The Canine Pancreatic Extracellular Matrix in Diabetes Mellitus and Pancreatitis: Its Essential Role and Therapeutic Perspective
Abstract
:Simple Summary
Abstract
1. Introduction
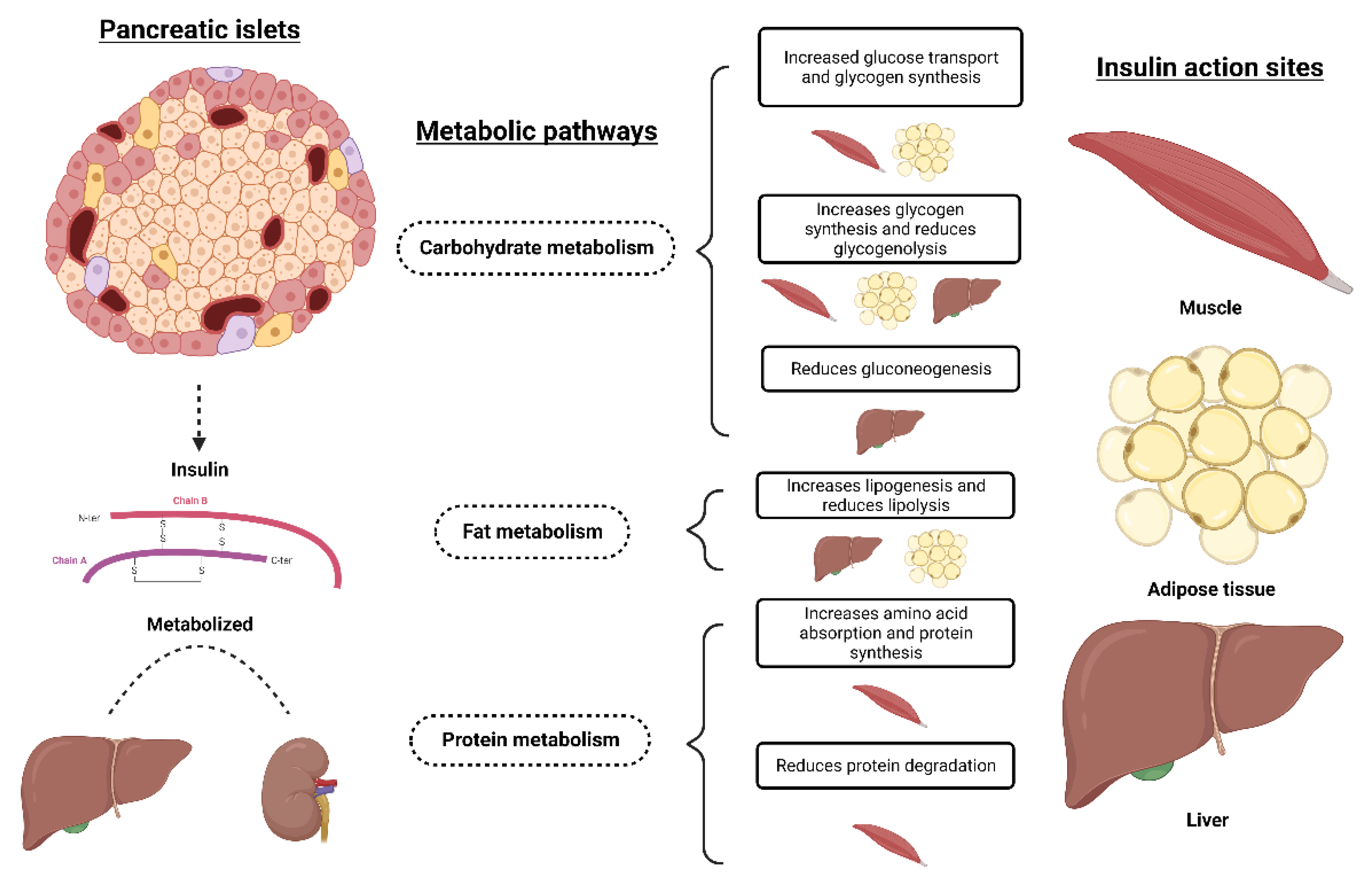
2. Canine Pancreas
3. Diabetes Mellitus
4. Pancreatitis
5. Extracellular Matrix Components
6. Extracellular Matrix Remodeling
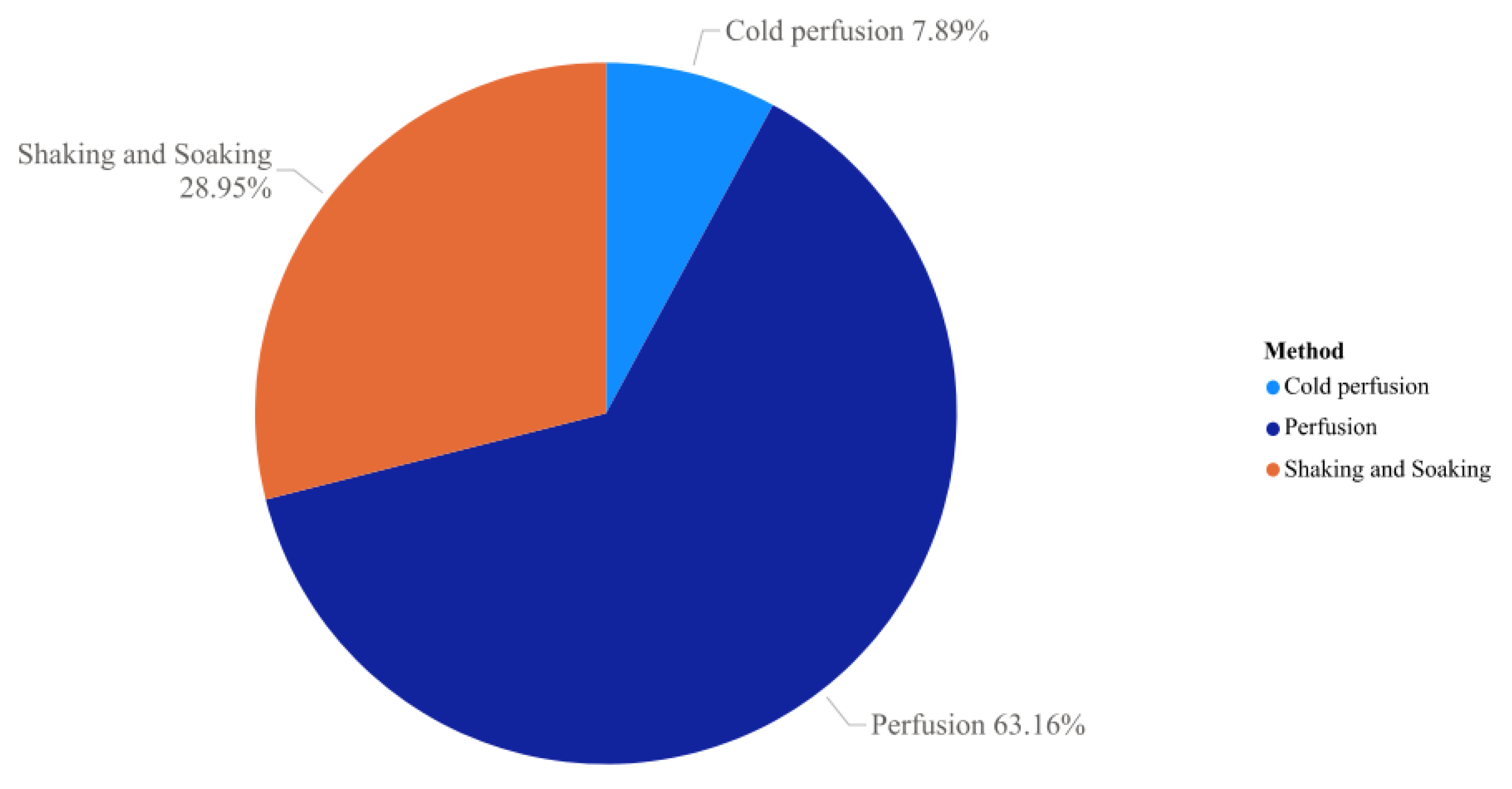
7. Tissue Decellularization
8. Tissue Recellularization
9. Use of Pancreatic ECM as a Therapeutic Possibility for the Treatment of DM and Pancreatitis
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Banfield Pet Hospital. A Obesidade em Animais de Estimação é uma Epidemia de Quarentena? | Banfield Pet Hospital®. Available online: https://www.banfield.com/about-banfield/newsroom/press-releases/2021/new-data-reveals-pet-obesity-epidemic-existed-long-before-quarantine (accessed on 13 December 2021).
