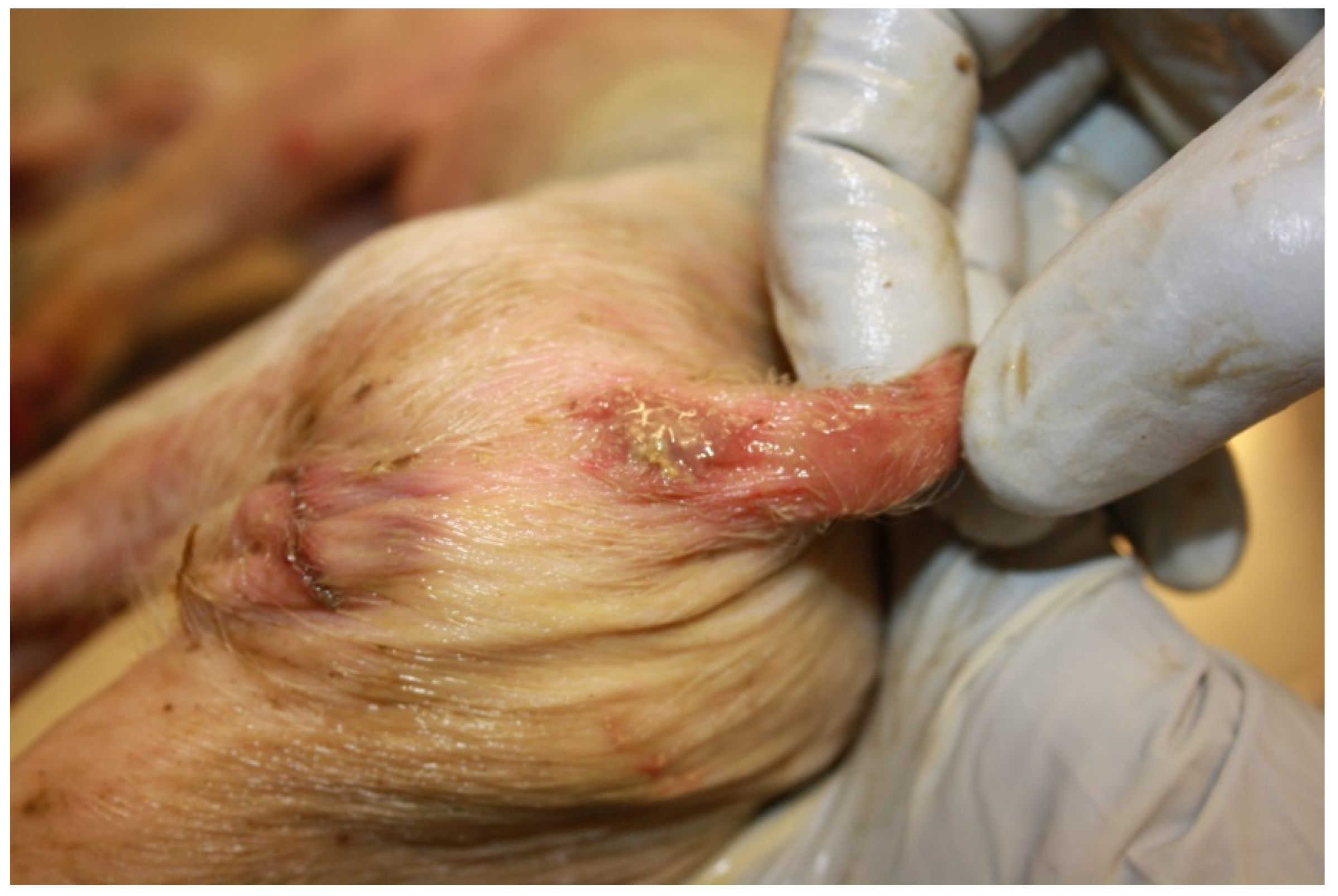
- at the onset: soft, yellow to grey diarrhoeic faeces, with large amounts of mucous and often flecks of blood and subsequently watery faeces containing blood, mucous and muco-fibrinous exudate (Figure 26A,B);
- (2)
- partial anorexia;
- (3)
- retarded growth rate;
- (4)
- fever (40–40.5 °C);
- (5)
- mortality rate of 50–90%.
