Simple Summary
The black soldier fly (BSF, Hermetia illucens) is one of the most studied insects for use in feed, whose larvae (BSFL) can be used to feed fish. In this study, the inclusion of 10.5% wholemeal BSFL meal in the diet of tambaqui (a neotropical fish) resulted in higher growth than fish fed conventional ingredients. The use of wholemeal BSFL meal is more advantageous because it has a lower production cost than defatted BSF meal, as there is no expense involved in the fat extraction process. Wholemeal BSFL meal can be considered a leading ingredient to meet the emerging need for protein ingredients by the feed industry.
Abstract
Black soldier fly (BSF, Hermetia illucens) larvae is a prominent ingredient in aquafeeds due to its high protein and energy contents. This study evaluated the digestibility of full-fat BSF larvae meal (FF-BSFL) and its inclusion in diets for tambaqui, Colossoma macropomum. The apparent digestibility coefficient of FF-BSFL for protein and energy was around of 88%, corresponding to 33.55% and 21.61 MJ kg−1 of digestible protein and energy, respectively. For the feeding trial, tambaqui juveniles (53.23 ± 1.07 g) were distributed in a completely randomized experimental design (n = 4; 150 L tanks; 10 fish per tank). Fish were fed diets including 0%, 5.25%, 10.50%, and 15.75% FF-BSFL to apparent satiation for 60 days. Fish fed 10.50% FF-BSFL dietary inclusion showed higher weight gain, feed intake, final biomass, and relative growth rate. The 10.50% FF-BSFL diet presented the highest index of economic profitability. Weight gain fitted a third-degree equation and the optimum FF-BSFL inclusion level was estimated at 11.6%. However, FF-BSFL dietary inclusion up to 15.75% did not impair growth fish performance. FF-BSFL seems to be a promising source of protein and energy for omnivorous fish aquafeed.
1. Introduction
The world population reached 8 billion people in 2022 and, according to the United Nations [1], the forecast is that it will reach 9 billion inhabitants in the next fifteen years. Increased life expectancy associated with advances in medicine, health and nutrition are among the reasons for this unprecedented population growth. Therefore, there is widespread concern about the emerging need to produce animal-based protein more efficiently and sustainably to meet the continuous growing human demand for food [2].
Insects are the most abundant macroscopic animals on the planet and represent 73% of the total described fauna [3]. The value of the insect market in 2023 is estimated at USD 2.6 billion. According to the Future Market Insights Global reports [4], the value of the insect market for animal nutrition worldwide is expected to increase at a compound annual growth rate of 20.9% from 2023 to 2033, reaching USD 17.4 billion by 2033. Insects as feed ingredients for aquafeeds are on the rise in science and industry sectors, since there is an emergent necessity for alternative protein sources to fish meal and fish oil [5].
Among the different species of insects, the black soldier fly (BSF, Hermetia illucens Linnaeus, 1758) has been proposed as one of the insects with the greatest potential as an ingredient for animal nutrition [6]. The production of BSF larvae (BSFL) is encouraged due to its characteristics that facilitate its handling, such as short development cycle, ability to live in high densities and take advantage of low-cost waste as substrate, fast growth, and low vulnerability to diseases, resulting in high survival rates [3,7].
BSFL can be processed in different ways which result in ingredients for the feed industry with different protein and fat contents. The defatted BSFL meal (D-BSFL) is produced by partial or total fat extraction using pressing or organic solvents, and the resulting defatted meal is then posteriorly dried and ground. D-BSFL has around 60% protein and 10–12% lipid content [8,9]. The full-fat BSFL (FF-BSFL) meal is easy to produce by drying and posteriorly grinding. The FF-BSFL is a low-cost technology when compared to D-BSFL as it avoids expenses associated with fat extraction processes. FF-BSFL has an average content of 42% crude protein and 30% lipids [10,11].
BSFL meal was evaluated positively when included or replacing fish meal in diets for Atlantic salmon (Salmo salar Linnaeus, 1758) [12], rainbow trout (Oncorhynchus mykiss Walbaum, 1792) [13], Nile tilapia (Oreochromis niloticus Walbaum, 1792) [14] and Siberian sturgeon (Acipenser baerii Brandt, 1869) [5]. The use of seven species of insects in the aquafeed industry was approved by the European Union (Regulation no. 2017/893), and it is a prominent issue in the world literature [15].
Tambaqui, Colossoma macropomum (Cuvier, 1818), is an omnivorous fish, native to the basins of the Amazon and Orinoco rivers and is one of the most reared species in South America and has already been introduced in the United States, China, Thailand, and the Philippines for aquaculture purposes [16,17]. In Brazil, tambaqui production reached 100.6 thousand tons in 2020 [18]. Tambaqui easily adapts to feed formulations and aquaculture production systems [16]; in addition, it can be reared with the replacement of 50% commercial feed for whole BSFL without impairing its growth performance [19]. Furthermore, tambaqui digests 86% of the crude protein of the defatted BSFL meal, and levels of up to 30% defatted BSFL meal can be used in its fishmeal-free diet [9]. However, there are no published data on the use of full-fat BSFL meal in tambaqui diets.