- Nelson, R.W.; Maggiore, A.-M. Della. Disorders. In Small Animal Internal Medicine; Elsevier: St. Louis, MO, USA, 2019; pp. 806–856. [Google Scholar]
- Nelson, R.W.; Reusch, C.E. Animal Models of Disease: Classification and Etiology of Diabetes in Dogs and Cats. J. Endocrinol. 2014, 222, T1–T9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- OMIA. Online Mendelian Inheritance in Animals. Available online: http://www.omia.org/home/ (accessed on 12 October 2022).
- Clause, K.C.; Barker, T.H. Extracellular Matrix Signaling in Morphogenesis and Repair. Curr. Opin. Biotechnol. 2013, 24, 830–833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frantz, C.; Stewart, K.M.; Weaver, V.M. The Extracellular Matrix at a Glance. J. Cell Sci. 2010, 123, 4195–4200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Theocharis, A.; Gialeli, C.; Hascall, V.; Karamanos, N.K. Extracellular Matrix: A Functional Scaffold. In Extracellular Matrix: Pathobiology and Signaling; De Gruyter: Berlin, Germany, 2012; pp. 3–19. [Google Scholar] [CrossRef]
- Theocharis, A.D.; Skandalis, S.S.; Gialeli, C.; Karamanos, N.K. Extracellular Matrix Structure. Adv. Drug Deliv. Rev. 2016, 97, 4–27. [Google Scholar] [CrossRef]
- Morais, L.K.; Machado, F.M.E.; Alberto, H.; Coelho, H.E. Estudo Macro e Microscópico de Pâncreas Em Cães. PUBVET 2014, 8, 0084–0229. [Google Scholar] [CrossRef] [Green Version]
- Tonon, B.P.; Bianchi, I. Diferenças Anatômicas do Pulmão, Fígado, rim, baço e Pâncreas Entre Bovinos e Cães Anatomical Differences of the Lung, Liver, Kidney, Base and Pancreas between Bovine and Dogs. Rev. Dimens. Acad. 2018, 3, 115–129. [Google Scholar]
- Dyce, K.; Sack, W.; Wensing, C. Textbook of Veterinary Anatomy, 5th ed.; Elsevier/Saunders: St. Louis, MO, USA, 2016. [Google Scholar]
- Hyttel, P.; Sinowatz, F.; Vejlsted, M. Essentials of Domestic Animal Embryology, 1st ed.; Elsevier/Saunders: Edinburgh, UK, 2010. [Google Scholar]
- Sisson, S.; Grossman, J. Anatomía de Los Animales Domésticos, 5th ed.; Elsevier Masson: Issy Les Moulineaux, France, 1982. [Google Scholar]
- Maiochi, A.M.; Machado, D.C.; Daineze, V.H.; Romão, F.G. Diabetes Mellitus em Cães e Gatos: Revisão de Literatura Diabetes Mellitus in Dogs and Cats: A Review. Alm. Med. Vet. Zoo. 2015, 1, 1–8. [Google Scholar]
- Junqueira, L.C.U.; Carneiro, J.; Abrahamsohn, P. Histologia Básica: Texto e Atlas, 13th ed.; Guanabara Koogan: Rio de Janeiro, Brazil, 2017. [Google Scholar]
- Colville, T.; Bassert, J.M. Clinical Anatomy and Physiology for Veterinary Technicians, 3rd ed.; Elsevier/Mosby: St. Louis, MO, USA, 2015. [Google Scholar]
- Bonner-Weir, S. Islets of Langerhans: Morphology and Postnatal Growth. In Joslin’s Diabetes Mellitus; Lippincott Williams and Wilkins: Boston, MA, USA, 2005; pp. 41–50. [Google Scholar]
- Martin, P.A.; Crump, M.H. The Endocrine Pancreas. In McDonald’s Veterinary Endocrinology and Reproduction; Iowa State Press: Ames, IA, USA, 2003; pp. 141–160. [Google Scholar]
- Steiner, D.J.; Kim, A.; Miller, K.; Hara, M. Pancreatic Islet Plasticity: Interspecies Comparison of Islet Architecture and Composition. Islets 2010, 2, 135–145. [Google Scholar] [CrossRef] [Green Version]
- Faria, P.F. Diabetes Mellitus Em Cães. Acta Vet. Bras. 2007, 1, 8–22. [Google Scholar] [CrossRef]
- Cunningham, J.G.; Klein, B.G. Textbook of Veterinary Physiology, 6th ed.; Elsevier/Saunders: St. Louis, MO, USA, 2020. [Google Scholar]
- Catchpole, B.; Ristic, J.M.; Fleeman, L.M.; Davison, L.J. Canine Diabetes Mellitus: Can Old Dogs Teach Us New Tricks? Diabetologia 2005, 48, 1948–1956. [Google Scholar] [CrossRef]
- Jericó, M.; Marco, V. Insulina e Hipoglicemiantes Orais. In Farmacologia Aplicada à Medicina Veterinária; Guanabara Koogan: Rio de Janeiro, Brazil, 2017; pp. 619–935. [Google Scholar]
- Redondo, M.J.; Eisenbarth, G.S. Genetic Control of Autoimmunity in Type I Diabetes and Associated Disorders. Diabetologia 2002, 45, 605–622. [Google Scholar] [CrossRef] [PubMed]
- Rand, J.S.; Fleeman, L.M.; Farrow, H.A.; Appleton, D.J.; Lederer, R. Canine and Feline Diabetes Mellitus: Nature or Nurture? J. Nutr. 2004, 134, 2072S–2080S. [Google Scholar] [CrossRef] [Green Version]
- Fleeman, L.M.; Rand, J.S. Management of Canine Diabetes. Vet. Clin. N. Am. Small Anim. Pract. 2001, 31, 855–880. [Google Scholar] [CrossRef] [PubMed]
- Hoenig, M. Comparative Aspects of Diabetes Mellitus in Dogs and Cats. Mol. Cell. Endocrinol. 2002, 197, 221–229. [Google Scholar] [CrossRef] [PubMed]
- ESVE. Project ALIVE | ESVE. Available online: https://esve.org/alive/intro.aspx (accessed on 13 December 2021).