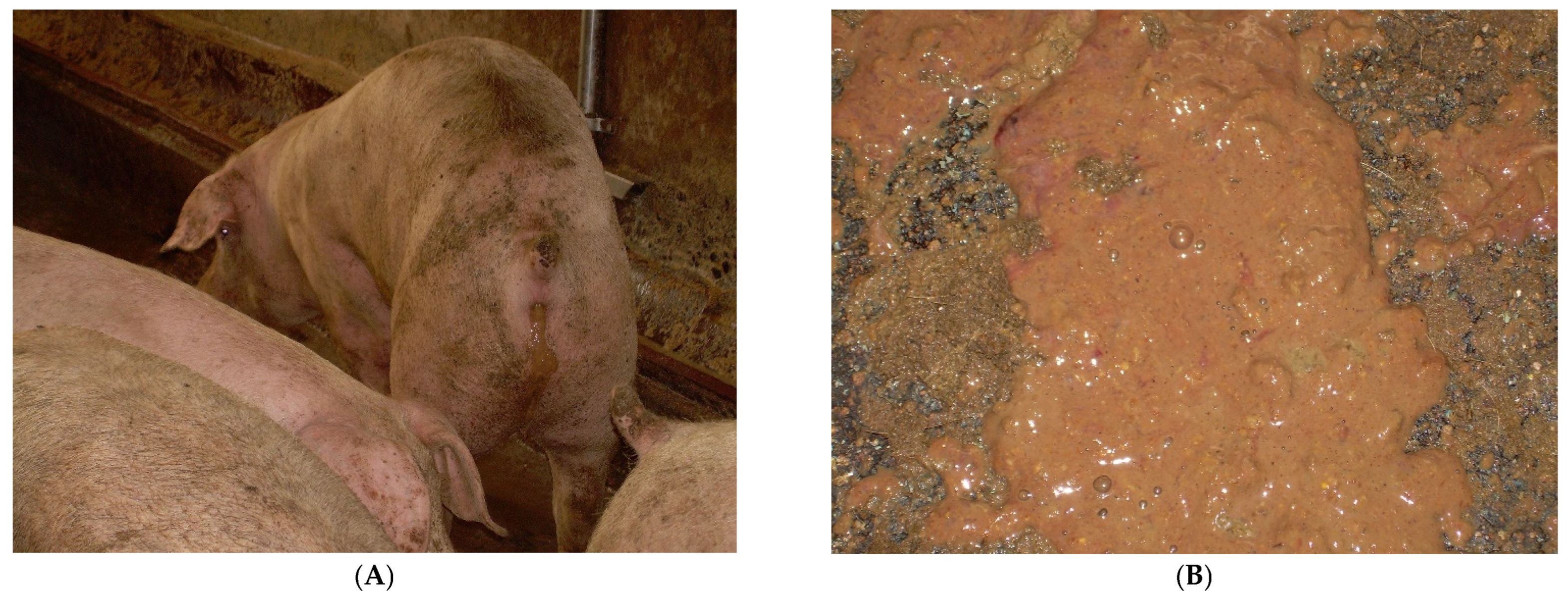
3.9.2. Pathological Changes
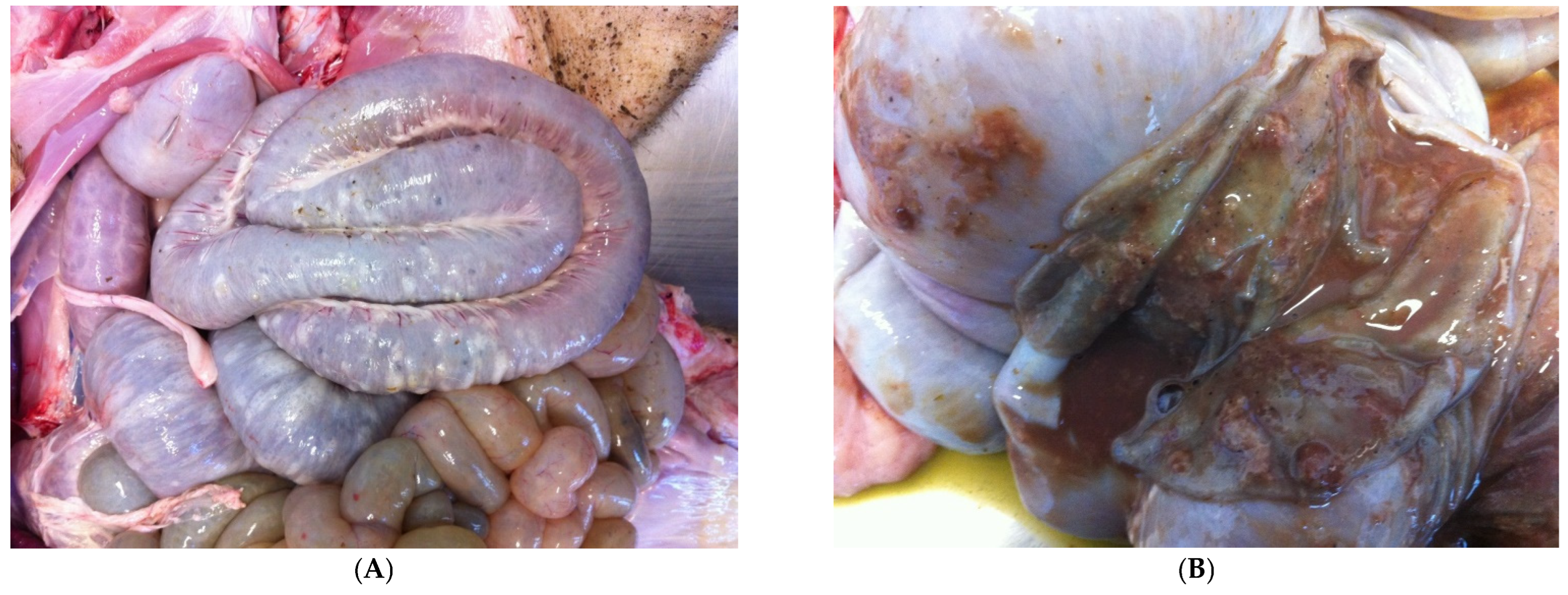
- (1)
- superficial mucosal necrosis;
- (2)
- neutrophilic infiltration of the lamina propria;
- (3)
- crypt elongation;
- (4)
- haemorrhage;
- (5)
- goblet cells hyperplasia.