This study evaluated the inclusion of full-fat BSFL meal as an ingredient in tambaqui diets and the effects on nutrient and energy digestibility, growth performance, whole body composition, and economic analysis of diets.
2. Materials and Methods
The experiment was carried out at the Aquaculture Experimental Station of the Technology and Innovation Coordination-COTEI/INPA (3°05′26.7″ S and 59°59′41.1″ W), Amazon State, Brazil. The study complied with the standards required by the National Council for the Control of Animal Experimentation (CONCEA, 2018) and was approved by the Ethics Committee in Research on the Use of Animals of the National Institute for Research in the Amazon (INPA) with protocol no. 056/2017 and 010/2021.
2.1. Production and Processing of Full-Fat Black Soldier Fly Larvae Meal (FF-BSFL)
BSFL were produced in starter feed for broilers (crude protein: 21%; ether extract: 2.5%; crude fiber: 5%; moisture: 13%) as a substrate for standardizing its bromatological composition, and they were supplied by the Natuprotein company in the city of Manaus, Amazon, Brazil. BSFL were dehydrated in a forced air circulation oven at 55 °C for 24 h. Dehydrated larvae were ground in a multiprocessor with 0.8 mm mesh.
2.2. Digestibility Assay
A practical feed was formulated to meet the nutritional requirements of tambaqui and used as the reference diet (RD; Table 1). The test diet (TD) was obtained by replacing 30% of RD by FF-BSFL. The apparent digestibility coefficient (ADC) was determined by the indirect method using 0.5% chromium oxide III (Cr2O3), as a marker, in the experimental diets [20].

Table 1.
Formulation and chemical composition of the reference diet utilized in the digestibility assay (values expressed on dry matter basis).
Tambaqui juveniles (48.66 ± 8.02 g) were housed in six conical tanks (200 L; n = 3; 17 fish per tank) fitted with a collection tube at the bottom for feces collection [21]. The tanks formed part of an open system with water renewal from an artesian well and with constant aeration. Fish were fed twice daily at a rate of 3% biomass for a 22-day period. Decanted feces were collected twice daily (8:00 am and 5:00 pm) and stored in a freezer (−20 °C) for later laboratory analyses.
The analyses to determine the chromium III oxide content in the samples of feeds and feces were carried out by the colorimetric method, according to the methodology described by Furukawa and Hiroko [22]. The standard curve was calculated from the nitro-perchloric digestion of the samples with known chromium III oxide concentrations. A reading was taken by a spectrophotometer, adjusted to a wavelength of 350 nm. The Apparent Digestibility Coefficients (ADCs) of nutrients and the energy of the RD and TD diets were calculated according to the following equation:
ADC (%) = 100 − (100 × ((%Chromium III in diet)/(%Chromium III in feces)) × ((% nutrient (or energy) in feces)/(% nutrient (or energy) in diet)).
The nutrient and energy ADCs of the FF-BSFL meal were calculated following the equation proposed by Bureau and Hua [23]:
ADCi (%) = ADCtd + (ADCtd-ADCref) × ((r × Nref)/(i × Ni))
where:
ADCi = the apparent digestibility coefficient of the ingredient;
ADCtd = the apparent digestibility coefficient of the TD nutrient;
ADCref = the apparent digestibility coefficient of the RD nutrient;
r = the proportion of the RD in the TD (0.65);
i = the proportion of the test ingredient in the TD (0.3);
Nref = the nutrient concentration in the RD (%);
Ni = the nutrient concentration in the test ingredient (%).
2.3. Feeding Trial
Four experimental diets were formulated according to the nutritional requirements of tambaqui juveniles [24,25], i.e., a control diet without inclusion of FF-BSFL meal (0%) and three diets with the following levels of inclusion of FF-BSFL meal: 5.25%, 10.50%, or 15.75% (Table 2). All ingredients were ground, sieved (0.8 mm), hydrated (25%), and extruded in a single-screw extruder (model MX-80, INBRAMAQ®; São Paulo, Brazil) with a 3 mm die.

Table 2.
Formulation and composition of the experimental diets (on dry matter basis).
Tambaqui juveniles (53.23 ± 1.07 g; 14.92 ± 0.47 cm) were distributed in a completely randomized experimental design, with four treatments and four replicates (150 L tanks; 10 fish/tank), totaling 16 experimental units. The experimental units formed part of a recirculation system with phytoremediation, constant aeration, and natural photoperiod. The fish were fed 3 times a day (10 am, 2 pm, 5 pm) until apparent satiation for 60 days.
2.4. Water Quality Parameters
The water quality parameters of dissolved oxygen, temperature, and pH were monitored daily using a multiparameter probe (PRO ODO, YSI®; Yellow Springs, OH, USA). The ammonia, nitrite, and alkalinity levels were monitored weekly using colorimetric and titrimetric kits (Alfakit AT 101; Alfakit, Florianópolis, Santa Catarina, Brazil).
