Innovations in Cattle Farming: Application of Innovative Technologies and Sensors in the Diagnosis of Diseases
Abstract
Simple Summary
Abstract
1. Introduction
2. Importance of Early Diagnostic in Dairy Farming
3. Innovative Tools in Farm Animals’ Early Disease Diagnosis
3.1. Milk Analyzers
3.1.1. Somatic Cell Count
3.1.2. Milk Progesterone
3.2. Breath, Sweat and Saliva Analysis
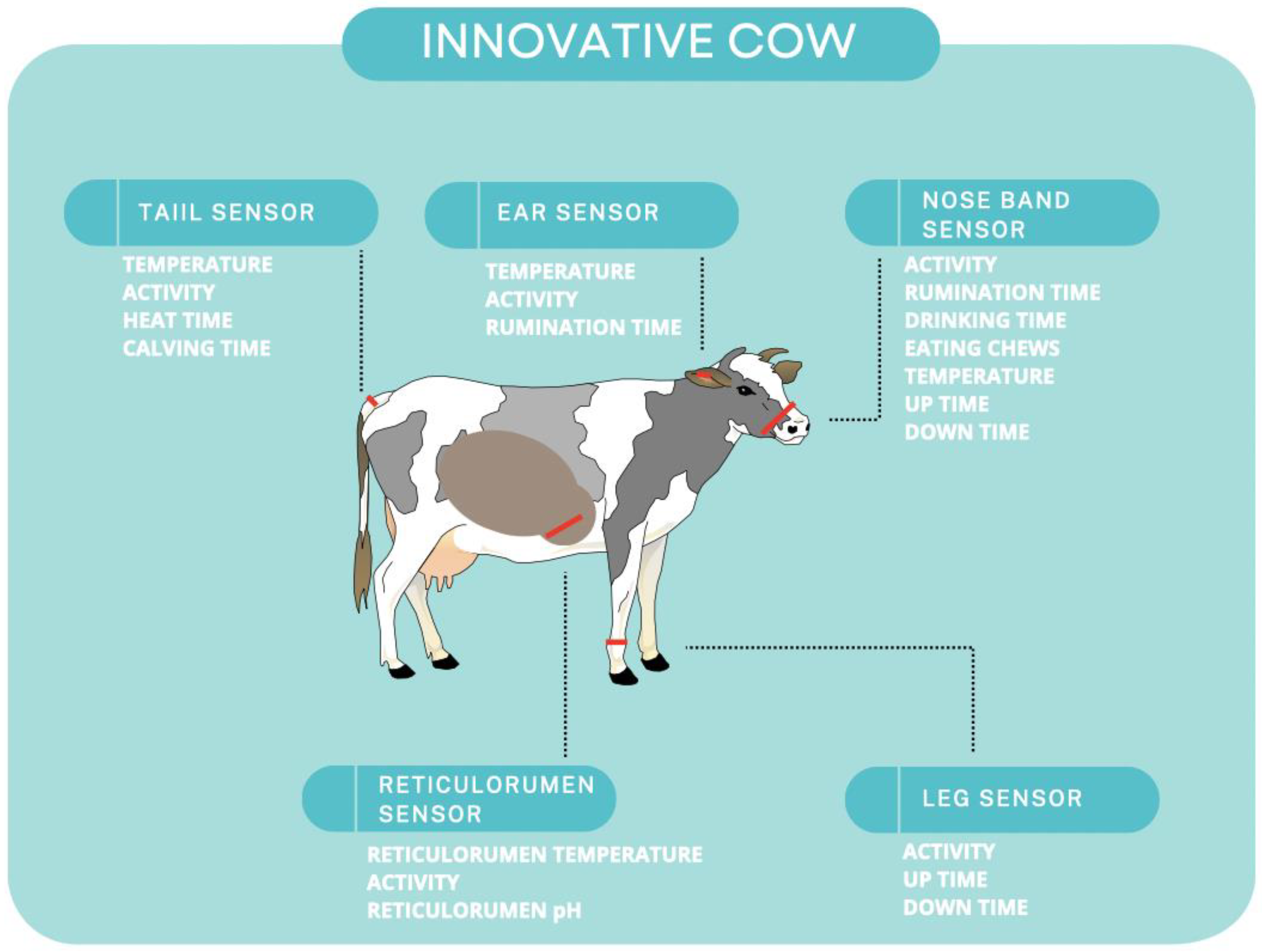
3.3. Wearable Devices for Animals
3.3.1. Head/Muzzle and Noseband Sensors
3.3.2. Motion, Movement, and Behavior Sensors
Pedometer
3.4. Other Analyzers: BCS Camera, Infrared Thermography, Sensors of Bolus
3.4.1. Infrared Thermography
3.4.2. Bolus Sensors
3.4.3. Body Condition Score
3.4.4. Animal Surveillance through Video and Imaging
3.4.5. Electronic Nose for Estrus Detection
4. Innovations for Common Procedures
5. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, M.; Wang, X.; Feng, H.; Huang, Q.; Xiao, X.; Zhang, X. Wearable Internet of Things enabled precision livestock farming in smart farms: A review of technical solutions for precise perception, biocompatibility, and sustainability monitoring. J. Clean. Prod. 2021, 312, 127712. [Google Scholar] [CrossRef]
- Stangaferro, M.L.; Wijma, R.; Caixeta, L.S.; Al-Abri, M.A.; Giordano, J.O. Use of rumination and activity monitoring for the identification of dairy cows with health disorders: Part I. Metabolic and digestive disorders. J. Dairy Sci. 2016, 99, 7395–7410. [Google Scholar] [CrossRef] [PubMed]
- Henchion, M.M.; Regan, Á.; Beecher, M.; MackenWalsh, Á. Developing ‘Smart’ Dairy Farming Responsive to Farmers and Consumer-Citizens: A Review. Animals 2022, 12, 360. [Google Scholar] [CrossRef]
- Lovarelli, D.; Bacenetti, J.; Guarino, M. A review on dairy cattle farming: Is precision livestock farming the compromise for an environmental, economic and social sustainable production? J. Clean. Prod. 2020, 262, 121409. [Google Scholar] [CrossRef]
- Vidic, J.; Manzano, M.; Chang, C.-M.; Jaffrezic-Renault, N. Advanced biosensors for detection of pathogens related to livestock and poultry. Vet. Res. 2017, 48, 11. [Google Scholar] [CrossRef] [PubMed]
- Neethirajan, S. Transforming the Adaptation Physiology of Farm Animals through Sensors. Animals 2020, 10, 1512. [Google Scholar] [CrossRef] [PubMed]
- Stygar, A.H.; Gómez, Y.; Berteselli, G.V.; Dalla Costa, E.; Canali, E.; Niemi, J.K.; Llonch, P.; Pastell, M. A Systematic Review on Commercially Available and Validated Sensor Technologies for Welfare Assessment of Dairy Cattle. Front. Vet. Sci. 2021, 8, 634338. [Google Scholar] [CrossRef] [PubMed]