- Verkest, K.R.; Rand, J.S.; Fleeman, L.M.; Morton, J.M. Spontaneously Obese Dogs Exhibit Greater Postprandial Glucose, Triglyceride, and Insulin Concentrations than Lean Dogs. Domest. Anim. Endocrinol. 2012, 42, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Borin-Crivellenti, S. Endocrinologia. In Casos de Rotina em Medicina Veterinária de Pequenos Animais; MedVet: São Paulo, Brazil, 2015; pp. 231–272. [Google Scholar]
- Davison, L.J. Canine Diabetes Mellitus. In Bsava Manual of Canine and Feline Endocrinology, 4th ed.; British Small Animal Veterinary Association: Gloucester, UK, 2012; pp. 184–206. [Google Scholar]
- Heeley, A.M.; O’Neill, D.G.; Davison, L.J.; Church, D.B.; Corless, E.K.; Brodbelt, D.C. Diabetes Mellitus in Dogs Attending UK Primary-Care Practices: Frequency, Risk Factors and Survival. Canine Med. Genet. 2020, 7, 6. [Google Scholar] [CrossRef]
- Eigenmann, J.E.; Eigenmann, R.Y.; Rijnberk, A.; van der Gaag, I.; Zapf, J.; Froesch, E.R. Progesterone-Controlled Growth Hormone Overproduction and Naturally Occurring Canine Diabetes and Acromegaly. Acta Endocrinol. 1983, 104, 167–176. [Google Scholar] [CrossRef]
- Todd, J.A.; Bell, J.I.; McDevitt, H.O. HLA-DQβ Gene Contributes to Susceptibility and Resistance to Insulin-Dependent Diabetes Mellitus. Nature 1987, 329, 599–604. [Google Scholar] [CrossRef]
- Kennedy, L.J.; Davison, L.J.; Barnes, A.; Short, A.D.; Fretwell, N.; Jones, C.A.; Lee, A.C.; Ollier, W.E.R.; Catchpole, B. Identification of Susceptibility and Protective Major Histocompatibility Complex Haplotypes in Canine Diabetes Mellitus. Tissue Antigens 2006, 68, 467. [Google Scholar] [CrossRef]
- Short, A.D.; Catchpole, B.; Kennedy, L.J.; Barnes, A.; Lee, A.C.; Jones, C.A.; Fretwell, N.; Ollier, W.E.R. T Cell Cytokine Gene Polymorphisms in Canine Diabetes Mellitus. Vet. Immunol. Immunopathol. 2009, 128, 137–146. [Google Scholar] [CrossRef]
- Dawra, R.; Sah, R.P.; Dudeja, V.; Rishi, L.; Talukdar, R.; Garg, P.; Saluja, A.K. Intra-Acinar Trypsinogen Activation Mediates Early Stages of Pancreatic Injury but Not Inflammation in Mice With Acute Pancreatitis. Gastroenterology 2011, 141, 2210–2217.e2. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Liu, Y.; Daniluk, J.; Gaiser, S.; Chu, J.; Wang, H.; Li, Z.; Logsdon, C.D.; Ji, B. Activation of Nuclear Factor-ΚB in Acinar Cells Increases the Severity of Pancreatitis in Mice. Gastroenterology 2013, 144, 202–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lack, E. Pathology of the Pancreas, Gallbladder, Extrahepatic Biliary Tract, and Ampullary Region; Oxford University Press: New York, NY, USA, 2003. [Google Scholar]
- Zhan, X.; Wang, F.; Bi, Y.; Ji, B. Animal Models of Gastrointestinal and Liver Diseases. Animal Models of Acute and Chronic Pancreatitis. Am. J. Physiol.—Gastrointest. Liver Physiol. 2016, 311, G343–G355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watson, P. Chronic Pancreatitis in Dogs. Top. Companion Anim. Med. 2012, 27, 133–139. [Google Scholar] [CrossRef]
- Santos, R.L.; Alessi, A.C. Patologia Veterinária, 2nd ed.; Roca: Rio de Janeiro, Brazil, 2016. [Google Scholar]
- Nelson, R.W.; Couto, C.G. Medicina Interna de Pequenos Animais, 5th ed.; Elsevier: Rio de Janeiro, Brazil, 2015. [Google Scholar]
- Bishop, M.A.; Xenoulis, P.G.; Levinski, M.D.; Suchodolski, J.S.; Steiner, J.M. Identification of Variants of the SPINK1 Gene and Their Association with Pancreatitis in Miniature Schnauzers. Am. J. Vet. Res. 2010, 71, 527–533. [Google Scholar] [CrossRef]
- Zadrozny Gouvêa da Costa, M.; Guarita, D.R.; Ono-Nita, S.K.; Paranaguá-Vezozzo, D.C.; Gonçalves Felga, G.E.; Arcon Pedroso, M.R.; de Souza, M.M.T.; Nasser, P.D.; Da Silva Ferreira, C.; Carrilho, F.J. Genetic Risk for Alcoholic Chronic Pancreatitis. Int. J. Environ. Res. Public Health 2011, 8, 2747–2757. [Google Scholar] [CrossRef] [Green Version]
- Cridge, H.; Twedt, D.C.; Marolf, A.J.; Sharkey, L.C.; Steiner, J.M. Advances in the Diagnosis of Acute Pancreatitis in Dogs. J. Vet. Intern. Med. 2021, 35, 2572. [Google Scholar] [CrossRef]
- João, C.F. Gastroenterologia e Hepatologia. In Casos de Rotina em Medicina Veterinária de Pequenos Animais; MedVet: São Paulo, Brazil, 2015; pp. 309–351. [Google Scholar]
- Windsor, J.A.; Hammodat, H. Metabolic Management of Severe Acute Pancreatitis. World J. Surg. 2000, 24, 664–672. [Google Scholar] [CrossRef]
- Mansfield, C. Pathophysiology of Acute Pancreatitis: Potential Application from Experimental Models and Human Medicine to Dogs. J. Vet. Intern. Med. 2012, 26, 875–887. [Google Scholar] [CrossRef]
- Fabrès, V.; Dossin, O.; Reif, C.; Campos, M.; Freiche, V.; Maurey, C.; Pilot-Storck, F.; Desquilbet, L.; Benchekroun, G. Development and Validation of a Novel Clinical Scoring System for Short-term Prediction of Death in Dogs with Acute Pancreatitis. J. Vet. Intern. Med. 2019, 33, 499. [Google Scholar] [CrossRef] [Green Version]
- Washabau, R.J.; Xenoulis, P.G.; Steiner, J.M.; Schaer, M.; Wiberg, M.; Axiak, S.; Hahn, K. Pancreas. In Canine Feline Gastroenterol; W.B. Saunders: Philadelphia, PA, USA, 2013; pp. 799–848. [Google Scholar] [CrossRef]