2.5. Growth Performance and Biometric Indices
Fish biometrics were performed at the beginning, 30th day, and 60th day of the experiment to monitor the growth of the fish. Before the biometrics, fish were fasted for 24 h and anesthetized with a solution of 100 mg of benzocaine L−1 [26] to facilitate handling and avoid injuries to the fish.
At the end of the experiment, the growth performance data were expressed by the parameters obtained by the following calculations:
- average weight gain (g) = final weight–initial weight;
- biomass weight gain (kg) = final biomass–initial biomass;
- final biomass (g) = final number of fish × final average weight;
- feed consumption (g) = total weight of feed consumed per tank;
- apparent feed conversion = feed intake ÷ weight gain;
- relative growth rate (% day−1) = (eg −1) × 100;
where “e” is the Neperian number and g = (ln (final weight)-ln (initial weight))/(number of experimental days);
- protein efficiency ratio (g g−1) = biomass weight gain ÷ protein consumption;
- protein conversion efficiency (%) = [(final body weight × final body protein %) − (initial body weight × initial body protein %)/protein intake (g)] × 100;
- condition factor = 100 × (body weight/total length3).
Two fish from each experimental unit were anesthetized by immersion in 300 mg benzocaine L−1 until the loss of reflex activity and no reaction to external stimuli were noted. Afterward, fish were euthanized by spinal medulla rupture according to the rules of the CONCEA [27] and destined for the collection and weighing of viscera and liver to determine the following indices: viscerosomatic index = 100 × (viscera weight/body weight); hepatosomatic index = 100 × (liver weight/body weight).
2.6. Proximate Composition
The proximate compositions of the FF-BSFL meal, diets, feces, and whole-body fish were determined according to AOAC [28]. Moisture content was determined by oven drying at 105 °C until constant weight; total lipids by solvent (hexane) extraction for 6 h (Soxhlet); ash content by burning in a muffle furnace at 500 °C for 4 h; crude protein (CP) by the micro-Kjeldahl method and correcting the total nitrogen content by multiplying by the factor 6.25. Specifically, for the FF-BSFL meal sample, CP was determined by the micro-Kjeldahl method and by correcting the total nitrogen content by multiplying by the factor 5.6, as recommended by Janssen et al. [29]. Chitin was analyzed following the modified method of Abidin et al. [30] with washing, deproteinization (NaOH), demineralization (HCl), and drying of samples. The gross energy of samples was determined by an IKA 2600 calorimeter bomb (IKA®-Werke GmbH & Co. KG, Staufen, Germany).
2.7. Economic Analysis of Feeds
The cost of producing BSFL meal was determined through fixed capital investment for small-scale production of 24 kg of BSFL in a 21-day production cycle. Investment in civil construction was not considered. Production costs were determined based on the total operating cost, which is the sum of the effect operating cost (EOC) and the depreciation of fixed capital items. In the EOC, labor, equipment maintenance (20% of the new value per year), and the initial feed for chickens that served as substrate for the growth of the BSFL were added.
The labor cost was calculated based on the time used to produce the BSFL and the cost per hour worked (USD 1.67 h) in Brazil. The cost per hour worked was calculated based on a salary of USD 225.70 (equivalent to a minimum wage of BRL 1212.00 currently practiced in Brazil), plus 48% social security contributions for 200 h worked per month. Depreciation of equipment was calculated based on the straight-line method. The production of 1 kg of BSFL was estimated at USD 3.50.
The cost of the diet was calculated based on the market prices of the ingredients used (Table 2). The cost of diet processing was not considered. The analysis was based on local market retail prices converted to USD (USD 1 = BRL 5.37; exchange as of 3 December 2022). The diets cost USD 0.93/kg (0% FF-BSFL), USD 0.97/kg (5.25% FF-BSFL), USD 1.00/kg (10.50% FF-BSFL), and USD 1.02/kg (15.75% FF-BSFL).
The economic efficiency of the diets was assessed using input–output analysis [12,31]. To determine the relative economic efficiency and cost benefits of the tested diet per unit of fish gain, the economic conversion ratio (ECR) was calculated as fellows: feed cost/kg weight gain = (feed intake (g)/body weight gain (g)) × cost of feed (USD/1 kg). The economic profit index (EPI) was calculated using the formula from Stejskal et al. (2020): EPI (USD/fish) = (gain of body weight (kg) × live fish selling price (USD/1 kg) − (body weight gain (kg) × (feed cost (USD/1 kg)).
The calculation of profitability (PRO) used the balance between the selling price of fish and the cost of feed per kg of fish gain (ECR): PRO (USD/1 kg of fish gain) = sale price of live fish (USD/1 kg) − economic conversion ratio (USD/1 kg of fish gain). Economic efficiency was calculated using the following ratio: economic efficiency = profit per kg gain/feed cost per kg gain.
