Molecular and Serological Detection of Anaplasma phagocytophilum in Dogs from Germany (2008–2020)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
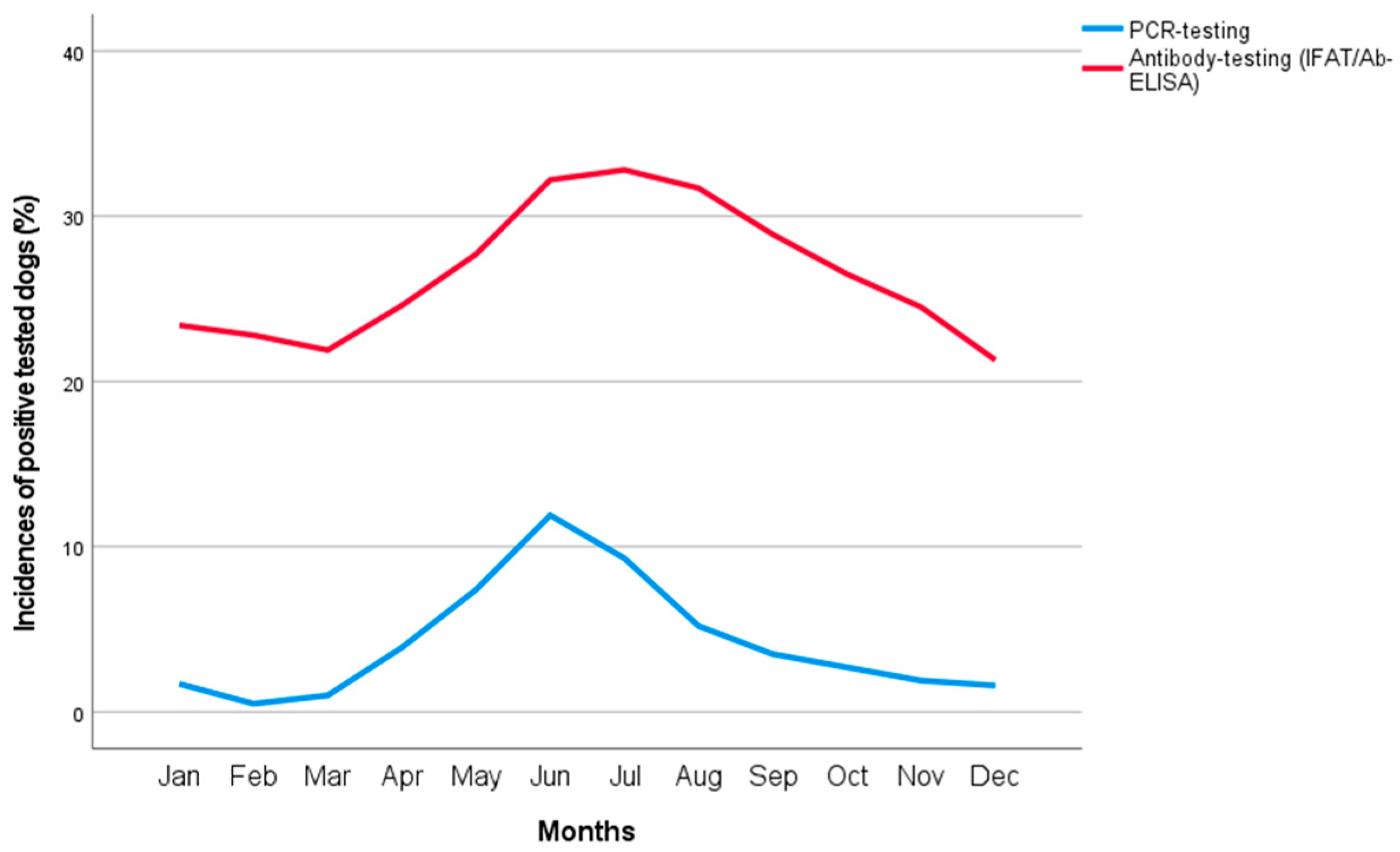
3. Results
4. Discussion
Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cohn, L.A. Ehrlichiosis and related infections. Vet. Clin. N. Am. Small Anim. Pract. 2003, 33, 863–884. [Google Scholar] [CrossRef] [PubMed]
- Dumler, J.S.; Barbet, A.F.; Bekker, C.P.; Dasch, G.A.; Palmer, G.H.; Ray, S.C.; Rikihisa, Y.; Rurangirwa, F.R. Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: Unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new species combinations and designation of Ehrlichia equi and ‘HGE agent’ as subjective synonyms of Ehrlichia phagocytophila. Int. J. Syst. Evol. Microbiol. 2001, 51, 2145–2165. [Google Scholar] [CrossRef] [PubMed]
- Katavolos, P.; Armstrong, P.M.; Dawson, J.E.; Telford, S.R. Duration of tick attachment required for transmission of granulocytic ehrlichiosis. J. Infect. Dis. 1998, 177, 1422–1425. [Google Scholar] [CrossRef] [PubMed]
- ESCCAP. Control of Vector-Borne Diseases in Dogs and Cats; European Scientific Counsel Companion Animal Parasites. 2019. Available online: https://www.esccap.org/uploads/docs/yhwdhifq_0775_ESCCAP_Guideline_GL5_v10_1p.pdf (accessed on 20 December 2022).
- Rubel, F.; Brugger, K.; Chitimia-Dobler, L.; Dautel, H.; Meyer-Kayser, E.; Kahl, O. Atlas of ticks (Acari: Argasidae, Ixodidae) in Germany. Exp. Appl. Acarol. 2021, 84, 183–214. [Google Scholar] [CrossRef] [PubMed]
- Egenvall, A.; Bjoersdorff, A.; Lilliehook, I.; Olsson Engvall, E.; Karlstam, E.; Artursson, K.; Hedhammar, A.; Gunnarsson, A. Early manifestations of granulocytic ehrlichiosis in dogs inoculated experimentally with a Swedish Ehrlichia species isolate. Vet. Rec. 1998, 143, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Kohn, B. Canine transfusion medicine. Kleintierprax 2010, 7, 387–395. [Google Scholar]
- Kemperman, M.; Neitzel, D.; Jensen, K.; Gorlin, J.; Perry, E.; Myers, T.; Miley, T.; McQuiston, J.; Eremeeva, M.E.; Nicholson, W.; et al. Anaplasma phagocytophilum transmitted through blood transfusion—Minnesota, 2007. JAMA-J. Am. Med. Assoc. 2008, 300, 2718–2720, reprinted from MMWR 2008, 57, 1145–1148. [Google Scholar]