- Neethirajan, S.; Tuteja, S.K.; Huang, S.-T.; Kelton, D. Recent advancement in biosensors technology for animal and livestock health management. Biosens. Bioelectron. 2017, 98, 398–407. [Google Scholar] [CrossRef]
- Neethirajan, S. Recent advances in wearable sensors for animal health management. Sens. BioSens. Res. 2017, 12, 15–29. [Google Scholar] [CrossRef]
- Antanaitis, R.; Juozaitienė, V.; Malašauskienė, D.; Televičius, M.; Urbutis, M.; Baumgartner, W. Relation of Automated Body Condition Scoring System and Inline Biomarkers (Milk Yield, β-Hydroxybutyrate, Lactate Dehydrogenase and Progesterone in Milk) with Cow’s Pregnancy Success. Sensors 2021, 21, 1414. [Google Scholar] [CrossRef]
- Gillund, P.; Reksen, O.; Gröhn, Y.T.; Karlberg, K. Body Condition Related to Ketosis and Reproductive Performance in Norwegian Dairy Cows. J. Dairy Sci. 2001, 84, 1390–1396. [Google Scholar] [CrossRef] [PubMed]
- Lou, W.; Zhang, H.; Luo, H.; Chen, Z.; Shi, R.; Guo, X.; Zou, Y.; Liu, L.; Brito, L.F.; Guo, G.; et al. Genetic analyses of blood β-hydroxybutyrate predicted from milk infrared spectra and its association with longevity and female reproductive traits in Holstein cattle. J. Dairy Sci. 2022, 105, 3269–3281. [Google Scholar] [CrossRef] [PubMed]
- Walsh, R.B.; Walton, J.S.; Kelton, D.F.; LeBlanc, S.J.; Leslie, K.E.; Duffield, T.F. The Effect of Subclinical Ketosis in Early Lactation on Reproductive Performance of Postpartum Dairy Cows. J. Dairy Sci. 2007, 90, 2788–2796. [Google Scholar] [CrossRef] [PubMed]
- Reist, M.; Erdin, D.K.; von Euw, D.; Tschümperlin, K.M.; Leuenberger, H.; Hammon, H.M.; Morel, C.; Philipona, C.; Zbinden, Y.; Künzi, N.; et al. Postpartum reproductive function: Association with energy, metabolic and endocrine status in high yielding dairy cows. Theriogenology 2003, 59, 1707–1723. [Google Scholar] [CrossRef] [PubMed]
- Malašauskienė, D.; Juozaitienė, V.; Televičius, M.; Rutkauskas, A.; Urbutis, M.; Kanapė, V.; Gerbutavičiūte, J.; Antanaitis, R. Changes in the inline lactate dehydrogenase according to the cow’s production and reproduction status. Acta Vet. Brno 2020, 88, 369–375. [Google Scholar] [CrossRef]
- Larsen, T.; Røntved, C.M.; Ingvartsen, K.L.; Vels, L.; Bjerring, M. Enzyme activity and acute phase proteins in milk utilized as indicators of acute clinical E. coli LPS-induced mastitis. Animal 2010, 4, 1672–1679. [Google Scholar] [CrossRef]