3.9.3. Diagnostic Tools and Criteria
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vidal, A.; Martín-Valls, G.E.; Tello, M.; Mateu, E.; Martín, M.; Darwich, L. Prevalence of enteric pathogens in diarrheic and non-diarrheic samples from pig farms with neonatal diarrhea in the North East of Spain. Vet. Microbiol. 2019, 237, 108419. [Google Scholar] [CrossRef] [PubMed]
- Sjölund, M.; Zoric, M.; Wallgren, P. Financial impact on pig production: III. In Proceedings of the Gastrointestinal Disorders: Proceedings of the 6th European Symposium of Porcine Health Management, Sorrento, Italy, 7–9 May 2014; p. 189. [Google Scholar]
- Thomson, J.R.; Friendship, R.M. Digestive system. In Disease of Swine, 11th ed.; Zimmerman, J.J., Karriker, L.A., Ramirez, A., Schwartz, K.J., Stevenson, G.W., Zhang, J., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2019; pp. 234–263. [Google Scholar]
- Arruda, P.H.E.; Gauger, P. Optimizing Sample Selection, Collection, and Submission to Optimize Diagnostic Value. In Disease of Swine, 11th ed.; Zimmerman, J.J., Karriker, L.A., Ramirez, A., Schwartz, K.J., Stevenson, G.W., Zhang, J., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2019; pp. 98–111. [Google Scholar]
- Looft, T.; Johnson, T.A.; Allen, H.K.; Bayles, D.O.; Alt, D.P.; Stedtfeld, R.D.; Sul, W.J.; Stedtfeld, T.M.; Chai, B.; Cole, J.R.; et al. In-feed antibiotic effects on the swine intestinal microbiome. Proc. Natl. Acad. Sci. USA 2012, 109, 1691–1696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Segalés, J.; Martinez, J.; Castellà, J.; Darwich, L.; Domingo, M.; Mateu, E.; Martin, M.; Sibila, M. Laboratory diagnosis of enteric disorders. In Handbook of Laboratory Diagnosis in Swine; Servet Editorial—Grupo Asis Biomedia: Zaraoza, Spain, 2013; pp. 64–73. [Google Scholar]
- Wilson, K.A. Diagnostic approach to enteric diseases of swine. Am. Assoc. Swine Vet. Swine Health Prod. 2000, 8, 235–236. [Google Scholar]
- Fairbrother, J.M.; Gyles, C.L. Colibacillosis. In Disease of Swine, 10th ed.; Zimmerman, J.J., Karriker, L.A., Ramirez, A., Schwartz, K.J., Stevenson, G.W., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2012; pp. 723–747. [Google Scholar]
- Fairbrother, J.M.; Nadeau, É. Colibacillosis. In Disease of Swine, 11th ed.; Zimmerman, J.J., Karriker, L.A., Ramirez, A., Schwartz, K.J., Stevenson, G.W., Zhang, J., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2019; pp. 807–834. [Google Scholar]
- Luppi, A. Swine enteric colibacillosis: Diagnosis, therapy and antimicrobial resistance. Porc. Health Manag. 2017, 3, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Larson, L.A.; Schwartz, K.J. Differential Diagnosis of Baby Pig Diarrhea. Iowa State Univ. Vet. 1987, 49, 84–90. [Google Scholar]
- Uzal, F.A.; Plattner, B.L.; Hostetter, J.M. The alimentary system. In Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals, 6th ed.; Grant Maxie, M., Ed.; Elsevier: St. Louis, MO, USA, 2016; Volume 2, pp. 1–257. [Google Scholar]
- Moredo, F.A.; Piñeyro, P.E.; Márquez, G.C.; Sanz, M.; Colello, R.; Etcheverría, A.; Padola, N.L.; Quiroga, M.A.; Perfumo, C.J.; Galli, L.; et al. Enterotoxigenic Escherichia coli Subclinical Infection in Pigs: Bacteriological and Genotypic Characterization and Antimicrobial Resistance Profiles. Foodborne Pathog. Dis. 2015, 12, 704–711. [Google Scholar] [CrossRef]
- Casey, T.A.; Bosworth, B.T. Design and evaluation of a multiplex polymerase chain reaction assay for the simultaneous identification of genes for nine different virulence factors associated with Escherichia coli that cause diarrhea and edema disease in swine. J. Vet. Diagn. Investig. 2009, 21, 25–30. [Google Scholar] [CrossRef] [Green Version]
- Uzal, F.A.; Songer, J.G. Clostridial Diseases. In Disease of Swine, 11th ed.; Zimmerman, J.J., Karriker, L.A., Ramirez, A., Schwartz, K.J., Stevenson, G.W., Zhang, J., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2019; pp. 792–805. [Google Scholar]
- Posthaus, H.; Kittl, S.; Tarek, B.; Bruggisser, J. Clostridium perfringens type C necrotic enteritis in pigs: Diagnosis, pathogenesis, and prevention. J. Vet. Diagn. Investig. 2020, 32, 203–212. [Google Scholar] [CrossRef] [Green Version]
- Songer, J.G.; Uzal, F.A. Clostridial Enteric Infections in Pigs. J. Vet. Diagn. Investig. 2005, 17, 528–536. [Google Scholar] [CrossRef] [Green Version]
- Collins, J.E.; Loula, T.J.; Yeske, P.E. Diagnosis of Clostridium perfringens type A enteritis in piglets. Swine Health Prod. 1994, 2, 24–25. [Google Scholar]