- Bakken, J.S.; Dumler, J.S. Clinical diagnosis and treatment of human granulocytotropic anaplasmosis. Ann. N. Y. Acad. Sci 2006, 1078, 236–247. [Google Scholar] [CrossRef]
- Courtney, J.W.; Kostelnik, L.M.; Zeidner, N.S.; Massung, R.F. Multiplex real-time PCR for detection of Anaplasma phagocytophilum and Borrelia burgdorferi. J. Clin. Microbiol. 2004, 42, 3164–3168. [Google Scholar] [CrossRef]
- Lappin, M.R.; Breitschwerdt, E.B.; Jensen, W.A.; Dunnigan, B.; Rha, J.Y.; Williams, C.R.; Brewer, M.; Fall, M. Molecular and serologic evidence of Anaplasma phagocytophilum infection in cats in North America. JAVMA 2004, 225, 893–896. [Google Scholar] [CrossRef]
- Sainz, A.; Roura, X.; Miro, G.; Estrada-Pena, A.; Kohn, B.; Harrus, S.; Solano-Gallego, L. Guideline for veterinary practitioners on canine ehrlichiosis and anaplasmosis in Europe. Parasit. Vectors 2015, 8, 75. [Google Scholar] [CrossRef] [PubMed]
- Egenvall, A.; Lilliehook, I.; Bjoersdorff, A.; Engvall, E.O.; Karlstam, E.; Artursson, K.; Heldtander, M.; Gunnarsson, A. Detection of granulocytic Ehrlichia species DNA by PCR in persistently infected dogs. Vet. Rec. 2000, 146, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.B.; Nelson, C.M.; Goodman, J.L. Antibiotic susceptibility of the newly cultivated agent of human granulocytic ehrlichiosis: Promising activity of quinolones and rifamycins. Antimicrob. Agents Chemother. 1997, 41, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Kohn, B.; Silaghi, C.; Galke, D.; Arndt, G.; Pfister, K. Infections with Anaplasma phagocytophilum in dogs in Germany. Res. Vet. Sci. 2011, 91, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Inokuma, H.; Nane, G.; Uechi, T.; Yonahara, Y.; Brouqui, P.; Okuda, M.; Onishi, T. Survey of tick infestation and tick-borne ehrlichial infection of dogs in Ishigaki Island, Japan. J. Vet. Med. Sci. 2001, 63, 1225–1227. [Google Scholar] [CrossRef]
- Scorpio, D.G.; Dumler, J.S.; Barat, N.C.; Cook, J.A.; Barat, C.E.; Stillman, B.A.; DeBisceglie, K.C.; Beall, M.J.; Chandrashekar, R. Comparative strain analysis of Anaplasma phagocytophilum infection and clinical outcomes in a canine model of granulocytic anaplasmosis. Vector-Borne Zoonotic Dis. 2011, 11, 223–229. [Google Scholar] [CrossRef]