- Hommeida, A.; Nakao, T.; Kubota, H. Luteal function and conception in lactating cows and some factors influencing luteal function after first insemination. Theriogenology 2004, 62, 217–225. [Google Scholar] [CrossRef]
- Ashraf, A.; Imran, M. Diagnosis of bovine mastitis: From laboratory to farm. Trop. Anim. Health Prod. 2018, 50, 1193–1202. [Google Scholar] [CrossRef]
- Neethirajan, S. The role of sensors, big data and machine learning in modern animal farming. Sens. Bio-Sens. Res. 2020, 29, 100367. [Google Scholar] [CrossRef]
- Ali, A.S.; Jacinto, J.G.P.; Mϋnchemyer, W.; Walte, A.; Gentile, A.; Formigoni, A.; Mammi, L.M.E.; Csaba Bajcsy, Á.; Abdu, M.S.; Kamel, M.M.; et al. Estrus Detection in a Dairy Herd Using an Electronic Nose by Direct Sampling on the Perineal Region. Vet. Sci. 2022, 9, 688. [Google Scholar] [CrossRef]
- Reith, S.; Hoy, S. Review: Behavioral signs of estrus and the potential of fully automated systems for detection of estrus in dairy cattle. Anim. Int. J. Anim. Biosci. 2018, 12, 398–407. [Google Scholar] [CrossRef]
- Wisnieski, L.; Norby, B.; Pierce, S.J.; Becker, T.; Gandy, J.C.; Sordillo, L.M. Predictive models for early lactation diseases in transition dairy cattle at dry-off. Prev. Vet. Med. 2019, 163, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Stone, A.E. Symposium review: The most important factors affecting adoption of precision dairy monitoring technologies. J. Dairy Sci. 2020, 103, 5740–5745. [Google Scholar] [CrossRef] [PubMed]
- Evangelista, C.; Basiricò, L.; Bernabucci, U. An Overview on the Use of Near Infrared Spectroscopy (NIRS) on Farms for the Management of Dairy Cows. Agriculture 2021, 11, 296. [Google Scholar] [CrossRef]
- Eckelkamp, E.A. Invited Review: Current state of wearable precision dairy technologies in disease detection. Appl. Anim. Sci. 2019, 35, 209–220. [Google Scholar] [CrossRef]
- Nogami, H.; Okada, H.; Miyamoto, T.; Maeda, R.; Itoh, T. Wearable Wireless Temperature Sensor Nodes Appressed to Base of a Calf’s Tail. Sens. Mater. 2014, 26, 539–545. [Google Scholar]
- Van Nuffel, A.; Zwertvaegher, I.; Van Weyenberg, S.; Pastell, M.; Thorup, V.; Bahr, C.; Sonck, B.; Saeys, W. Lameness Detection in Dairy Cows: Part 2. Use of Sensors to Automatically Register Changes in Locomotion or Behavior. Animals 2015, 5, 861–885. [Google Scholar] [CrossRef]
- Badwolf. CowAlert|Use Sensors to Manage Your Herd. IceRobotics. Available online: https://www.icerobotics.com/cowalert/ (accessed on 14 November 2022).