2.8. Statistical Analysis
The variables expressed as percentages were previously transformed by square root arcsine. Data were submitted to the Levene and Shapiro–Wilk tests to verify normality and homoscedasticity, respectively. If the data were not normally distributed, or had unequal variance, they were subjected to the nonparametric Kruskal–Wallis test, followed by the Dwass–Steel–Critchlow–Fligner test. The parametric data were submitted to one-way analysis of variance where diet (4 levels) was the factor. If there was a difference between treatments (diets), means were compared using the Tukey test at α = 0.05. The treatments were also treated as a continuous variable, and regression analysis was performed. If significant differences were found, a lack of fit test was performed to validate the regression. Variables were analyzed by regression using the CurveExpert Pro software, and models were selected for best fitting the data to the model by the coefficient of determination (r2) and by making a comparison by the F test and the AIC criterion.
3. Results
There were no significant differences in the water quality parameters during the experimentation period (Table 3). Tambaqui presented ADC above 88% for crude protein and energy of FF-BSFL, which resulted in 33.55% and 21.61 MJ kg−1 of digestible protein and energy, respectively. The ADC of lipids was 96.42% and resulted in 30.47% of digestible lipids for tambaqui. For chitin, tambaqui presented an ADC of 16.40%, which consisted of 1.72% digestible chitin (Table 4).

Table 3.
Water quality parameters in rearing tambaqui fed diets containing full-fat black soldier fly larvae (FF-BSFL) meal during the experimental period.

Table 4.
Composition, apparent digestibility coefficient (ADC), values of digestible nutrients, and energy of the full-fat black soldier larva meal (FF-BSFL).
All groups of fish accepted the diets formulated with FF-BSFL and there was no record of mortality. Fish fed 10.5% FF-BSFL diet showed higher weight gain, final biomass, and relative growth rate compared to fish fed the other levels of dietary FF-BSFL (Table 4). The inflection point of the regression curve (Figure 1) was at 11.6% FF-BSFL dietary inclusion, which corresponded to 80.51 g of weight gain of tambaqui juveniles after 60 days of the experiment. Tambaqui fed 5.25% or 15.75% FF-BSFL dietary inclusion showed a similar growth performance to fish fed with the control diet (0% FF-BSFL). Apparent feed conversion, protein efficiency rate, protein conversion efficiency, and condition factor did not differ between experimental groups (Table 5).
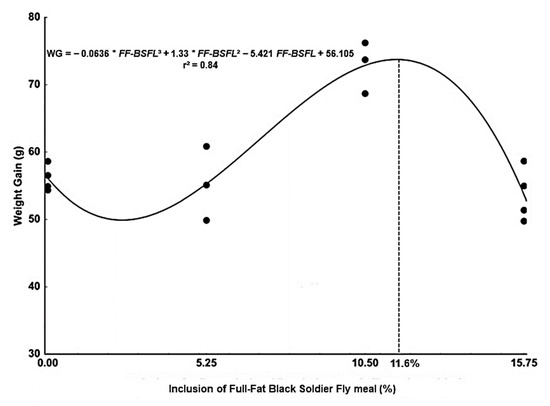
Figure 1.
Weight gain of tambaqui juveniles fed diets containing increasing levels of full-fat black soldier fly (FF-BSFL) meal.

Table 5.
Growth performance and biometric indices of tambaqui fed diets containing graded levels of full-fat black soldier fly larvae (FF-BSFL) meal for 60 days.
There was no statistically significant difference in the proximate composition and protein conversion efficiency of tambaqui fed diets containing up to 15.75% FF-BSFL meal (Table 6).

Table 6.
Proximate composition of whole body of tambaqui fed diets containing graded levels of full-fat black soldier fly larvae (FF-BSFL) meal for 60 days.
The economic profit index values were higher (p < 0.05) for diets with 10.50% FF-BSFL meal inclusion. No statistical differences were observed for economic conversion ratio, profitability, and economic efficiency of feeds (Table 7).

Table 7.
Economic analysis of feeds with the different inclusion levels of full-fat BSFL meal in the diets.
4. Discussion
The water quality parameters of all experiments were within acceptable limits for tambaqui [32]. For new ingredients to be considered promising for aquafeed formulation, it is necessary to observe their nutrient and energy contents, in addition to their digestibility, attractiveness, effect on growth performance, and impact on animal health [33]. The nutritional value of FF-BSFL (42.3% crude protein; 37.9% corrected protein; 31.6% crude lipids) was within the average nutrient content found in the literature. In a review article, Tran et al. [34] found that the composition of BSFL ranged from 41% to 44% for crude protein and from 15% to 34% for total lipids. The nutritional composition of BSFL can be influenced by the nutritional variation of the substrate on which the insect larvae grew [7]. In addition, the BSFL crude protein value can be overestimated if the factor 5.6 is not used to correct chitin nitrogen or non-protein nitrogen [29].