- Eberts, M.D.; Vissotto de Paiva Diniz, P.P.; Beall, M.J.; Stillman, B.A.; Chandrashekar, R.; Breitschwerdt, E.B. Typical and atypical manifestations of Anaplasma phagocytophilum infection in dogs. J. Am. Anim. Hosp. Assoc. 2011, 47, e86–e94. [Google Scholar] [CrossRef]
- Jensen, J.; Simon, D.; Murua Escobar, H.; Soller, J.T.; Bullerdiek, J.; Beelitz, P.; Pfister, K.; Nolte, I. Anaplasma phagocytophilum in dogs in Germany. Zoonoses Public Health 2007, 54, 94–101. [Google Scholar] [CrossRef]
- Chirek, A.; Silaghi, C.; Pfister, K.; Kohn, B. The occurrence of Anaplasma phagocytophilum in canine blood donors in Berlin/Brandenburg (2006–2012): Retrospective analysis of clinical data and relevance for transfusion medicine. Berl. Münchener Tierärztliche Wochenschr. 2017, 131, 124–130. [Google Scholar]
- Barutzki, D.; De Nicola, A.; Zeziola, M.; Reule, M. Seroprevalence of Anaplasma phagocytophilum infection in dogs in Germany. Berl. Münchener Tierärztliche Wochenschr. 2006, 119, 342–347. [Google Scholar]
- Schaarschmidt-Kiener, D.; Müller, W. Labordiagnostische und klinische Aspekte der kaninen Anaplasmose und Ehrlichiose. Tierärztliche Prax. 2007, 35, 129–136. [Google Scholar] [CrossRef]
- Preyss-Jageler, C.; Müller, E.; Straubinger, R.K.; Hartmann, K. Prevalence of antibodies against Borrelia burgdorferi, Anaplasma phagocytophilum, and Leptospira interrogans serovars in Bernese Mountain Dogs. Tierärztliche Prax. Ausg. K Kleintiere Heimtiere 2016, 44, 77–85. [Google Scholar] [CrossRef]
- Krupka, I.; Pantchev, N.; Weise, M.; Straubinger, R.K. Tick-transmitted, bacterial infections in dogs: Seroprevalences of Anaplasma phagocytophilum, Borrelia burgdorferi sensu lato and Ehrlichia canis in Germany. Prakt. Tierarzt 2007, 10, 776–787. [Google Scholar]
- Barth, C.; Straubinger, R.K.; Sauter-Louis, C.; Hartmann, K. Prevalence of antibodies against Borrelia burgdorferi sensu lato and Anaplasma phagocytophilum and their clinical relevance in dogs in Munich, Germany. Berl. Münchener Tierärztliche Wochenschr. 2012, 125, 337–344. [Google Scholar]
- afosa GmbH: Instructions for Use: Anaplasma-ELISA Dog. 2021. Available online: https://shop.indical.com/en/assays-and-reagents/companion-animals/anaplasma-elisa-dog-1-elisa-plate.html (accessed on 19 December 2022).