- Glennon, T.; O’Quigley, C.; McCaul, M.; Matzeu, G.; Beirne, S.; Wallace, G.G.; Stroiescu, F.; O’Mahoney, N.; White, P.; Diamond, D. ‘SWEATCH’: A Wearable Platform for Harvesting and Analysing Sweat Sodium Content. Electroanalysis 2016, 28, 1283–1289. [Google Scholar] [CrossRef]
- Heikenfeld, J. Technological leap for sweat sensing. Nature 2016, 529, 475–476. [Google Scholar] [CrossRef]
- U-Motion®—Monitor Your Herd’s Behavior. Available online: http://desamis.co.jp/en/ (accessed on 14 November 2022).
- Diagnostic Value of Milk Fat. BROLIS HerdLine. Available online: https://brolisherdline.com/milk-fat/ (accessed on 3 December 2022).
- Luo, T.; Steeneveld, W.; Nielen, M.; Zanini, L.; Zecconi, A. Linear Mixed-Effects Model to Quantify the Association between Somatic Cell Count and Milk Production in Italian Dairy Herds. Animals 2023, 13, 80. [Google Scholar] [CrossRef]
- Neculai-Valeanu, A.-S.; Ariton, A.-M. Udder Health Monitoring for Prevention of Bovine Mastitis and Improvement of Milk Quality. Bioengineering 2022, 9, 608. [Google Scholar] [CrossRef]
- Wang, Y.; Kang, X.; He, Z.; Feng, Y.; Liu, G. Accurate detection of dairy cow mastitis with deep learning technology: A new and comprehensive detection method based on infrared thermal images. Animal 2022, 16, 100646. [Google Scholar] [CrossRef] [PubMed]
- Carvalho-Sombra, T.C.; Fernandes, D.D.; Bezerra, B.M.; Nunes-Pinheiro, D.C. Systemic inflammatory biomarkers and somatic cell count in dairy cows with subclinical mastitis. Vet. Anim. Sci. 2021, 11, 100165. [Google Scholar] [CrossRef] [PubMed]
- Alhussien, M.N.; Dang, A.K. Milk somatic cells, factors influencing their release, future prospects, and practical utility in dairy animals: An overview. Vet. World 2018, 11, 562–577. [Google Scholar] [CrossRef]
- Balaine, L.; Dillon, E.J.; Läpple, D.; Lynch, J. Can technology help achieve sustainable intensification? Evidence from milk recording on Irish dairy farms. Land Use Policy 2020, 92, 104437. [Google Scholar] [CrossRef] [PubMed]
- Martins, S.A.M.; Martins, V.C.; Cardoso, F.A.; Germano, J.; Rodrigues, M.; Duarte, C.; Bexiga, R.; Cardoso, S.; Freitas, P.P. Biosensors for On-Farm Diagnosis of Mastitis. Front. Bioeng. Biotechnol. 2019, 7, 186. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Dhama, K.; Tiwari, R.; Iqbal Yatoo, M.; Khurana, S.K.; Khandia, R.; Munjal, A.; Munuswamy, P.; Kumar, M.A.; Singh, M.; et al. Technological interventions and advances in the diagnosis of intramammary infections in animals with emphasis on bovine population—A review. Vet. Q 2019, 39, 76–94. [Google Scholar] [CrossRef] [PubMed]
- Zachut, M.; Šperanda, M.; de Almeida, A.M.; Gabai, G.; Mobasheri, A.; Hernández-Castellano, L.E. Biomarkers of fitness and welfare in dairy cattle: Healthy productivity. J. Dairy Res. 2020, 87, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Rutten, C.J.; Velthuis, A.G.J.; Steeneveld, W.; Hogeveen, H. Invited review: Sensors to support health management on dairy farms. J. Dairy Sci. 2013, 96, 1928–1952. [Google Scholar] [CrossRef]
- Muncan, J.; Miyazaki, M.; Kuroki, S.; Ikuta, K.; Tsenkova, R. Adaptive Spectral Model for abnormality detection based on physiological status monitoring of dairy cows. Talanta 2023, 253, 123893. [Google Scholar] [CrossRef]