The values of ADCs of the protein and energy of the FF-BSFL meal (higher than 88%) were similar to the values reported for Nile tilapia (Oreochromis niloticus) juveniles (70.0–82.1%) fed different insect meals [35]. Chitin can inhibit the absorption of lipids in the intestine and increase their excretion [36]. However, this was not observed in this study, since the FF-BSFL lipid ADC was greater than 95%. The low ADC values of chitin suggest that tambaqui has a low chitinase activity, as demonstrated in Nile tilapia, another neotropical freshwater species [34]. However, it is possible that a prolonged period of feeding FF-BSFL-based diets collaborates to increase the digestibility of chitin, since the digestive enzymes of tambaqui can specialize in digesting this carbohydrate [37]. In nature, tambaqui consume a diversified diet and, like most omnivorous fish, they have morphological adaptations such as numerous pyloric caeca and plasticity of digestive enzymes that contribute to the better utilization of nutrients [38,39].
Extrusion processing of feeds can make complex carbohydrates, such as chitin, more available. In fact, extrusion cooking can break down complex polysaccharides into smaller components, such as mono- or disaccharides, which are more digestible. The levels of chitin present in the experimental diets did not affect the growth performance of tambaqui, as was also observed in Atlantic salmon fed with FF-BSFL meal and paste of up to 12.5% dietary inclusion [40].
The higher consumption of the 10.50% FF-BSFL diet reflected better growth performance results for tambaqui. Insects have pheromones on their surface, known as “natural attractants”, which may have acted as a palatability agent in the experimental diets [41,42]. However, the inclusion level of 15.75% may indicate a limit to the palatability of FF-BSFL dietary inclusion or fish satiety due to the chitin content.
Chitin is a carbohydrate resistant to chemical degradation, but it presents an energy content of approximately 17.1 kJ g−1 [43]. This fraction of energy was accumulated in diets with increasing levels of FF-BSFL inclusion; however, as chitin digestibility was low (16.40%), it may have been a kind of filler with low digestible energy content [44]. This may have kept the fish satiated for a longer period of time, influencing their feed intake and weight gain. Similarly, Siberian sturgeon (Acipenser baerii) fed FF-BSFL meal-based diets showed improvements in growth performance parameters [5].
In aquaculture, feeding costs amount to more than half of production costs [45]. The reduction in fish feeding costs contributes to greater profitability and economical sustainability of fish farming. Studies on the effects of using ingredients from BSFL production in diets for neotropical fish related to the economic analysis are still limited. In this study, the improvement in feed conversion of fish that consumed 10.50% FF-BSFL dietary inclusion indicates a contribution of the use of FF-BSFL meal to improve the efficiency of tambaqui production.
Despite the feed price increasing according to the inclusion of FF-BSFL meal, feeding tambaqui with 10.50% FF-BSFL meal diet increased the economic profit index by an average of 6% in relation to the diet with 0% FF-BSFL meal. In the case of Nile tilapia fed diets with 75% replacement of fish meal by BSFL meal, an increase of 3.97% in the economic profit index was observed [46], and greater economic efficiency with replacement of 100% fish meal was found [31]. When diets with FF-BSFL were tested in sturgeon, the economic profit index increased according to the level of inclusion [5]. In these studies, the price of BSFL meal was the main factor that had an impact on the variation in diet prices.
There is a worldwide tendency to produce BSFL on a large scale, which can reduce the costs of production, positively impacting the economic efficiency rates of feeds formulated with BSF ingredients. Furthermore, it must be considered that full-fat BSFL meal has a lower production cost than the defatted BSF meal, as there is no expense associated with the fat extraction process. The optimization of growth performance of tambaqui fed FF-BSFL meal-based diets and the economic profit index of its production indicate the possibility of more sustainable aquaculture, which can strengthen the bioeconomy around the world to produce more fish as food for humans.
5. Conclusions
Full-fat black soldier fly larvae meal is well digested by tambaqui and the inclusion of 11.6% FF-BSFL in diet yielded the maximum weight gain in tambaqui juveniles. Based on the results obtained, FF-BSFL meal can be considered as a prominent ingredient to meet the emerging need for protein sources by the aquafeed industry.
Author Contributions
Conceptualization, L.U.G., D.K.M.d.S., O.R.d.F., and F.A.L.d.F.; methodology, D.K.M.d.S., C.A.O., and L.U.G.; validation, O.R.d.F., D.K.M.d.S., and C.A.O.; formal analysis, L.U.G., D.K.M.d.S., and F.A.L.d.F.; investigation, all authors; resources, L.U.G. and G.P.; data curation, F.A.L.d.F., D.K.M.d.S., and L.U.G.; writing—original draft preparation, D.K.M.d.S. and L.U.G.; writing—review and editing, G.P., F.A.L.d.F., and L.U.G.; visualization, L.U.G. and D.K.M.d.S.; supervision, L.U.G. and G.P.; project administration, L.U.G. and G.P.; funding acquisition, L.U.G. All authors have read and agreed to the published version of the manuscript.
Funding
The authors would like to thank the Fundação de Amparo à Pesquisa do Estado do Amazonas (Amazonas State Research Foundation-FAPEAM), who granted the research funds (008/2021-PROSPAM/FAPEAM); FAPEAM for the doctoral scholarship and Conselho Nacional de Desenvolvimento Científico e Tecnológico (Brazil’s National Council for Scientific and Technological Development—CNPq) (process 205749/2018-6) for the doctoral sandwich scholarship, both to D.K.M.d.S.; and CNPq for the research fellowship to L.U.G (process 312492/2021-9).