- Gethmann, J.; Hoffmann, B.; Kasbohm, E.; Süss, J.; Habedank, B.; Conraths, F.J.; Beer, M.; Klaus, C. Research paper on abiotic factors and their influence on Ixodes ricinus activity-observations over a two-year period at several tick collection sites in Germany. Parasitol. Res. 2020, 119, 1455–1466. [Google Scholar] [CrossRef] [PubMed]
- Pantchev, N.; Pluta, S.; Huisinga, E.; Nather, S.; Scheufelen, M.; Vrhovec, M.G.; Schweinitz, A.; Hampel, H.; Straubinger, R.K. Tick-borne diseases (Borreliosis, Anaplasmosis, Babesiosis) in German and Austrian dogs: Status quo and review of distribution, transmission, clinical findings, diagnostics and prophylaxis. Parasitol. Res. 2015, 114, S19–S54. [Google Scholar] [CrossRef] [PubMed]
- Jore, S.; Vanwambeke, S.O.; Viljugrein, H.; Isaksen, K.; Kristoffersen, A.B.; Woldehiwet, Z.; Johansen, B.; Brun, E.; Brun-Hansen, H.; Westermann, S.; et al. Climate and environmental change drives Ixodes ricinus geographical expansion at the northern range margin. Parasit. Vectors 2014, 7, 11. [Google Scholar] [CrossRef]
- Jaenson, T.G.; Jaenson, D.G.; Eisen, L.; Petersson, E.; Lindgren, E. Changes in the geographical distribution and abundance of the tick Ixodes ricinus during the past 30 years in Sweden. Parasit. Vectors 2012, 5, 8. [Google Scholar] [CrossRef]
- Martello, E.; Mannelli, A.; Ragagli, C.; Ambrogi, C.; Selmi, M.; Ceballos, L.A.; Tomassone, L. Range expansion of Ixodes ricinus to higher altitude, and co-infestation of small rodents with Dermacentor marginatus in the Northern Apennines, Italy. Ticks Tick Borne Dis. 2014, 5, 970–974. [Google Scholar] [CrossRef]
- Lindgren, E.; Talleklint, L.; Polfeldt, T. Impact of climatic change on the northern latitude limit and population density of the disease-transmitting European tick Ixodes ricinus. Environ. Health Perspect. 2000, 108, 119–123. [Google Scholar] [CrossRef]
- Diniz, P.P.; Breitschwerdt, E.B. Anaplasma phagocytophilum infection (canine granulocytotropic anaplasmosis). In Infectious Diseases of the Dog and Cat; Greene, C.E., Ed.; Elsevier: St. Louis, MI, USA, 2012; Volume 4, pp. 244–254. [Google Scholar]
- Greig, B.; Asanovich, K.M.; Armstrong, P.J.; Dumler, J.S. Geographic, clinical, serologic, and molecular evidence of granulocytic ehrlichiosis, a likely zoonotic disease, in Minnesota and Wisconsin dogs. J. Clin. Microbiol. 1996, 34, 44–48. [Google Scholar] [CrossRef]