- Faulk, D.M.; Wildemann, J.D.; Badylak, S.F. Decellularization and Cell Seeding of Whole Liver Biologic Scaffolds Composed of Extracellular Matrix. J. Clin. Exp. Hepatol. 2015, 5, 69–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stendahl, J.C.; Kaufman, D.B.; Stupp, S.I. Extracellular Matrix in Pancreatic Islets: Relevance to Scaffold Design and Transplantation. Cell Transplant. 2009, 18, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Llacua, L.A.; Faas, M.M.; de Vos, P. Extracellular Matrix Molecules and Their Potential Contribution to the Function of Transplanted Pancreatic Islets. Diabetologia 2018, 61, 1261. [Google Scholar] [CrossRef] [Green Version]
- White, S.A.; Hughes, D.P.; Contractor, H.H.; London, N.J.M. An Investigation into the Distribution of Different Collagen Types within Adult and Juvenile Porcine Pancreata. J. Mol. Med. 1999, 77, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Hughes, S.J.; Clark, A.; McShane, P.; Contractor, H.H.; Gray, D.W.R.; Johnson, P.R.V. Characterisation of Collagen VI within the Islet-Exocrine Interface of the Human Pancreas: Implications for Clinical Islet Isolation? Transplantation 2006, 81, 423–426. [Google Scholar] [CrossRef]
- Otonkoski, T.; Banerjee, M.; Korsgren, O.; Thornell, L.E.; Virtanen, I. Unique Basement Membrane Structure of Human Pancreatic Islets: Implications for β-Cell Growth and Differentiation. Diabetes Obes. Metab. 2008, 10 (Suppl. 4), 119–127. [Google Scholar] [CrossRef]
- Lin, H.Y.; Tsai, C.C.; Chen, L.L.; Chiou, S.H.; Wang, Y.J.; Hung, S.C. Fibronectin and Laminin Promote Differentiation of Human Mesenchymal Stem Cells into Insulin Producing Cells through Activating Akt and ERK. J. Biomed. Sci. 2010, 17, 56. [Google Scholar] [CrossRef] [Green Version]
- Leite, A.R.; Corrêa-Giannella, M.L.; Dagli, M.L.Z.; Fortes, M.A.Z.; Vegas, V.M.T.; Giannella-Neto, D. Fibronectin and Laminin Induce Expression of Islet Cell Markers in Hepatic Oval Cells in Culture. Cell Tissue Res. 2007, 327, 529–537. [Google Scholar] [CrossRef]
- Riopel, M.; Stuart, W.; Wang, R. Fibrin Improves Beta (INS-1) Cell Function, Proliferation and Survival through Integrin Avβ3. Acta Biomater. 2013, 9, 8140–8148. [Google Scholar] [CrossRef]
- Park, S.H.; Park, S.R.; Chung, S., II; Pai, K.S.; Min, B.H. Tissue-Engineered Cartilage Using Fibrin/Hyaluronan Composite Gel and Its In Vivo Implantation. Artif. Organs 2005, 29, 838–845. [Google Scholar] [CrossRef]
- Weber, L.M.; Hayda, K.N.; Anseth, K.S. Cell–Matrix Interactions Improve β-Cell Survival and Insulin Secretion in Three-Dimensional Culture. Tissue Eng. Part A 2008, 14, 1959–1968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, P.; Takai, K.; Weaver, V.M.; Werb, Z. Extracellular Matrix Degradation and Remodeling in Development and Disease. Cold Spring Harb. Perspect. Biol. 2011, 3, a005058. [Google Scholar] [CrossRef] [PubMed]
- Bonnans, C.; Chou, J.; Werb, Z. Remodelling the Extracellular Matrix in Development and Disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 786–801. [Google Scholar] [CrossRef]
- Page-McCaw, A.; Ewald, A.J.; Werb, Z. Matrix Metalloproteinases and the Regulation of Tissue Remodelling. Nat. Rev. Mol. Cell Biol. 2007, 8, 221–233. [Google Scholar] [CrossRef]
- Roy, R.; Morad, G.; Jedinak, A.; Moses, M.A. Metalloproteinases and Their Roles in Human Cancer. Anat. Rec. 2020, 303, 1557–1572. [Google Scholar] [CrossRef]
- Nyren-Erickson, E.K.; Jones, J.M.; Srivastava, D.K.; Mallik, S. A Disintegrin and Metalloproteinase-12 (ADAM12): Function, Roles in Disease Progression, and Clinical Implications. Biochim. Biophys. Acta 2013, 1830, 4445–4455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kruse, M.N.; Becker, C.; Lottaz, D.; Köhler, D.; Yiallouros, I.; Krell, H.W.; Sterchi, E.E.; Stöcker, W. Human Meprin Alpha and Beta Homo-Oligomers: Cleavage of Basement Membrane Proteins and Sensitivity to Metalloprotease Inhibitors. Biochem. J. 2004, 378 Pt 2, 383. [Google Scholar] [CrossRef] [Green Version]
- Hynes, R.O. The Extracellular Matrix: Not Just Pretty Fibrils. 2009, 326, 1216–1219. Science 2009, 326, 1216–1219. [Google Scholar] [CrossRef] [Green Version]
- Hayden, M.R.; Sowers, J.R. Isletopathy in Type 2 Diabetes Mellitus: Implications of Islet RAS, Islet Fibrosis, Islet Amyloid, Remodeling, and Oxidative Stress. Antioxid. Redox Signal. 2007, 9, 891–910. [Google Scholar] [CrossRef]
- Hayden, M.R.; Patel, K.; Habibi, J.; Gupta, D.; Tekwani, S.S.; Whaley-Connell, A.; Sowers, J.R. Attenuation of Endocrine-Exocrine Pancreatic Communication in Type 2 Diabetes: Pancreatic Extracellular Matrix Ultrastructural Abnormalities. J. Cardiometab. Syndr. 2008, 3, 234–243. [Google Scholar] [CrossRef]
- Whaley-Connell, A.; Govindarajan, G.; Habibi, J.; Hayden, M.R.; Cooper, S.A.; Wei, Y.; Ma, L.; Qazi, M.; Link, D.; Karuparthi, P.R.; et al. Angiotensin II-Mediated Oxidative Stress Promotes Myocardial Tissue Remodeling in the Transgenic (MRen2) 27 Ren2 Rat. Am. J. Physiol. Metab. 2007, 293, E355–E363. [Google Scholar] [CrossRef] [PubMed]