Institutional Review Board Statement
The procedures of this study were in compliance with the Ethical Committee of Research on the Use of Animals of the INPA (no. 056/2017 and 010/2021).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data from the study are available from the corresponding authors upon reasonable request.
Acknowledgments
We are grateful to Nelson Poli Teixeira Filho for donating insects to produce raw material, and to all the students of the Gigas Project at the National Institute for Research in the Amazon, who provided assistance in this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- United Nations Day of Eight Billion. Available online: https://www.un.org/en/dayof8billion; (accessed on 5 December 2022).
- Verbeke, W.; Marcu, A.; Rutsaert, P.; Gaspar, R.; Seibt, B.; Fletcher, D.; Barnett, J. “Would you eat cultured meat?”: Consumers’ reactions and attitude formation in Belgium, Portugal and the United Kingdom. Meat Sci. 2015, 102, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Van Huis, A.; Van Itterbeeck, J.; Klunder, H.; Mertens, E.; Halloran, A.; Muir, G.; Vantomme, P. Edible Insects: Future Prospects for Food and Feed Security; FAO: Rome, Italy, 2013; p. 183. [Google Scholar]
- Globe Newswire Insect Feed Market is Estimated to Reach a Value of US$ 17.4 billion by 2033, Registering a CAGR of 20.9% during the forecast period 2023 to 2033|Future Market Insights, Inc. Globe Newswire. 2023. Available online: https://www.futuremarketinsights.com/reports/sample/rep-gb-11604 (accessed on 10 January 2023).
- Rawski, M.; Mazurkiewicz, J.; Kierończyk, B.; Józefiak, D. Black soldier fly full-fat larvae meal is more profitable than fish meal and fish oil in Siberian sturgeon farming: The effects on aquaculture sustainability, economy and fish git development. Animals 2021, 11, 604. [Google Scholar] [CrossRef] [PubMed]
- Van der Fels, H.J. Risk profile related to production and consumption of insects as food and feed: EFSA Scientific Committee’. EFSA J. 2015, 13, 4257. [Google Scholar] [CrossRef]
- Spranghers, T.; Ottoboni, M.; Klootwijk, C.; Ovyn, A.; Deboosere, S.; De Meulenaer, B.; Michiels, J.; Eeckhout, M.; De Clercq, P.; De Smet, S. Nutritional composition of black soldier fly (Hermetia illucens) prepupae reared on different organic waste substrates. J. Sci. Food Agric. 2017, 97, 2594–2600. [Google Scholar] [CrossRef] [PubMed]
- Barroso, F.G.; de Haro, C.; Sánchez-Muros, M.J.; Venegas, E.; Martínez Sánchez, A.; Pérez-Bañón, C. The potential of various insect species for use as food for fish. Aquaculture 2014, 422–423, 193–201. [Google Scholar] [CrossRef]
- Monteiro dos Santos, D.K.; Santana, T.M.; de Matos Dantas, F.; Farias AB de, S.; Epifânio, C.M.F.; Prestes, A.G.; da Fonseca, F.A.L.; Parisi, G.; Viegas, E.M.M.; Gonçalves, L.U. Defatted black soldier fly larvae meal as a dietary ingredient for tambaqui (Colossoma macropomum): Digestibility, growth performance, haematological parameters, and carcass composition. Aquac. Res. 2022, 53, 6762–6770. [Google Scholar] [CrossRef]
- Veldkamp, T.; van Duinkerken, G.; van Huis, A.; Ottevanger, E.; Bosch, G.; van Boekel, T. Insects as a sustainable feed ingredient in pig and poultry diets: A feasibility study. Food Chem. 2012, 50, 192–195. [Google Scholar]
- Magalhães, R.; Sánchez-López, A.; Leal, R.S.; Martínez-Llorens, S.; Oliva-Teles, A.; Peres, H. Black soldier fly (Hermetia illucens) pre-pupae meal as a fish meal replacement in diets for European seabass (Dicentrarchus labrax). Aquaculture 2017, 476, 79–85. [Google Scholar] [CrossRef]
- Lock, E.R.; Arsiwalla, T.W. Insect larvae meal as an alternative source of nutrients in the diet of Atlantic salmon (Salmo salar) postsmolt. Aquac. Nutr. 2016, 22, 1202–1213. [Google Scholar] [CrossRef]
- St-Hilaire, S.; Sheppard, C.; Tomberlin, J.K.; Irving, S.; Newton, L.; McGuire, M.A.; Mosley, E.E.; Hardy, R.W.; Sealey, W. Fly prepupae as a feedstuff for rainbow trout, Oncorhynchus mykiss. J. World Aquac. Soc. 2007, 38, 59–67. [Google Scholar] [CrossRef]
- Muin, H.; Taufek, N.M.; Kamarudin, M.S.; Razak, S.A. Growth performance, feed utilization and body composition of Nile tilapia, Oreochromis niloticus (Linnaeus, 1758) fed with different levels of black soldier fly, Hermetia illucens (Linnaeus, 1758) maggot meal diet. Iran. J. Fish. Sci. 2017, 16, 567–577. [Google Scholar]
- Gasco, L.; Biasato, I.; Enes, P.; Gai, F. Potential and challenges for the use of insects as feed for aquaculture. In Mass Production of Beneficial Organisms, 2nd ed.; Academic Press: Cambridge, MA, USA; Elsevier Inc.: Amsterdam, Netherlands, 2023; pp. 465–492. [Google Scholar] [CrossRef]
- Hilsdorf, A.W.S.; Hallerman, E.; Valladão, G.M.R.; Zaminhan-Hassemer, M.; Hashimoto, D.T.; Dairiki, J.K.; Takahashi, L.S.; Albergaria, F.C.; Gomes ME de, S.; Venturieri, R.L.L.; et al. The farming and husbandry of Colossoma macropomum: From Amazonian waters to sustainable production. Rev. Aquac. 2022, 14, 993–1027. [Google Scholar] [CrossRef]
- Grim, J.; Jenkins, W.; Tucker, M.E. Routledge Handbook of Religion and Ecology; Routledge: Abingdon, UK, 2017. [Google Scholar] [CrossRef]
- Instituto Brasileiro de Geografia e Estatística (IBGE). 2021. Available online: https://sidra.ibge.gov.br/Tabela/3940 (accessed on 29 November 2022).