- Burciaga-Robles, L.O.; Holland, B.P.; Step, D.L.; Krehbiel, C.R.; McMillen, G.L.; Richards, C.J.; Sims, L.E.; Jeffers, J.D.; Namjou, K.; McCann, P.J. Evaluation of breath biomarkers and serum haptoglobin concentration for diagnosis of bovine respiratory disease in heifers newly arrived at a feedlot. Am. J. Vet. Res. 2009, 70, 1291–1298. [Google Scholar] [CrossRef]
- Garner, C.E.; Smith, S.; Bardhan, P.K.; Ratcliffe, N.M.; Probert, C.S.J. A pilot study of faecal volatile organic compounds in faeces from cholera patients in Bangladesh to determine their utility in disease diagnosis. Trans. R. Soc. Trop. Med. Hyg. 2009, 103, 1171–1173. [Google Scholar] [CrossRef] [PubMed]
- Leopold, J.H.; van Hooijdonk, R.T.; Sterk, P.J.; Abu-Hanna, A.; Schultz, M.J.; Bos, L.D. Glucose prediction by analysis of exhaled metabolites—A systematic review. BMC Anesthesiol. 2014, 14, 46. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Bandodkar, A.J.; Valdés-Ramírez, G.; Windmiller, J.R.; Yang, Z.; Ramírez, J.; Chan, G.; Wang, J. Electrochemical tattoo biosensors for real-time noninvasive lactate monitoring in human perspiration. Anal. Chem. 2013, 85, 6553–6560. [Google Scholar] [CrossRef] [PubMed]
- Bandodkar, A.J.; Wang, J. Non-invasive wearable electrochemical sensors: A review. Trends Biotechnol. 2014, 32, 363–371. [Google Scholar] [CrossRef]
- Mojsym, W.; Wawrzykowski, J.; Jamioł, M.; Chrobak, Ł.; Kankofer, M. Comparative Analysis of Saliva and Plasma Proteins Patterns in Pregnant Cows—Preliminary Studies. Animals 2022, 12, 2850. [Google Scholar] [CrossRef]
- Global Survey of the Bovine Salivary Proteome: Integrating Multidimensional Prefractionation, Targeted, and Glycocapture Strategies. Journal of Proteome Research. Available online: https://pubs.acs.org/doi/10.1021/pr200516d (accessed on 4 January 2023).
- Malon, R.S.; Sadir, S.; Balakrishnan, M.; Córcoles, E.P. Saliva-based biosensors: Noninvasive monitoring tool for clinical diagnostics. BioMed Res. Int. 2014, 2014, 962903. [Google Scholar] [CrossRef]
- Gautam, S.S.; Singh, R.P.; Karsauliya, K.; Sonker, A.K.; Reddy, P.J.; Mehrotra, D.; Gupta, S.; Singh, S.; Kumar, R.; Singh, S.P. Label-free plasma proteomics for the identification of the putative biomarkers of oral squamous cell carcinoma. J. Proteom. 2022, 259, 104541. [Google Scholar] [CrossRef]
- Singh, L.K.; Pandey, M.; Baithalu, R.K.; Fernandes, A.; Ali, S.A.; Jaiswal, L.; Pannu, S.; Bhatia, N.; Mohanty, T.K.; Kumaresan, A.; et al. Comparative Proteome Profiling of Saliva Between Estrus and Non-Estrus Stages by Employing Label-Free Quantitation (LFQ) and Tandem Mass Tag (TMT)-LC-MS/MS Analysis: An Approach for Estrus Biomarker Identification in Bubalus bubalis. Front. Genet. 2022, 13, 969. [Google Scholar] [CrossRef]
- Contreras-Aguilar, M.D.; Vallejo-Mateo, P.J.; Želvytė, R.; Tecles, F.; Rubio, C.P. Changes in Saliva Analytes Associated with Lameness in Cows: A Pilot Study. Animals 2020, 10, 2078. [Google Scholar] [CrossRef]
- Hendriks, S.J.; Phyn, C.V.; Huzzey, J.M.; Mueller, K.R.; Turner, S.A.; Donaghy, D.J.; Roche, J.R. Graduate Student Literature Review: Evaluating the appropriate use of wearable accelerometers in research to monitor lying behaviors of dairy cows. J. Dairy Sci. 2020, 103, 12140–12157. [Google Scholar] [CrossRef] [PubMed]
- Andriamandroso, A.; Bindelle, J.; Mercatoris, B.; Lebeau, F. A review on the use of sensors to monitor cattle jaw movements and behavior when grazing. Biotechnol. Agron. Société Environ. 2016, 20. [Google Scholar] [CrossRef]