- Meyer, S.R.; Chiu, B.; Churchill, T.A.; Zhu, L.; Lakey, J.R.T.; Ross, D.B. Comparison of Aortic Valve Allograft Decellularization Techniques in the Rat. J. Biomed. Mater. Res.—Part A 2006, 79, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Hayden, M.R.; Karuparthi, P.R.; Habibi, J.; Lastra, G.; Patel, K.; Wasekar, C.; Manrique, C.M.; Ozerdem, U.; Stas, S.; Sowers, J.R. Ultrastructure of Islet Microcirculation, Pericytes and the Islet Exocrine Interface in the HIP Rat Model of Diabetes. Exp. Biol. Med. 2008, 233, 1109–1123. [Google Scholar] [CrossRef] [Green Version]
- Yoon, J.-W.; Jun, H.-S. Cellular and Molecular Pathogenic Mechanisms of Insulin-Dependent Diabetes Mellitus. Ann. N. Y. Acad. Sci. 2006, 928, 200–211. [Google Scholar] [CrossRef]
- Descamps, F.J.; Van Den Steen, P.E.; Martens, E.; Ballaux, F.; Geboes, K.; Opdenakker, G. Gelatinase B Is Diabetogenic in Acute and Chronic Pancreatitis by Cleaving Insulin. FASEB J. 2003, 17, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, J.D.; Yang, S.; Zhang, J.; Zhu, T.H. BMP6 Reverses TGF-Β1-Induced Changes in HK-2 Cells: Implications for the Treatment of Renal Fibrosis. Acta Pharmacol. Sin. 2009, 30, 994. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.-H.; Mellado-Gil, J.M.; Bahn, Y.J.; Pathy, S.M.; Zhang, Y.E.; Rane, S.G. Protection from β-Cell Apoptosis by Inhibition of TGF-β/Smad3 Signaling. Cell Death Dis. 2020, 11, 184. [Google Scholar] [CrossRef] [Green Version]
- Peloso, A.; Citro, A.; Oldani, G.; Brambilla, S.; Piemonti, L.; Cobianchi, L. Bioengineering the Pancreas: Cell-on-Scaffold Technology. In Scaffolds in Tissue Engineering—Materials, Technologies and Clinical Applications; InTech: Rang-Du-Fliers, France, 2017. [Google Scholar] [CrossRef] [Green Version]
- Gilbert, T.; Sellaro, T.; Badylak, S. Decellularization of Tissues and Organs. Biomaterials 2006, 27, 3675–3683. [Google Scholar] [CrossRef]
- Asthana, A.; Tamburrini, R.; Chaimov, D.; Gazia, C.; Walker, S.J.; Van Dyke, M.; Tomei, A.; Lablanche, S.; Robertson, J.; Opara, E.C.; et al. Comprehensive Characterization of the Human Pancreatic Proteome for Bioengineering Applications. Biomaterials 2021, 270, 120613. [Google Scholar] [CrossRef]
- Sackett, S.D.; Tremmel, D.M.; Ma, F.; Feeney, A.K.; Maguire, R.M.; Brown, M.E.; Zhou, Y.; Li, X.; O’Brien, C.; Li, L.; et al. Extracellular Matrix Scaffold and Hydrogel Derived from Decellularized and Delipidized Human Pancreas. Sci. Rep. 2018, 8, 10452. [Google Scholar] [CrossRef] [Green Version]
- Ma, F.; Sun, R.; Tremmel, D.M.; Sackett, S.D.; Odorico, J.; Li, L. Large-Scale Differentiation and Site Specific Discrimination of Hydroxyproline Isomers by Electron Transfer/Higher-Energy Collision Dissociation (EThcD) Mass Spectrometry. Anal. Chem. 2018, 90, 5857. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Tremmel, D.M.; Li, Z.; Lietz, C.B.; Sackett, S.D.; Odorico, J.S.; Li, L. In Depth Quantification of Extracellular Matrix Proteins from Human Pancreas. J. Proteome Res. 2019, 18, 3156–3165. [Google Scholar] [CrossRef] [PubMed]
- Tamburrini, R.; Chaimov, D.; Asthana, A.; Enck, K.; Muir, S.M.; Aziz, J.M.; Lablanche, S.; Tubbs, E.; Tomei, A.A.; Dyke, M.; et al. Detergent-Free Decellularization of the Human Pancreas for Soluble Extracellular Matrix (Ecm) Production. J. Vis. Exp. 2020, 2020, e61663. [Google Scholar] [CrossRef]
- Berman, A.; Klak, M.; Adamiok, A.; Kaczyński, Ł.; Tymicki, G.; Gomółka, M.; Kowalska, P.; Kosowska, K.; Cywoniuk, P.; Turowski, P.; et al. The Influence of the Flow of Detergent and Donor Characteristics on the Extracellular Matrix Composition After Human Pancreas Decellularization. Transplant. Proc. 2020, 52, 2043–2049. [Google Scholar] [CrossRef]
- Peloso, A.; Urbani, L.; Cravedi, P.; Katari, R.; Maghsoudlou, P.; Fallas, M.E.A.; Sordi, V.; Citro, A.; Purroy, C.; Niu, G.; et al. The Human Pancreas as a Source of Protolerogenic Extracellular Matrix Scaffold for a New-Generation Bioartificial Endocrine Pancreas. Ann. Surg. 2016, 264, 169–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uday Chandrika, K.; Tripathi, R.; Kameshwari, Y.; Rangaraj, N.; Mahesh Kumar, J.; Singh, S. Refunctionalization of Decellularized Organ Scaffold of Pancreas by Recellularization: Whole Organ Regeneration into Functional Pancreas. Tissue Eng. Regen. Med. 2021, 18, 99–112. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Chen, Y.; Kong, H.; He, Q.; Sun, H.; Bhugul, P.A.; Zhang, Q.; Chen, B.; Zhou, M. The Rat Pancreatic Body Tail as a Source of a Novel Extracellular Matrix Scaffold for Endocrine Pancreas Bioengineering. J. Biol. Eng. 2018, 12, 6. [Google Scholar] [CrossRef] [Green Version]
- Goh, S.K.; Bertera, S.; Olsen, P.; Candiello, J.E.; Halfter, W.; Uechi, G.; Balasubramani, M.; Johnson, S.A.; Sicari, B.M.; Kollar, E.; et al. Perfusion-Decellularized Pancreas as a Natural 3D Scaffold for Pancreatic Tissue and Whole Organ Engineering. Biomaterials 2013, 34, 6760–6772. [Google Scholar] [CrossRef] [Green Version]
- Napierala, H.; Hillebrandt, K.-H.; Haep, N.; Tang, P.; Tintemann, M.; Gassner, J.; Noesser, M.; Everwien, H.; Seiffert, N.; Kluge, M.; et al. Engineering an Endocrine Neo-Pancreas by Repopulation of a Decellularized Rat Pancreas with Islets of Langerhans. Sci. Rep. 2017, 7, 41777. [Google Scholar] [CrossRef]