- Ordoñez, B.M.; Santana, T.M.; Carneiro, D.P.; dos Santos, D.K.M.; Parra, G.A.P.; Moreno, L.C.C.; Teixeira Filho, N.P.; Aguilar, F.A.A.; Yamamoto, F.Y.; Gonçalves, L.U. Whole black soldier fly larvae (Hermetia illucens) as dietary replacement of extruded feed for tambaqui (Colossoma macropomum) juveniles. Aquac. J. 2022, 2, 14. [Google Scholar] [CrossRef]
- Bureau, D.P.; Harris, A.M.; Cho, C.Y. Apparent digestibility of rendered animal protein ingredients for rainbow trout (Oncorhynchus mykiss). Aquaculture 1999, 180, 345–358. [Google Scholar] [CrossRef]
- Tibbetts, S.M.; Milley, J.E.; Lall, S.P. Apparent protein and energy digestibility of common and alternative feed ingredients by Atlantic cod, Gadus morhua (Linnaeus, 1758). Aquaculture 2006, 261, 1314–1327. [Google Scholar] [CrossRef]
- Furukawa, A.; Hiroko, T. On the acid digestion method for determination of chromic oxide as an indicator substance in the study of digestibility in fish. Bull. Jpn. Soc. Sci. Fish. 1966, 32, 502–506. [Google Scholar] [CrossRef]
- Bureau, D.P.; Hua, K. Letter to the editor of Aquaculture. Aquaculture 2006, 252, 103–105. [Google Scholar] [CrossRef]
- Oishi, C.A.; Nwanna, L.C.; Pereira Filho, M. Optimum dietary protein requirement for Amazonian Tambaqui, Colossoma macropomum Cuvier, 1818, fed fish meal free diets. Acta Amaz. 2010, 40, 757–762. [Google Scholar] [CrossRef]
- Rodrigues, A.P.O. Nutrition and feeding of tambaqui (Colossoma macropomum). Bol. Do Inst. De Pesca 2014, 40, 135–145. [Google Scholar]
- Gomes, L.C.; Chippari-Gomes, A.R.; Lopes, N.P.; Roubach, R.; Araujo-Lima, C.A.R.M. Efficacy of benzocaine as an anesthetic in juvenile tambaqui Colossoma macropomum. J. World Aquac. Soc. 2001, 32, 426–431. [Google Scholar] [CrossRef]
- CONCEA Normative Resolution n. 37, of 15 February 2018. Available online: https://www.mctic.gov.br/mctic/opencms/institucional/concea/paginas/legislacao.html (accessed on 21 July 2022).