- Chan, G.; Farzan, A.; Prescott, J.F.; Friendship, R. How do swine practitioners and veterinary pathologists arrive at a diagnosis of Clostridium perfringens type A enteritis in neonatal piglets? Can. Vet. J. 2013, 54, 504–506. [Google Scholar]
- Weese, J.S. Clostridium (Clostridioides) difficile in animals. J. Vet. Diagn. Investig. 2020, 32, 213–221. [Google Scholar] [CrossRef]
- Yaeger, M.J.; Kinyon, J.M.; Glenn Songer, J. A prospective, case control study evaluating the association between Clostridium difficile toxins in the colon of neonatal swine and gross and microscopic lesions. J. Vet. Diagn. Investig. 2007, 19, 52–59. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, G.M.; Ramos, C.P.; Lobato, F.C.F.; Guedes, R.M.C.; Giaretta, P.R.; Silva, R.O.S. Laboratory diagnosis of Clostridioides (Clostridium) difficile infection in domestic animals: A short review. Anaerobe 2022, 75, 102574. [Google Scholar] [CrossRef]
- Ramos, C.P.; Lopes, E.O.; Oliveira Júnior, C.A.; Diniz, A.N.; Lobato, F.C.F.; Silva, R.O.S. Immunochromatographic test and ELISA for the detection of glutamate dehydrogenase (GDH) and A/B toxins as an alternative for the diagnosis of Clostridioides (Clostridium) difficile-associated diarrhea in foals and neonatal piglets. Braz. J. Microbiol. 2020, 51, 1459–1462. [Google Scholar] [CrossRef]
- Wilkins, T.D.; Lyerly, D.M. Clostridium difficile testing: After 20 Years, still challenging. J. Clin. Microbiol. 2003, 41, 531–534. [Google Scholar] [CrossRef] [Green Version]
- Vancanneyt, M.; Snauwaert, C.; Cleenwerck, I.; Baele, M.; Descheemaeker, P.; Goossens, H.; Pot, B.; Vandamme, P.; Swings, J.; Haesebrouck, F.; et al. Enterococcus villorum sp. nov., an enteroadherent bacterium associated with diarrhoea in piglets. Int. J. Syst. Evol. Microbiol. 2001, 51, 393–400. [Google Scholar] [CrossRef] [Green Version]
- Cheon, D.S.; Chae, C.H. Outbreak of diarrhea associated with Enterococcus durans in piglets. J. Vet. Diagn. Investig. 1996, 8, 123–124. [Google Scholar] [CrossRef] [Green Version]
- Larsson, J.; Lindberg, R.; Aspán, A.; Grandon, R.; Westergren, E.; Jacobson, M. Neonatal piglet diarrhoea associated with enteroadherent Enterococcus hirae. J. Comp. Pathol. 2014, 151, 137–147. [Google Scholar] [CrossRef]
- Kongsted, H.; Pedersen, K.; Hjulsager, C.K.; Larsen, L.E.; Pedersen, K.S.; Jorsal, S.E.; Bækbo, P. Diarrhoea in neonatal piglets: A case control study on microbiological findings. Porc. Health Manag. 2018, 4, 1–7. [Google Scholar] [CrossRef]
- Jang, S.; Shin, S.; Kim, S.H.; Kim, H.; Moon, C. Diagnosis of Enterococcus hirae infection in association with piglet diarrhea. J. Biomed. Transl. Res. 2019, 20, 115–120. [Google Scholar] [CrossRef]
- Joachim, A.; Shrestha, A. Coccidiosis of pigs. In Coccidiosis in Livestock, Companion Animals and Humans; Dubey, J.P., Ed.; CRC Press, Taylor and Francis: Boca Raton, FL, USA, 2020; pp. 125–145. [Google Scholar]
- Worliczek, H.L.; Buggelsheim, M.; Saalmüller, A.; Joachim, A. Porcine isosporosis: Infection dynamics, pathophysiology and immunology of experimental infections. Wien. Klin. Wochenschr. 2007, 119, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Maes, D.; Vyt, P.; Rabaeys, P.; Gevaert, D. Effects of toltrazuril on the growth of piglets in herds without clinical isosporosis. Vet. J. 2007, 173, 197–199. [Google Scholar] [CrossRef] [PubMed]
- Joachim, A.; Ruttkowski, B.; Sperling, D. Detection of Cystoisospora suis in faeces of suckling piglets—When and how? A comparison of methods. Porc. Health Manag. 2018, 4, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, D.S.; Dubey, J.P.; Santín-Durán, M. Coccidia and Other Protozoa. In Disease of Swine, 11th ed.; Zimmerman, J.J., Karriker, L.A., Ramirez, A., Schwartz, K.J., Stevenson, G.W., Zhang, J., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2019; pp. 1015–1027. [Google Scholar]
- Daugschies, A.; Bialek, R.; Joachim, A.; Mundt, H.C. Autofluorescence microscopy for the detection of nematode eggs and protozoa, in particular Isospora suis, in swine faeces. Parasitol. Res. 2001, 87, 409–412. [Google Scholar] [CrossRef]
- Henriksen, S.A.; Christensen, J.P.B. Demonstration of Isospora suis oocysts in faecal samples. Vet. Rec. 1992, 131, 443–444. [Google Scholar] [CrossRef]
- Lindsay, D.S.; Current, W.L.; Taylor, J.R. Effects of experimentally induced Isospora suis infection on morbidity, mortality, and weight gains in nursing pigs. Am. J. Vet. Res. 1985, 46, 1511–1512. [Google Scholar]