- Raynor, E.J.; Derner, J.D.; Soder, K.J.; Augustine, D.J. Noseband sensor validation and behavioural indicators for assessing beef cattle grazing on extensive pastures. Appl. Anim. Behav. Sci. 2021, 242, 105402. [Google Scholar] [CrossRef]
- Linnane, M.I.; Brereton, A.J.; Giller, P.S. Seasonal changes in circadian grazing patterns of Kerry cows (Bos taurus) in semi-feral conditions in Killarney National Park, Co. Kerry, Ireland. Appl. Anim. Behav. Sci. 2001, 71, 277–292. [Google Scholar] [CrossRef] [PubMed]
- Antanaitis, R.; Juozaitienė, V.; Malašauskienė, D.; Televičius, M.; Urbutis, M.; Rutkaukas, A.; Šertvytytė, G.; Baumgartner, W. Identification of Changes in Rumination Behavior Registered with an Online Sensor System in Cows with Subclinical Mastitis. Vet. Sci. 2022, 9, 454. [Google Scholar] [CrossRef] [PubMed]
- Pezeshki, A.; Stordeur, P.; Wallemacq, H.; Schynts, F.; Stevens, M.; Boutet, P.; Peelman, L.J.; De Spiegeleer, B.; Duchateau, L.; Bureau, F.; et al. Variation of inflammatory dynamics and mediators in primiparous cows after intramammary challenge with Escherichia coli. Vet. Res. 2011, 42, 15. [Google Scholar] [CrossRef]
- Zehner, N.; Umstätter, C.; Niederhauser, J.J.; Schick, M. System specification and validation of a noseband pressure sensor for measurement of ruminating and eating behavior in stable-fed cows. Comput. Electron. Agric. 2017, 136, 31–41. [Google Scholar] [CrossRef]
- Fadul, M.; Bogdahn, C.; Alsaaod, M.; Hüsler, J.; Starke, A.; Steiner, A.; Hirsbrunner, G. Prediction of calving time in dairy cattle. Anim. Reprod. Sci. 2017, 187, 37–46. [Google Scholar] [CrossRef]
- Herd Monitoring Software|SMARTBOW. Available online: https://www.smartbow.com/en/Home.aspx (accessed on 23 November 2022).
- Moonsyst. Available online: https://moonsyst.com/home (accessed on 3 December 2022).
- How It Works. Available online: https://smaxtec.com/en/function/ (accessed on 4 January 2023).
- CattleEye|Autonomous Livestock Monitoring. Available online: https://cattleeye.com/ (accessed on 3 December 2022).
- Technology. Cainthus. Available online: https://www.cainthus.com/technology (accessed on 3 December 2022).
- Saint-Dizier, M.; Chastant-Maillard, S. Potential of connected devices to optimize cattle reproduction. Theriogenology 2018, 112, 53–62. [Google Scholar] [CrossRef]
- Richeson, J.T.; Lawrence, T.E.; White, B.J. Using advanced technologies to quantify beef cattle behavior1. Transl. Anim. Sci. 2018, 2, 223–229. [Google Scholar] [CrossRef]
- Lewis Baida, B.E.; Swinbourne, A.M.; Barwick, J.; Leu, S.T.; van Wettere, W.H.E.J. Technologies for the automated collection of heat stress data in sheep. Anim. Biotelemetry 2021, 9, 4. [Google Scholar] [CrossRef]
- Rivero, M.J.; Grau-Campanario, P.; Mullan, S.; Held, S.D.E.; Stokes, J.E.; Lee, M.R.F.; Cardenas, L.M. Factors Affecting Site Use Preference of Grazing Cattle Studied from 2000 to 2020 through GPS Tracking: A Review. Sensors 2021, 21, 2696. [Google Scholar] [CrossRef] [PubMed]
- Cabezas, J.; Yubero, R.; Visitación, B.; Navarro-García, J.; Algar, M.J.; Cano, E.L.; Ortega, F. Analysis of Accelerometer and GPS Data for Cattle Behaviour Identification and Anomalous Events Detection. Entropy 2022, 24, 336. [Google Scholar] [CrossRef] [PubMed]
- Augustine, D.J.; Derner, J.D. Assessing Herbivore Foraging Behavior with GPS Collars in a Semiarid Grassland. Sensors 2013, 13, 3711–3723. [Google Scholar] [CrossRef] [PubMed]