- AOAC. Official Methods of Analysis of the AOAC, 18th ed.; AOAC International: Gaithersburg, MD, USA, 2010. [Google Scholar]
- Janssen, R.H.; Vincken, J.P.; Van Den Broek, L.A.M.; Fogliano, V.; Lakemond, C.M.M. Nitrogen-to-protein conversion factors for three edible insects: Tenebrio molitor, Alphitobius diaperinus, and Hermetia illucens. J. Agric. Food Chem. 2017, 65, 2275–2278. [Google Scholar] [CrossRef] [PubMed]
- Abidin, N.A.Z.; Kormin, F.; Abidin, N.A.Z.; Anuar, N.A.F.M.; Bakar, M.F.A. The potential of insects as alternative sources of chitin: An overview on the chemical method of extraction from various sources. Int. J. Mol. Sci. 2020, 21, 4978. [Google Scholar] [CrossRef]
- Kishawy, A.T.Y.; Mohammed, H.A.; Zaglool, A.W.; Attia, M.S.; Hassan, F.A.M.; Roushdy, E.M.; Ismail, T.A.; Ibrahim, D. Partial defatted black solider larvae meal as a promising strategy to replace fish meal protein in diet for Nile tilapia (Oreochromis niloticus): Performance, expression of protein and fat transporters, and cytokines related genes and economic efficiency. Aquaculture 2022, 555, 738195. [Google Scholar] [CrossRef]
- de Farias Lima, J.; Montagner, D.; Duarte, S.S.; Yoshioka, E.T.O.; Dias, M.K.R.; Tavares-Dias, M. Recirculating system using biological aerated filters on tambaqui fingerling farming. Pesqui. Agropecu. Bras. 2019, 54, e00294. [Google Scholar] [CrossRef]
- Sørensen, M. A review of the effects of ingredient composition and processing conditions on the physical qualities of extruded high-energy fish feed as measured by prevailing methods. Aquac. Nutr. 2012, 18, 233–248. [Google Scholar] [CrossRef]
- Tran, G.; Gnaedinger, C.; Mélin, C. Black soldier fly larvae (Hermetia illucens). Feedipedia, a programme by INRAE CIRAD, AFZ and FAO. Last updated on 20 October 2015, 11:10. Available online: https://www.feedipedia.org/node/16388 (accessed on 20 July 2022).
- Fontes, T.V.; de Oliveira, K.R.B.; Almeida, I.L.G.; Orlando, T.M.; Rodrigues, P.B.; da Costa, D.V.; Rosa, P.V. Digestibility of insect meals for Nile tilapia fingerlings. Animals 2019, 9, 181. [Google Scholar] [CrossRef]
- Tanaka, Y.; Tanioka, S.I.; Tanaka, M.; Tanigawa, T.; Kitamura, Y.; Minami, S.; Okamoto, Y.; Miyashita, M.; Nanno, M. Effects of chitin and chitosan particles on BALB/c mice by oral and parenteral administration. Biomaterials 1997, 18, 591–595. [Google Scholar] [CrossRef]
- Ikeda, M.; Kakizaki, H.; Matsumiya, M. Biochemistry of fish stomach chitinase. Int. J. Biol. Macromol. 2017, 104, 1672–1681. [Google Scholar] [CrossRef]
- Oliveira, A.C.B.; Martinelli, L.A.; Moreira, M.Z.; Soares, M.G.M.; Cyrino, J.E.P. Seasonality of energy sources of Colossoma macropomum in a floodplain lake in the Amazon—Lake Camaleão, Amazonas, Brazil. Fish. Manag. Ecol. 2006, 13, 135–142. [Google Scholar] [CrossRef]
- Corrêa, C.F.; de Aguiar, L.H.; Lundstedt, L.M.; Moraes, G. Responses of digestive enzymes of tambaqui (Colossoma macropomum) to dietary cornstarch changes and metabolic inferences. Comp. Biochem. Physiol.—A Mol. Integr. Physiol. 2007, 147, 857–862. [Google Scholar] [CrossRef]
- Weththasinghe, P.; Hansen, J.; Nøkland, D.; Lagos, L.; Rawski, M.; Øverland, M. Full-fat black soldier fly larvae (Hermetia illucens) meal and paste in extruded diets for Atlantic salmon (Salmo salar): Effect on physical pellet quality, nutrient digestibility, nutrient utilization and growth performances. Aquaculture 2021, 530, 735785. [Google Scholar] [CrossRef]
- Ramos-Elorduy, J.; Menzel, P. Creepy Crawly Cuisine: The Gourmet Guide to Edible Insects; Inner Traditions/Bear & Co.: Rochester, VT, USA, 1998. [Google Scholar]
- Nogales-Mérida, S.; Gobbi, P.; Józefiak, D.; Mazurkiewicz, J.; Dudek, K.; Rawski, M.; Kierończyk, B.; Józefiak, A. Insect meals in fish nutrition. Rev. Aquac. 2019, 11, 1080–1103. [Google Scholar] [CrossRef]
- Gutowska, M.A.; Drazen, J.C.; Robison, B.H. Digestive chitinolytic activity in marine fishes of Monterey Bay, California. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2004, 139, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Karlsen, Ø.; Amlund, H.; Berg, A.; Olsen, R.E. The effect of dietary chitin on growth and nutrient digestibility in farmed Atlantic cod, Atlantic salmon and Atlantic halibut. Aquac. Res. 2017, 48, 123–133. [Google Scholar] [CrossRef]
- Limbu, S.M. The effects of on-farm produced feeds on growth, survival, yield and feed cost of juvenile African sharptooth catfish (Clarias gariepinus). Aquac. Fish. 2020, 5, 58–64. [Google Scholar] [CrossRef]
- Limbu, S.M.; Shoko, A.P.; Ulotu, E.E.; Luvanga, S.A.; Munyi, F.M.; John, J.O.; Opiyo, M.A. Black soldier fly (Hermetia illucens, L.) larvae meal improves growth performance, feed efficiency and economic returns of Nile tilapia (Oreochromis niloticus, L.) fry. Aquac. Fish Fish. 2022, 2, 167–178. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).