- Baptista, P.M.; Orlando, G.; Mirmalek-Sani, S.-H.; Siddiqui, M.; Atala, A.; Soker, S. Whole Organ Decellularization—A Tool for Bioscaffold Fabrication and Organ Bioengineering. In Proceedings of the 2009 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Minneapolis, MN, USA, 3–6 September 2009; IEEE: Piscataway, NJ, USA, 2009; Volume 2009, pp. 6526–6529. [Google Scholar] [CrossRef]
- Katsuki, Y.; Yagi, H.; Okitsu, T.; Kitago, M.; Tajima, K.; Kadota, Y.; Hibi, T.; Abe, Y.; Shinoda, M.; Itano, O.; et al. Endocrine Pancreas Engineered Using Porcine Islets and Partial Pancreatic Scaffolds. Pancreatology 2016, 16, 922–930. [Google Scholar] [CrossRef]
- Gaetani, R.; Aude, S.; Demaddalena, L.L.; Strassle, H.; Dzieciatkowska, M.; Wortham, M.; Bender, R.H.F.; Nguyen-Ngoc, K.V.; Schmid-Schöenbein, G.W.; George, S.C.; et al. Evaluation of Different Decellularization Protocols on the Generation of Pancreas-Derived Hydrogels. Tissue Eng.—Part C Methods 2018, 24, 697–708. [Google Scholar] [CrossRef] [PubMed]
- Klak, M.; Łojszczyk, I.; Berman, A.; Tymicki, G.; Adamiok-Ostrowska, A.; Sierakowski, M.; Olkowski, R.; Szczepankiewicz, A.A.; Kamiński, A.; Dobrzyń, A.; et al. Impact of Porcine Pancreas Decellularization Conditions on the Quality of Obtained DECM. Int. J. Mol. Sci. 2021, 22, 7005. [Google Scholar] [CrossRef] [PubMed]
- Elebring, E.; Kuna, V.K.; Kvarnström, N.; Sumitran-Holgersson, S. Cold-Perfusion Decellularization of Whole-Organ Porcine Pancreas Supports Human Fetal Pancreatic Cell Attachment and Expression of Endocrine and Exocrine Markers. J. Tissue Eng. 2017, 8, 204173141773814. [Google Scholar] [CrossRef]
- Kuna, V.K.; Kvarnström, N.; Elebring, E.; Holgersson, S.S. Isolation and Decellularization of a Whole Porcine Pancreas. J. Vis. Exp. 2018, 2018, 58302. [Google Scholar] [CrossRef] [Green Version]
- Dong, X.; Wei, X.; Yi, W.; Gu, C.; Kang, X.; Liu, Y.; Li, Q.; Yi, D. RGD-Modified Acellular Bovine Pericardium as a Bioprosthetic Scaffold for Tissue Engineering. J. Mater. Sci. Mater. Med. 2009, 20, 2327–2336. [Google Scholar] [CrossRef]
- Reing, J.E.; Brown, B.N.; Daly, K.A.; Freund, J.M.; Gilbert, T.W.; Hsiong, S.X.; Huber, A.; Kullas, K.E.; Tottey, S.; Wolf, M.T. The Effects of Processing Methods upon Mechanical and Biologic Properties of Porcine Dermal Extracellular Matrix Scaffolds. Biomaterials 2010, 31, 8626–8633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orwick-Rydmark, M.; Arnold, T.; Linke, D. The Use of Detergents to Purify Membrane Proteins. Curr. Protoc. Protein Sci. 2016, 2016, 4–8. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Ko, H.; Kwon, I.K.; Shin, K. Extracellular Matrix Revisited: Roles in Tissue Engineering. Int. Neurourol. J. 2016, 20 (Suppl. 1), S23–S29. [Google Scholar] [CrossRef]
- Peloso, A.; Katari, R.; Zambon, J.P.; Defrancesco, A.; Moore, A.; Holton, C.; Mogul, A.; Manzia, T.M.; Orlando, G. Abdominal Organ Bioengineering: Current Status and Future Perspectives. Minerva Chir. 2015, 70, 43–55. [Google Scholar]
- Rieder, E.; Kasimir, M.T.; Silberhumer, G.; Seebacher, G.; Wolner, E.; Simon, P.; Weigel, G. Decellularization Protocols of Porcine Heart Valves Differ Importantly in Efficiency of Cell Removal and Susceptibility of the Matrix to Recellularization with Human Vascular Cells. J. Thorac. Cardiovasc. Surg. 2004, 127, 399–405. [Google Scholar] [CrossRef] [Green Version]
- He, M.; Callanan, A.; Lagaras, K.; Steele, J.A.M.; Stevens, M.M. Optimization of SDS Exposure on Preservation of ECM Characteristics in Whole Organ Decellularization of Rat Kidneys. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 1352–1360. [Google Scholar] [CrossRef] [PubMed]
- Woods, T.; Gratzer, P.F. Effectiveness of Three Extraction Techniques in the Development of a Decellularized Bone-Anterior Cruciate Ligament-Bone Graft. Biomaterials 2005, 26, 7339–7349. [Google Scholar] [CrossRef]
- Keane, T.J.; Swinehart, I.T.; Badylak, S.F. Methods of Tissue Decellularization Used for Preparation of Biologic Scaffolds and in Vivo Relevance. Methods 2015, 84, 25–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roth, S.P.; Glauche, S.M.; Plenge, A.; Erbe, I.; Heller, S.; Burk, J. Automated Freeze-Thaw Cycles for Decellularization of Tendon Tissue—A Pilot Study. BMC Biotechnol. 2017, 17, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grauss, R.; Hazekamp, M.; Oppenhuizen, F.; Vanmunsteren, C.; Gittenbergerdegroot, A.; Deruiter, M. Histological Evaluation of Decellularised Porcine Aortic Valves: Matrix Changes Due to Different Decellularisation Methods. Eur. J. Cardio-Thoracic Surg. 2005, 27, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Pellegata, A.F.; Asnaghi, M.A.; Zonta, S.; Zerbini, G.; Mantero, S. A Novel Device for the Automatic Decellularization of Biological Tissues. Int. J. Artif. Organs 2012, 35, 191–198. [Google Scholar] [CrossRef]
- Fu, Y.; Fan, X.; Tian, C.; Luo, J.; Zhang, Y.; Deng, L.; Qin, T.; Lv, Q. Decellularization of Porcine Skeletal Muscle Extracellular Matrix for the Formulation of a Matrix Hydrogel: A Preliminary Study. J. Cell. Mol. Med. 2016, 20, 740–749. [Google Scholar] [CrossRef]
- Funamoto, S.; Nam, K.; Kimura, T.; Murakoshi, A.; Hashimoto, Y.; Niwaya, K.; Kitamura, S.; Fujisato, T.; Kishida, A. The Use of High-Hydrostatic Pressure Treatment to Decellularize Blood Vessels. Biomaterials 2010, 31, 3590–3595. [Google Scholar] [CrossRef]