- Poitout, F.M.; Shinozaki, J.K.; Stockwell, P.J.; Holland, C.J.; Shukla, S.K. Genetic variants of Anaplasma phagocytophilum infecting dogs in Western Washington State. J. Clin. Microbiol. 2005, 43, 796–801. [Google Scholar] [CrossRef] [PubMed]
- Kohn, B.; Galke, D.; Beelitz, P.; Pfister, K. Clinical features of canine granulocytic anaplasmosis in 18 naturally infected dogs. J. Vet. Intern. Med. 2008, 22, 1289–1295. [Google Scholar] [CrossRef] [PubMed]
- Granick, J.L.; Armstrong, P.J.; Bender, J.B. Anaplasma phagocytophilum infection in dogs: 34 cases (2000-2007). J. Am. Vet. Med. Assoc. 2009, 234, 1559–1565. [Google Scholar] [CrossRef] [PubMed]
- Moroff, S.; Sokolchik, I.; Woodring, T.; Woodruff, C.; Atkinson, B.; Lappin, M.R. Detection of antibodies against Anaplasma phagocytophilum in dogs using an automated fluorescence based system. Vet. J. 2014, 202, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Egenvall, A.E.; Hedhammar, A.A.; Bjoersdorff, A.I. Clinical features and serology of 14 dogs affected by granulocytic ehrlichiosis in Sweden. Vet. Rec. 1997, 140, 222–226. [Google Scholar] [CrossRef]
- Egenvall, A.; Bonnett, B.N.; Gunnarsson, A.; Hedhammar, A.; Shoukri, M.; Bornstein, S.; Artursson, K. Sero-prevalence of granulocytic Ehrlichia spp. and Borrelia burgdorferi sensu lato in Swedish dogs 1991-94. Scand. J. Infect. Dis. 2000, 32, 19–25. [Google Scholar] [CrossRef]
- Kirtz, G.; Czettel, B.; Thum, D.; Leidinger, E. Anaplasma phagocytophilum in dogs in Austria: A serological prevalence study (2001–2006). Kleintierprax 2007, 52, 562–568. [Google Scholar]
- Nelder, M.P.; Russell, C.B.; Lindsay, L.R.; Dibernardo, A.; Brandon, N.C.; Pritchard, J.; Johnson, S.; Cronin, K.; Patel, S.N. Recent emergence of Anaplasma phagocytophilum in Ontario, Canada: Early serological and entomological indicators. Am. J. Trop. Med. Hyg. 2019, 101, 1249–1258. [Google Scholar] [CrossRef]
- Greig, B.; Armstrong, P.J. Canine granulocytotrophic anaplasmosis (A. phagocytophilum infection). In Infectious Diseases of the Dog and the Cat, 4th ed.; Greene, C.G., Ed.; Saunders Elsevier: St. Louis, MI, USA, 2012; pp. 244–254. [Google Scholar]
| Age-Group | PCR | IFAT/ELISA |
|---|---|---|
| 0–2 years (junior) | 206/4934 (4.2 [3.7; 4.8]) | 2557/20,749 (12.3 [11.9; 12.8]) |
| >2–7 years (adult) | 478/9529 (5.0 [4.6; 5.5]) | 8924/31,198 (28.6 [28.1; 29.1]) |
| >7–10 years (mature) | 285/6019 (4.7 [4.2; 5.3]) | 6473/16,349 (39.6 [38.8; 40.4]) |
| >10–13 years (senior) | 213/3809 (5.6 [4.9; 6.4]) | 3704/8757 (42.3 [41.3; 43.3]) |