- Vítovec, J.; Koudela, B. Double alteration of the small intestine in conventional and gnotobiotic piglets experimentally infected with the coccidium Isospora suis (Apicomplexa, Eimeriidae). Folia Parasitol. 1990, 37, 21–33. [Google Scholar]
- Huang, C.; Wen, F.; Yue, L.; Chen, R.; Zhou, W.; Hu, L.; Chen, M.; Wang, S. Exploration of fluorescence-based real-time loop-mediated isothermal amplification (LAMP) assay for detection of Isospora suis oocysts. Exp. Parasitol. 2016, 165, 1–6. [Google Scholar] [CrossRef]
- Lalonde, L.F.; Gajadhar, A.A. Detection and differentiation of coccidian oocysts by real-time PCR and melting curve analysis. J. Parasitol. 2011, 97, 725–730. [Google Scholar] [CrossRef]
- Gong, Q.L.; Zhao, W.X.; Wang, Y.C.; Zong, Y.; Wang, Q.; Yang, Y.; Yang, Y.; Shi, K.; Li, J.M.; Leng, X.; et al. Prevalence of coccidia in domestic pigs in China between 1980 and 2019: A systematic review and meta-analysis. Parasites Vectors 2021, 14, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Vlasova, A.N.; Amimo, J.O.; Saif, L.J. Porcine Rotaviruses: Epidemiology, Immune Responses and Control Strategies. Viruses 2017, 9, 48. [Google Scholar] [CrossRef] [PubMed]
- Theuns, S.; Conceicao-Neto, N.; Zeller, M.; Heylen, E.; Roukaerts, I.D.; Desmarets, L.M.; Van Ranst, M.; Nauwynck, H.J.; Matthijnssens, J. Characterization of a genetically heterogeneous porcine rotavirus C, and other viruses present in the fecal virome of a non-diarrheic Belgian piglet. Infect. Genet. Evol. 2016, 43, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Cooper, V.L. Diagnosis of Neonatal Pig Diarrhea, Veterinary Clinics of North America. Food Anim. Pract. 2000, 16, 117–133. [Google Scholar] [CrossRef] [PubMed]
- Amimo, J.O.; Machuka, E.M.; Okoth, E. First Detection of Rotavirus Group C in Asymptomatic Pigs of Smallholder Farms in East Africa. Pathogens 2017, 6, 37. [Google Scholar] [CrossRef] [Green Version]
- Shepherd, F.K.; Freeman, M.J.; Culhane, M.R.; Marthaler, D.G. Reoviruses (Rotaviruses and Reoviruses). In Disease of Swine, 11th ed.; Zimmerman, J.J., Karriker, L.A., Ramirez, A., Schwartz, K.J., Stevenson, G.W., Zhang, J., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2019; pp. 715–727. [Google Scholar]
- Dewey, C.; Carman, S.; Pasma, T.; Josephson, G.; McEwen, B. Relationship between group A porcine rotavirus and management practices in swine herds in Ontario. Can. Vet. J. 2003, 44, 649–653. [Google Scholar]
- Almeida, P.R.; Lorenzetti, E.; Cruz, R.S.; Watanabe, T.T.; Zlotowski, P.; Alfieri, A.A.; Driemeier, D. Diarrhea caused by rotavirus A, B, and C in suckling piglets from southern Brazil: Molecular detection and histologic and immunohistochemical characterization. J. Vet. Diagn. Investig. 2018, 30, 370–376. [Google Scholar] [CrossRef]
- Chepngeno, J.; Diaz, A.; Paim, F.C.; Saif, L.J.; Vlasova, A.N. Rotavirus C: Prevalence in suckling piglets and development of virus-like particles to assess the influence of maternal immunity on the disease development. Vet. Res. 2019, 50, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Crowley, A. Diagnostic Testing and the Challenges Associated with Vaccine Development for Porcine Rotavirus; Master of Science, Department of Diagnostic Medicine/Pathobiology, College of Veterinary Medicine, Kansas State University: Manhattan, KS, USA, 2021; pp. 1–51. [Google Scholar]
- Masuda, T.; Tsuchiaka, S.; Ashiba, T.; Yamasato, H.; Fukunari, K.; Omatsu, T.; Furuya, T.; Shirai, J.; Mizutani, T.; Nagai, M. Development of one-step real-time reverse transcriptase-PCR-based assays for the rapid and simultaneous detection of four viruses causing porcine diarrhoea. Jpn. J. Vet. Res. 2016, 64, 5–14. [Google Scholar]
- Marthaler, D.; Homwong, N.; Rossow, K.; Culhane, M.; Goyal, S.; Collins, J.; Matthijnssens, J.; Ciarlet, M. Rapid detection and high occurrence of porcine rotavirus A, B and C by RT-qPCR in diagnostic samples. J. Virol. Methods 2014, 209, 30–34. [Google Scholar] [CrossRef]
- Miyabe, F.M.; Dall Agnol, A.M.; Leme, R.A.; Oliveira, T.E.S.; Headley, S.A.; Fernandes, T.; de Oliveira, A.G.; Fernandes Alferi, A.; Amauri AlcindoAlferi, A.A. Porcine rotavirus B as primary causative agent of diarrhea outbreaks in newborn piglets. Sci. Rep. 2020, 10, 1–9. [Google Scholar]
- Resende, T.P.; Marthaler, D.; Vannucci, F.A. In situ hybridization detection and subtyping of rotaviruses in swine samples. J. Vet. Diagn. Investig. 2019, 31, 113–117. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Vlasova, A.N.; Kenney, S.P.; Saif, L.J. Emerging and reemerging coronaviruses in pigs. Curr. Opin. Virol. 2019, 34, 39–49. [Google Scholar] [CrossRef]