- Tudorache, I.; Cebotari, S.; Sturz, G.; Kirsch, L.; Hurschler, C.; Hilfiker, A.; Haverich, A.; Lichtenberg, A. Tissue Engineering of Heart Valves: Biomechanical and Morphological Properties of Decellularized Heart Valves. J. Heart Valve Dis. 2007, 16, 567–573; discussion 574. [Google Scholar]
- Wainwright, J.M.; Czajka, C.A.; Patel, U.B.; Freytes, D.O.; Tobita, K.; Gilbert, T.W.; Badylak, S.F. Preparation of Cardiac Extracellular Matrix from an Intact Porcine Heart. Tissue Eng.—Part C Methods 2010, 16, 525–532. [Google Scholar] [CrossRef]
- Gui, L.; Chan, S.A.; Breuer, C.K.; Niklason, L.E. Novel Utilization of Serum in Tissue Decellularization. Tissue Eng.—Part C Methods 2010, 16, 173–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steffens, D.; Braghirolli, D.I.; Maurmann, N.; Pranke, P. Update on the Main Use of Biomaterials and Techniques Associated with Tissue Engineering. Drug Discov. Today 2018, 23, 1474–1488. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Wang, L.; Huang, Y.; Fan, H.; Chen, C.; Yuan, X.; Guo, Y.; Yin, L. Using GRGDSPC Peptides to Improve Re-Endothelialization of Decellularized Pancreatic Scaffolds. Artif. Organs 2020, 44, E172–E180. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Qiu, H.; Wang, D.; Miao, H.; Zhu, Y.; Guo, Q.; Guo, Y.; Wang, Z. Enhanced Vascularization and Biocompatibility of Rat Pancreatic Decellularized Scaffolds Loaded with Platelet-Rich Plasma. J. Biomater. Appl. 2020, 35, 313–330. [Google Scholar] [CrossRef]
- Xu, L.; Guo, Y.; Huang, Y.; Xiong, Y.; Xu, Y.; Li, X.; Lu, J.; Wang, L.; Wang, Y.; Lu, Y.; et al. Constructing Heparin-Modified Pancreatic Decellularized Scaffold to Improve Its Re-Endothelialization. J. Biomater. Appl. 2018, 32, 1063–1070. [Google Scholar] [CrossRef]
- Huang, Y.; Mei, J.; Yu, Y.; Ding, Y.; Xia, W.; Yue, T.; Chen, W.; Zhou, M.; Yang, Y. Comparative Decellularization and Recellularization of Normal Versus Streptozotocin-Induced Diabetes Mellitus Rat Pancreas. Artif. Organs 2019, 43, 399–412. [Google Scholar] [CrossRef]
- Citro, A.; Moser, P.T.; Dugnani, E.; Rajab, T.K.; Ren, X.; Evangelista-Leite, D.; Charest, J.M.; Peloso, A.; Podesser, B.K.; Manenti, F.; et al. Biofabrication of a Vascularized Islet Organ for Type 1 Diabetes. Biomaterials 2019, 199, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, M.; Hwang, D.G.; Shim, I.K.; Kim, S.C.; Jang, J. Pancreatic Tissue-Derived Extracellular Matrix Bioink for Printing 3D Cell-Laden Pancreatic Tissue Constructs. J. Vis. Exp. 2019, 2019, e60434. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Chaimov, D.; Patel, S.N.; Liang, J.-P.; Wiggins, S.C.; Samojlik, M.M.; Rubiano, A.; Simmons, C.S.; Stabler, C.L. 3-D Physiomimetic Extracellular Matrix Hydrogels Provide a Supportive Microenvironment for Rodent and Human Islet Culture. Biomaterials 2019, 198, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Guruswamy Damodaran, R.; Vermette, P. Decellularized Pancreas as a Native Extracellular Matrix Scaffold for Pancreatic Islet Seeding and Culture. J. Tissue Eng. Regen. Med. 2018, 12, 1230–1237. [Google Scholar] [CrossRef]
- Mahmoud, A.I.; Galdos, F.X.; Dinan, K.A.; Jedrychowski, M.P.; Davis, J.C.; Vujic, A.; Rachmin, I.; Shigley, C.; Pancoast, J.R.; Lee, S.; et al. Apolipoprotein E Is a Pancreatic Extracellular Factor That Maintains Mature β-Cell Gene Expression. PLoS ONE 2018, 13, e0204595. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Chen, S.; Xu, L.; Huang, Y.; Xu, Y.; Lu, Y.; Wang, Z. Decellularized and Solubilized Pancreatic Stroma Promotes the in Vitro Proliferation, Migration and Differentiation of BMSCs into IPCs. Cell Tissue Bank. 2019, 20, 389–401. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Huang, Y.; Wang, D.; Zhu, S.; Wang, Z.; Yang, Y.; Guo, Y. Reseeding Endothelial Cells with Fibroblasts to Improve the Re-Endothelialization of Pancreatic Acellular Scaffolds. J. Mater. Sci. Mater. Med. 2019, 30, 85. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wu, C.; Xu, L.; Xu, Y.; Xiaohong, L.; Hui, Z.; Jingjing, L.; Lu, Y.; Wang, Z. Vascularization of Pancreatic Decellularized Scaffold with Endothelial Progenitor Cells. J. Artif. Organs 2018, 21, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Huang, Y.; Zhou, P.; Guo, Y.; Wu, C.; Zhu, S.; Wang, Y.; Wang, L.; Lu, Y.; Wang, Z. Culture of IPSCs Derived Pancreatic β -Like Cells In Vitro Using Decellularized Pancreatic Scaffolds: A Preliminary Trial. Biomed Res. Int. 2017, 2017, 4276928. [Google Scholar] [CrossRef] [Green Version]
- Sabetkish, S.; Kajbafzadeh, A.-M.; Sabetkish, N.; Khorramirouz, R.; Akbarzadeh, A.; Seyedian, S.L.; Pasalar, P.; Orangian, S.; Beigi, R.S.H.; Aryan, Z.; et al. Whole-Organ Tissue Engineering: Decellularization and Recellularization of Three-Dimensional Matrix Liver Scaffolds. J. Biomed. Mater. Res. Part A 2015, 103, 1498–1508. [Google Scholar] [CrossRef]