| >13 years (geriatric) | 49/762 (6.4 [4.8; 8.5]) | 600/1475 (40.7 [38.2; 43.2]) |
| Total | 1231/25,053 (4.9 [4.7; 5.2]) | 22,258/78,528 (28.3 [28.0; 28.7]) |
| Mann–Whitney U test | p < 0.001 | p < 0.001 |
| Year | PCR | IFAT/ELISA | IFAT | ELISA |
|---|---|---|---|---|
| 2008 | 40/850 (4.7 [3.4; 6.4]) | 531/2988 (17.8 [16.4; 19.2]) | 531/2988 (17.8 [16.4; 19.2]) | |
| 2009 | 53/1194 (4.4 [3.4; 5.8]) | 1054/4082 (25.8 [24.5; 27.2]) | 1054/4082 (25.8 [24.5; 27.2]) | |
| 2010 | 42/1245 (3.4 [2.5; 4.6]) | 1187/4160 (28.5 [27.2; 29.9]) | 1187/4160 (28.5 [27.2; 29.9]) | |
| 2011 | 55/1182 (4.7 [3.6; 6.1]) | 1491/5174 (28.8 [27.6; 30.1]) | 1491/5174 (28.8 [27.6; 30.1]) | |
| 2012 | 58/1355 (4.3 [3.3; 5.5]) | 1669/6187 (27.0 [25.9; 28.1]) | 1669/6187 (27.0 [25.9; 28.1]) | |
| 2013 | 46/1461 (3.1 [2.3; 4.2]) | 1698/6539 (26.0 [24.9; 27.1]) | 1698/6539 (26.0 [24.9; 27.1]) | |
| 2014 | 84/1907 (4.4 [3.5; 5.4]) | 1534/7875 (19.5 [18.6; 20.4]) | 1534/7875 (19.5 [18.6; 20.4]) | |
| 2015 | 100/1887 (5.3 [4.4; 6.4]) | 984/7616 (12.9 [12.2; 13.7]) | 984/7616 (12.9 [12.2; 13.7]) | |
| 2016 | 124/2378 (5.2 [4.4; 6.2]) | 1208/7487 (16.1 [15.3; 17.0]) | 1208/7487 (16.1 [15.3; 17.0]) | |
| 2017 | 167/2955 (5.7 [4.9; 6.6]) | 3383/9146 (37.0 [36.0; 38.0]) | 451/1365 (33.0 [30.6; 35.6]) | 3206/8949 (35.8 [34.8; 36.8]) |
| 2018 | 157/3160 (5.0 [4.3; 5.8]) | 3183/8892 (35.8 [34.8; 36.8]) | 305/1085 (28.1 [25.5; 30.9]) | 3032/8729 (34.7 [33.7; 35.7]) |
| 2019 | 190/3598 (5.3 [4.6; 6.1]) | 2998/9390 (31.9 [31.0; 32.9]) | 248/964 (25.7 [23.0; 28.6]) | 2848/9235 (30.8 [29.9; 31.8]) |
| 2020 | 216/4196 (5.1 [4.5; 5.9]) | 3800/10,840 (35.1 [34.2; 36.0]) | 254/1092 (23.3 [20.8; 25.9]) | 3639/10,668 (34.1 [33.2; 35.0]) |
| Total | 1332/27,368 (4.9 [4.6; 5.1]) | 24,720/90,376 (27.4 [27.1; 27.6]) | 12,614/56,614 (22.3 [21.9; 22.6]) | 12,725/37,581 (33.9 [33.4; 34.3]) |
| B | SE | Wald | p | Odds Ratio | 95%-CI for Odds Ratio | ||
|---|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | ||||||
| PCR testing | |||||||
| Sex | 0.000 | 0.060 | 0.000 | 0.995 | 1.000 | 0.888 | 1.125 |
| Age | 0.094 | 0.061 | 2.388 | 0.122 | 1.099 | 0.975 | 1.238 |
| Years | 0.029 | 0.009 | 10.582 | 0.001 | 1.029 | 1.011 | 1.047 |
| Season | 1.173 | 0.061 | 370.629 | <0.001 | 3.233 | 2.869 | 3.643 |
| Constant | −61.158 | 17.721 | 11.910 | <0.001 | |||
| Antibody testing (IFAT/ELISA) | |||||||
| Sex | 0.238 | 0.017 | 203.113 | <0.001 | 1.269 | 1.228 | 1.312 |
| Age | 0.872 | 0.017 | 2656.929 | <0.001 | 2.392 | 2.314 | 2.472 |