- Turlewicz-Podbielska, H.; Pomorska-Mól, M. Porcine Coronaviruses: Overview of the State of the Art. Virol. Sin. 2021, 36, 833–851. [Google Scholar] [CrossRef]
- Fenner, F. Coronaviridae. In Fenner’s Veterinary Virology, 5th ed.; Maclachlan, N.J., Dubovi, E.J., Eds.; Academic Press: New York, NY, USA, 2017; pp. 435–461. [Google Scholar]
- Saif, L.J.; Wang, Q.; Vlasova, A.N.; Jung, K.; Xiao, S. Coronaviruses. In Disease of Swine, 11th ed.; Zimmerman, J.J., Karriker, L.A., Ramirez, A., Schwartz, K.J., Stevenson, G.W., Zhang, J., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2019; pp. 488–523. [Google Scholar]
- Chen, F.; Knutson, T.P.; Rossow, S.; Saif, L.J.; Marthaler, D.G. Decline of transmissible gastroenteritis virus and its complex evolutionary relationship with porcine respiratory coronavirus in the United States. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Niederwerder, M.C.; Hesse, R.A. Swine enteric coronavirus disease: A review of 4 years with porcine epidemic diarrhoea virus and porcine deltacoronavirus in the United States and Canada. Transbound. Emerg. Dis. 2018, 65, 660–675. [Google Scholar] [CrossRef] [Green Version]
- Feng, Y.; Xu, Z.; Zhu, L. Prevalence and phylogenetic analysis of porcine deltacoronavirus in Sichuan province, China. Arch. Virol. 2020, 165, 2883–2889. [Google Scholar] [CrossRef]
- Zhang, J. Porcine deltacoronavirus: Overview of infection dynamics, diagnostic methods, prevalence and genetic evolution. Virus Res. 2016, 226, 71–84. [Google Scholar] [CrossRef]
- Jung, K.; Hu, H.; Saif, L.J. Porcine deltacoronavirus infection: Etiology, cell culture for virus isolation and propagation, molecular epidemiology and pathogenesis. Virus Res. 2016, 226, 50–59. [Google Scholar] [CrossRef]
- Zhou, Z.; Sun, Y.; Yan, X.; Tang, X.; Li, Q.; Tan, Y.; Lan, T.; Ma, J. Swine acute diarrhea syndrome coronavirus (SADS-CoV) antagonizes interferon-β production via blocking IPS-1 and RIG-I. Virus Res. 2020, 278, 1–8. [Google Scholar] [CrossRef]
- Zappulli, V.; Ferro, S.; Bonsembiante, F.; Brocca, G.; Calore, A.; Cavicchioli, L.; Centelleghe, C.; Corazzola, G.; De Vreese, S.; Gelain, M.E.; et al. Pathology of Coronavirus Infections: A Review of Lesions in Animals in the One-Health Perspective. Animals 2020, 10, 2377. [Google Scholar] [CrossRef] [PubMed]
- Shoup, D.I.; Swayne, D.E.; Jackwood, D.J.; Saif, L.J. Immunohistochemistry of transmissible gastroenteritis virus antigens in fixed paraffin-embedded tissues. J. Vet. Diagn. Investig. 1996, 8, 161–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madson, D.M.; Magstadt, D.R.; Arruda, P.H.E.; Hoang, H.; Sun, D.; Bower, L.P.; Bhandari, M.; Burrough, E.R.; Gauger, P.C.; Pillatzki, A.E.; et al. Pathogenesis of porcine epidemic diarrhea virus isolate in 3-week-old weaned pigs. Vet. Microbiol. 2014, 174, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.; Wang, Q.; Scheuer, K.A.; Lu, Z.; Zhang, Y.; Saif, L.J. Pathology of US porcine epidemic diarrhea virus strain PC21A in gnotobiotic pigs. Emerg. Infect. Dis. 2014, 20, 662–665. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hayes, J.; Sarver, C.; Byrum, B.; Zhang, Y. Porcine deltacoronavirus: Histological lesions and genetic characterization. Arch. Virol. 2016, 161, 171–175. [Google Scholar] [CrossRef]
- Jung, K.; Hu, H.; Eyerly, B.; Lu, Z.; Chepngeno, J.; Saif, L.J. Pathogenicity of 2 porcine deltacoronavirus strains in gnotobiotic pigs. Emerg. Infect. Dis. 2015, 21, 650–654. [Google Scholar] [CrossRef] [Green Version]
- Zhou, P.; Fan, H.; Lan, T.; Yang, X.L.; Shi, W.F.; Zhang, W.; Zhu, Y.; Zhang, Y.W.; Xie, Q.M.; Mani, S.; et al. Fatal swine acute diarrhoea syndrome caused by an HKU2-related coronavirus of bat origin. Nature 2018, 556, 255–258. [Google Scholar] [CrossRef] [Green Version]
- Vitosh-Sillman, S.; Loy, J.D.; Brodersen, B.; Kelling, C.; Doster, A.; Topliff, C.; Nelson, E.; Bai, J.; Schirtzinger, E.; Poulsen, E.; et al. Experimental infection of conventional nursing pigs and their dams with Porcine deltacoronavirus. J. Vet. Diagn. Investig. 2016, 28, 486–497. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Wang, H.Y. Porcine enteric coronaviruses: An updated overview of the pathogenesis, prevalence, and diagnosis. Vet. Res. Commun. 2021, 45, 75–86. [Google Scholar] [CrossRef]