- Song, L.; Zhou, Q.; Duan, P.; Guo, P.; Li, D.; Xu, Y.; Li, S.; Luo, F.; Zhang, Z. Successful Development of Small Diameter Tissue-Engineering Vascular Vessels by Our Novel Integrally Designed Pulsatile Perfusion-Based Bioreactor. PLoS ONE 2012, 7, e42569. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Cui, J.; Zhang, B.-Q.; Zhang, H.; Bi, Y.; Kang, Q.; Wang, N.; Bie, P.; Yang, Z.; Wang, H.; et al. Decellularized Liver Scaffolds Effectively Support the Proliferation and Differentiation of Mouse Fetal Hepatic Progenitors. J. Biomed. Mater. Res. Part A 2014, 102, 1017–1025. [Google Scholar] [CrossRef] [Green Version]
- Ott, H.C.; Clippinger, B.; Conrad, C.; Schuetz, C.; Pomerantseva, I.; Ikonomou, L.; Kotton, D.; Vacanti, J.P. Regeneration and Orthotopic Transplantation of a Bioartificial Lung. Nat. Med. 2010, 16, 927–933. [Google Scholar] [CrossRef]
- Scarrit, M.E. A Review of Cellularization Strategies for Tissue Engineering of Whole Organs. Front. Bioeng. Biotechnol. 2015, 3, 43. [Google Scholar] [CrossRef]
- O’Neill, J.D.; Anfang, R.; Anandappa, A.; Costa, J.; Javidfar, J.; Wobma, H.M.; Singh, G.; Freytes, D.O.; Bacchetta, M.D.; Sonett, J.R.; et al. Decellularization of Human and Porcine Lung Tissues for Pulmonary Tissue Engineering. Ann. Thorac. Surg. 2013, 96, 1046–1056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ott, H.C.; Matthiesen, T.S.; Goh, S.-K.; Black, L.D.; Kren, S.M.; Netoff, T.I.; Taylor, D.A. Perfusion-Decellularized Matrix: Using Nature’s Platform to Engineer a Bioartificial Heart. Nat. Med. 2008, 14, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Uygun, B.E.; Soto-Gutierrez, A.; Yagi, H.; Izamis, M.L.; Guzzardi, M.A.; Shulman, C.; Milwid, J.; Kobayashi, N.; Tilles, A.; Berthiaume, F.; et al. Organ Reengineering through Development of a Transplantable Recellularized Liver Graft Using Decellularized Liver Matrix. Nat. Med. 2010, 16, 814–820. [Google Scholar] [CrossRef] [PubMed]
- Palakkan, A.A.; Hay, D.C.; PR, A.K.; TV, K.; Ross, J.A. Liver Tissue Engineering and Cell Sources: Issues and Challenges. Liver Int. 2013, 33, 666–676. [Google Scholar] [CrossRef] [PubMed]
- Mizukami, A.; Swiech, K. Mesenchymal Stromal Cells: From Discovery to Manufacturing and Commercialization. Stem Cells Int. 2018, 2018, 4083921. [Google Scholar] [CrossRef]
- Porzionato, A.; Stocco, E.; Barbon, S.; Grandi, F.; Macchi, V.; De Caro, R. Tissue-Engineered Grafts from Human Decellularized Extracellular Matrices: A Systematic Review and Future Perspectives. Int. J. Mol. Sci. 2018, 19, 4117. [Google Scholar] [CrossRef] [Green Version]
- Cui, H.; Chai, Y.; Yu, Y. Progress in Developing Decellularized Bioscaffolds for Enhancing Skin Construction. J. Biomed. Mater. Res. Part A 2019, 107, 1849–1859. [Google Scholar] [CrossRef]
- Bruni, A.; Gala-Lopez, B.; Pepper, A.R.; Abualhassan, N.S.; James Shapiro, A.M. Islet Cell Transplantation for the Treatment of Type 1 Diabetes: Recent Advances and Future Challenges. Diabetes Metab. Syndr. Obes. Targets Ther. 2014, 7, 211. [Google Scholar] [CrossRef] [Green Version]
- Smink, A.M.; de Vos, P. Therapeutic Strategies for Modulating the Extracellular Matrix to Improve Pancreatic Islet Function and Survival After Transplantation. Curr. Diab. Rep. 2018, 18, 39. [Google Scholar] [CrossRef] [Green Version]
- Guruswamy Damodaran, R.; Vermette, P. Tissue and Organ Decellularization in Regenerative Medicine. Biotechnol. Prog. 2018, 34, 1494–1505. [Google Scholar] [CrossRef]
- Mirmalek-Sani, S.H.; Orlando, G.; McQuilling, J.P.; Pareta, R.; Mack, D.L.; Salvatori, M.; Farney, A.C.; Stratta, R.J.; Atala, A.; Opara, E.C.; et al. Porcine Pancreas Extracellular Matrix as a Platform for Endocrine Pancreas Bioengineering. Biomaterials 2013, 34, 5488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pantoja, B.T.S.; Silva, I.G.R.; Fernandes, L.A.; Miglino, M.A.; Carreira, A.C.O. Development of a Biomaterial from Canine Pancreatic Extracellular Matrix Functionalized with Canine and Murine Mesenchymal Stem Cells. Cytotherapy 2022, 24, S30. [Google Scholar] [CrossRef]
- Pantoja, B.T.S.; Silva, D.R.S.; Silva, M.G.K.C.; Carvalho, R.C.; Miglino, M.A.; Carreira, A.C.O. Ultrastructural Analysis of Decellularized Diabetic and Non-Diabetic Canine Pancreas for the Production of Biological Scaffolds. Cytotherapy 2021, 23, 37. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pantoja, B.T.d.S.; Carvalho, R.C.; Miglino, M.A.; Carreira, A.C.O. The Canine Pancreatic Extracellular Matrix in Diabetes Mellitus and Pancreatitis: Its Essential Role and Therapeutic Perspective. Animals 2023, 13, 684. https://doi.org/10.3390/ani13040684
Pantoja BTdS, Carvalho RC, Miglino MA, Carreira ACO. The Canine Pancreatic Extracellular Matrix in Diabetes Mellitus and Pancreatitis: Its Essential Role and Therapeutic Perspective. Animals. 2023; 13(4):684. https://doi.org/10.3390/ani13040684
Chicago/Turabian StylePantoja, Bruna Tássia dos Santos, Rafael Cardoso Carvalho, Maria Angelica Miglino, and Ana Claudia Oliveira Carreira. 2023. "The Canine Pancreatic Extracellular Matrix in Diabetes Mellitus and Pancreatitis: Its Essential Role and Therapeutic Perspective" Animals 13, no. 4: 684. https://doi.org/10.3390/ani13040684
APA StylePantoja, B. T. d. S., Carvalho, R. C., Miglino, M. A., & Carreira, A. C. O. (2023). The Canine Pancreatic Extracellular Matrix in Diabetes Mellitus and Pancreatitis: Its Essential Role and Therapeutic Perspective. Animals, 13(4), 684. https://doi.org/10.3390/ani13040684