| Years | 0.054 | 0.002 | 623.234 | <0.001 | 1.056 | 1.051 | 1.060 |
| Season | 0.338 | 0.017 | 379.519 | <0.001 | 1.403 | 1.356 | 1.451 |
| Constant | −117.757 | 4.868 | 585.175 | <0.001 | |||
| Federal State | PCR | IFAT/ELISA |
|---|---|---|
| Baden-Wuerttemberg | 118/2887 (4.1 [3.4; 4.9]) | 2111/8968 (23.5 [22.7; 24.4]) |
| Bavaria | 139/2721 (5.1 [4.3; 6.0]) | 2511/7215 (34.8 [33.7; 35.9]) |
| Berlin-Brandenburg | 231/3038 (7.6 [6.7; 8.6]) | 1062/3475 (30.6 [29.0; 32.1]) |
| Hesse | 74/2496 (3.0 [2.4; 3.7]) | 1943/9739 (20.0 [19.2; 20.8]) |
| Lower Saxony/Bremen | 247/4341 (5.7 [5.0; 6.4]) | 5733/17,056 (33.6 [32.9;34.3]) |
| Mecklenburg Western Pomerania | 28/419 (6.7 [4.6; 9.6]) | 158/676 (23.4 [20.3; 26.8]) |
| North Rhine Westphalia | 237/6124 (3.9 [3.4; 4.4]) | 6913/26,786 (25.8 [25.3;26.3]) |
| Rhineland Palatinate | 55/1734 (3.2 [2.4; 4.1]) | 1358/6286 (21.6 [20.6; 22.6]) |
| Saarland | 28/748 (3.7 [2.5; 5.4]) | 672/2216 (30.3 [28.4; 32.3]) |
| Saxony | 76/1006 (7.6 [6.0; 9.4]) | 471/1817 (25.9 [23.9; 28.0]) |
| Saxony-Anhalt | 16/383 (4.2 [2.5; 6.8]) | 273/930 (29.4 [26.5; 32.4]) |
| Schleswig-Holstein/Hamburg | 52/1051 (4.9 [3.8; 6.5]) | 1176/4046 (29.1 [27.7; 30.5]) |
| Thuringia | 31/420 (7.4 [5.2; 10.4]) | 339/1166 (29.1 [26.5; 31.8]) |
| Total | 1332/27,368 (4.9 [4.6; 5.1]) | 24,720/90,376 (27.4 [27.1; 27.6]) |
| Chi-square test | p < 0.001 | p < 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schäfer, I.; Kohn, B.; Silaghi, C.; Fischer, S.; Marsboom, C.; Hendrickx, G.; Müller, E. Molecular and Serological Detection of Anaplasma phagocytophilum in Dogs from Germany (2008–2020). Animals 2023, 13, 720. https://doi.org/10.3390/ani13040720
Schäfer I, Kohn B, Silaghi C, Fischer S, Marsboom C, Hendrickx G, Müller E. Molecular and Serological Detection of Anaplasma phagocytophilum in Dogs from Germany (2008–2020). Animals. 2023; 13(4):720. https://doi.org/10.3390/ani13040720
Chicago/Turabian StyleSchäfer, Ingo, Barbara Kohn, Cornelia Silaghi, Susanne Fischer, Cedric Marsboom, Guy Hendrickx, and Elisabeth Müller. 2023. "Molecular and Serological Detection of Anaplasma phagocytophilum in Dogs from Germany (2008–2020)" Animals 13, no. 4: 720. https://doi.org/10.3390/ani13040720
APA StyleSchäfer, I., Kohn, B., Silaghi, C., Fischer, S., Marsboom, C., Hendrickx, G., & Müller, E. (2023). Molecular and Serological Detection of Anaplasma phagocytophilum in Dogs from Germany (2008–2020). Animals, 13(4), 720. https://doi.org/10.3390/ani13040720