- Huang, X.; Chen, J.; Yao, G.; Guo, Q.; Wang, J.; Liu, G. A TaqManprobe-based multiplex real-time RT-qPCR for simultaneous detection of porcine enteric coronaviruses. Appl. Microbiol. Biotechnol. 2019, 103, 4943–4952. [Google Scholar] [CrossRef] [Green Version]
- Stevenson, G.W.; Hoang, H.; Schwartz, K.J.; Burrough, E.R.; Sun, D.; Madson, D.; Cooper, V.L.; Pillatzki, A.; Gauger, P.; Schmitt, B.J.; et al. Emergence of Porcine epidemic diarrhea virus in the United States: Clinical signs, lesions, and viral genomic sequences. J. Vet. Diagn. Investig. 2013, 25, 649–654. [Google Scholar] [CrossRef] [Green Version]
- Fu, Y.; Li, B.; Liu, G. Rapid and efcient detection methods of pathogenic swine enteric coronaviruses. Appl. Microbiol. Biotechnol. 2020, 104, 6091–6100. [Google Scholar] [CrossRef]
- Griffith, R.W.; Carlson, S.A.; Krull, A.C. Salmonellosis. In Disease of Swine, 11th ed.; Zimmerman, J.J., Karriker, L.A., Ramirez, A., Schwartz, K.J., Stevenson, G.W., Zhang, J., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2019; pp. 912–925. [Google Scholar]
- Alban, L.; Stege, H.; Dahl, J. The new classification system for slaughter-pig herds in the Danish Salmonella surveillance-and-control program. Prev. Vet. Med. 2002, 53, 133–146. [Google Scholar] [CrossRef]
- Vannucci, F.A.; Gebhart, C.J.; McOrist, S. Proliferative Enteropathy. In Disease of Swine, 11th ed.; Zimmerman, J.J., Karriker, L.A., Ramirez, .A., Schwartz, K.J., Stevenson, G.W., Zhang, J., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2019; pp. 898–911. [Google Scholar]
- Campillo, M.; Smith, S.H.; Gally, D.L.; Opriessnig, T. Review of methods for the detection of Lawsonia intracellularis infection in pigs. J. Vet. Diagn. Investig. 2021, 33, 621–631. [Google Scholar] [CrossRef]
- D’Annunzio, G.; Bardini, R.; Ostanello, F.; Sarli, G. Enteropatia proliferativa da Lawsonia intracellularis nel suino. Large Anim. Rev. 2021, 27, 149–163. [Google Scholar]
- Guedes, R.M.; Gebhart, C.J.; Winkelman, N.L.; Mackie-Nuss, R.A.; Marsteller, T.A.; Deen, J. Comparison of different methods for diagnosis of porcine proliferative enteropathy. Can. J. Vet. Res. 2002, 66, 99–107. [Google Scholar]
- Lawson, G.H.; Gebhart, C.J. Proliferative enteropathy. J. Comp. Pathol. 2000, 122, 77–100. [Google Scholar] [CrossRef]
- Rowland, A.C.L.; Lawson, G.H.K. Porcine proliferative enteropathies. In Diseases of Swine, 7th ed.; Leman, A.D., Straw, B.E., Mengeline, W.L., Eds.; Iowa State University Press: Ames, IA, USA, 1992; pp. 560–569. [Google Scholar]
- McOrist, S.; Gebhart, C.J.; Bosworth, B.T. Evaluation of porcine ileum models of enterocyte infection by Lawsonia intracellularis. Can. J. Vet. Res. 2006, 70, 155–159. [Google Scholar]
- Huerta, B.; Arenas, A.; Carrasco, L.; Maldonado, A.; Tarradas, C.; Carbonero, A.; Perea, A. Comparison of diagnostic techniques for porcine proliferative enteropathy (Lawsonia intracellularis infection). J. Comp. Pathol. 2003, 129, 179–185. [Google Scholar] [CrossRef]
- Burrough, E.R.; Rotolo, M.L.; Gauger, P.C.; Madson, D.M. Correlation of Lawsonia intracellularis semi-quantitative fecal polymerase chain reaction assay results with the presence of histologic lesions of proliferative enteropathy and positive immunohistochemical staining. J. Swine Health Prod. 2015, 23, 204–207. [Google Scholar]
- Collins, A.M. Advances in Ileitis Control, Diagnosis, Epidemiology and the Economic Impacts of Disease in Commercial Pig Herds. Agriculture 2013, 3, 536–555. [Google Scholar] [CrossRef] [Green Version]
- Hampson, D.J.; Burrough, E.R. Swine Dysentery and Brachyspiral Colitis. In Diseases of Swine, 11th ed.; Zimmerman, J.J., Karriker, L.A., Ramirez, A., Schwartz, K.J., Stevenson, G.W., Zhang, J., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2019; pp. 951–970. [Google Scholar]
- Hampson, D.J.; Fellstrom, C.; Thomson, J.R. Swine dysentery. In Diseases of Swine, 9th ed.; Straw, B.E., Zimmerman, J.J., D’Allaire, S., Eds.; Blackwell Publishing Professional: Ames, IA, USA, 2006; pp. 785–805. [Google Scholar]
- Burrough, E.R. Swine Dysentery: Etiopathogenesis and Diagnosis of a Reemerging Disease. Vet. Pathol. 2017, 54, 22–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hampson, D.J. The Spirochete Brachyspira pilosicoli, Enteric Pathogen of Animals and Humans. Clin. Microbiol. Rev. 2017, 31, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Thomson, J.R.; Smith, W.J.; Murray, B.P. Investigations into field cases of porcine colitis with particular reference to infection with Serpulina pilosicoli. Vet. Rec. 1998, 142, 235–239. [Google Scholar] [CrossRef]
- Wilberts, B.L.; Arruda, P.H.; Kinyon, J.M.; Frana, T.S.; Wang, C.; Magstadt, D.R.; Madson, D.M.; Patience, J.F.; Burrough, E.R. Investigation of the impact of increased dietary insoluble fiber through the feeding of distillers dried grains with solubles (DDGS) on the incidence and severity of Brachyspira-associated colitis in pigs. PLoS ONE 2014, 9, e114741. [Google Scholar] [CrossRef]
- Prohaska, S.; Pflüger, V.; Ziegler, D.; Scherrer, S.; Frei, D.; Lehmann, A.; Wittenbrink, M.M.; Huber, H. MALDI-TOF MS for identification of porcine Brachyspira species. Lett. Appl. Microbiol. 2014, 58, 292–298. [Google Scholar] [CrossRef]
- Burrough, E.R.; Strait, E.L.; Kinyon, J.M.; Bower, L.P.; Madson, D.M.; Wilberts, B.L.; Schwartz, K.J.; Frana, T.S.; Songer, J.G. Comparative virulence of clinical Brachyspira spp. isolates in inoculated pigs. J. Vet. Diagn. Investig. 2012, 24, 1025–1034. [Google Scholar] [CrossRef] [Green Version]
- Boye, M.; Jensen, T.K.; Møller, K.; Leser, T.D.; Jorsal, S.E. Specific detection of the genus Serpulina, S. hyodysenteriae and S. pilosicoli in porcine intestines by fluorescent rRNA in situ hybridization. Mol. Cell Probes. 1998, 12, 323–330. [Google Scholar] [CrossRef]
- Burrough, E.R.; Wilberts, B.L.; Bower, L.P.; Jergens, A.E.; Schwartz, K.J. Fluorescent in situ hybridization for detection of "Brachyspira hampsonii" in porcine colonic tissues. J. Vet. Diagn. Investig. 2013, 25, 407–412. [Google Scholar] [CrossRef]







| Tissue/Sample | Specimen Collection |
|---|---|
| Lymph node | Mesenteric—1 cm thickness |
| Tonsils | Half of a tonsil |
| Spleen | 1 cm thickness |
| Liver | 1 sample 2 × 2 × 0.5 cm |
| Kidney | Half of a kidney, 0.5 cm slice through the centre |
| Stomach | 3 × 3 × 3 cm piece 1 cm thickness |
| Jejunum | Three sections, 2 cm long |
| Ileum | Three sections, 2 cm long |
| Spiral colon | Three sections, 2 cm long |
| Disease/Aetiological Agent | Main Clinical and Anatomopathological Characters |
|---|---|
| Proliferative enteropathy (PE) L. Intracellularis | Clinically resembles SD, but SD does not affect the small intestine |
| Enteric salmonellosis S. Typhimurium and its monophasic variant | Clinical signs and gross lesions can be similar. Parenchymatous organs and lymph nodes necrosis, fibrinous and ulcerative lesions in the small intestine not observed in SD |
| Trichuriasis Trichuris suis | Large numbers of Trichuris suis in the cecum |
| Gastric ulcers and other haemorrhagic conditions | Digested blood in the faeces, “tarry” appearance; the large intestine has no lesions. |
| Porcine intestinal spirochetosis B. Pilosicoli | The differential diagnosis is difficult, being similar to mild cases of SD |
| Disease/Aetiological Agent | Age | Clinical and Pathological Findings | Diagnostic Tools |
|---|---|---|---|
| Neonatal and Post-weaning Colibacillosis E.coli (ETEC) | Neonatal: mostly 0–4 days Post-weaning: mostly 28–60 days |
|
|
| Clostridiosis C. perfrigens type C | Neonates (until 3 weeks of age) |
|
|
| Clostridiosis C. perfrigens type A | Neonates/suckling piglets |
|
|
| Clostridiosis Clostridioides difficile | 1–7 days of life |
|
|
| Coccidiosis Cystoisospora suis | Commonly in the second week of life |
|
|
| Rotavirosis Rotavirus | Commonly in 2 to 6 weeks old pigs |
|
|
| Coronaviruses (PECs) | All |
|
|
| Salmonellosis (Salmonella Typhimurium and its monophasic variant S. 1,4,[5],12:i:-) | Mostly in growing period |
|
|
| Proliferative enteropathy Lawsonia intracellularis | 4–12 months of age (PHE); 6–20 weeks (PIA) |
|
|
| Swine dysentery (SD) Brachyspira hyodysenteriae (B. hampsonii; B. suanatina) Intestinal spirochetosis (PIS) B. pilosicoli | Mainly in grower and finisher pigs |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luppi, A.; D’Annunzio, G.; Torreggiani, C.; Martelli, P. Diagnostic Approach to Enteric Disorders in Pigs. Animals 2023, 13, 338. https://doi.org/10.3390/ani13030338
Luppi A, D’Annunzio G, Torreggiani C, Martelli P. Diagnostic Approach to Enteric Disorders in Pigs. Animals. 2023; 13(3):338. https://doi.org/10.3390/ani13030338
Chicago/Turabian StyleLuppi, Andrea, Giulia D’Annunzio, Camilla Torreggiani, and Paolo Martelli. 2023. "Diagnostic Approach to Enteric Disorders in Pigs" Animals 13, no. 3: 338. https://doi.org/10.3390/ani13030338
APA StyleLuppi, A., D’Annunzio, G., Torreggiani, C., & Martelli, P. (2023). Diagnostic Approach to Enteric Disorders in Pigs. Animals, 13(3), 338. https://doi.org/10.3390/ani13030338