- Ganskopp, D.C.; Bohnert, D.W. Landscape nutritional patterns and cattle distribution in rangeland pastures. Appl. Anim. Behav. Sci. 2009, 116, 110–119. [Google Scholar] [CrossRef]
- Riaboff, L.; Relun, A.; Petiot, C.-E.; Feuilloy, M.; Couvreur, S.; Madouasse, A. Identification of discriminating behavioural and movement variables in lameness scores of dairy cows at pasture from accelerometer and GPS sensors using a Partial Least Squares Discriminant Analysis. Prev. Vet. Med. 2021, 193, 105383. [Google Scholar] [CrossRef]
- Riaboff, L.; Couvreur, S.; Madouasse, A.; Roig-Pons, M.; Aubin, S.; Massabie, P.; Chauvin, A.; Bédère, N.; Plantier, G. Use of Predicted Behavior from Accelerometer Data Combined with GPS Data to Explore the Relationship between Dairy Cow Behavior and Pasture Characteristics. Sensors 2020, 20, 4741. [Google Scholar] [CrossRef]
- Caja, G.; Castro-Costa, A.; Salama, A.A.K.; Oliver, J.; Baratta, M.; Ferrer, C.; Knight, C.H. Sensing solutions for improving the performance, health and wellbeing of small ruminants. J. Dairy Res. 2020, 87, 34–46. [Google Scholar] [CrossRef]
- LokeshBabu, D.S.; Jeyakumar, S.; Vasant, P.J.; Sathiyabarathi, M.; Manimaran, A.; Kumaresan, A.; Pushpadass, H.A.; Sivaram, M.; Ramesha, K.P.; Kataktalware, M.A.; et al. Monitoring foot surface temperature using infrared thermal imaging for assessment of hoof health status in cattle: A review. J. Therm. Biol. 2018, 78, 10–21. [Google Scholar]
- McManus, C.; Tanure, C.B.; Peripolli, V.; Seixas, L.; Fischer, V.; Gabbi, A.M.; Menegassi, S.R.O.; Stumpf, M.T.; Kolling, G.J.; Dias, E.; et al. Infrared thermography in animal production: An overview. Comput. Electron. Agric. 2016, 123, 10–16. [Google Scholar] [CrossRef]
- Alsaaod, M.; Schaefer, A.L.; Büscher, W.; Steiner, A. The Role of Infrared Thermography as a Non-Invasive Tool for the Detection of Lameness in Cattle. Sensors 2015, 15, 14513–14525. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Zhang, X.D.; Liu, G. A Review: Development of Computer Vision-Based Lameness Detection for Dairy Cows and Discussion of the Practical Applications. Sensors 2021, 21, 753. [Google Scholar] [CrossRef] [PubMed]
- Berry, R.J.; Kennedy, A.D.; Scott, S.L.; Kyle, B.L.; Schaefer, A.L. Daily variation in the udder surface temperature of dairy cows measured by infrared thermography: Potential for mastitis detection. Can. J. Anim. Sci. 2003, 83, 687–693. [Google Scholar] [CrossRef]
- Zaninelli, M.; Redaelli, V.; Luzi, F.; Bronzo, V.; Mitchell, M.; Dell’Orto, V.; Bontempo, V.; Cattaneo, D.; Savoini, G. First Evaluation of Infrared Thermography as a Tool for the Monitoring of Udder Health Status in Farms of Dairy Cows. Sensors 2018, 18, 862. [Google Scholar] [CrossRef] [PubMed]
- Salles, M.S.V.; da Silva, S.C.; Salles, F.A.; Roma, L.C.; El Faro, L.; Bustos Mac Lean, P.A.; Lins de Oliveira, C.E.; Martello, L.S. Mapping the body surface temperature of cattle by infrared thermography. J. Therm. Biol. 2016, 62, 63–69. [Google Scholar] [CrossRef]
- Stokes, J.E.; Leach, K.A.; Main, D.C.J.; Whay, H.R. An investigation into the use of infrared thermography (IRT) as a rapid diagnostic tool for foot lesions in dairy cattle. Vet. J. 2012, 193, 674–678. [Google Scholar] [CrossRef]
- Lowe, G.; McCane, B.; Sutherland, M.; Waas, J.; Schaefer, A.; Cox, N.; Stewart, M. Automated Collection and Analysis of Infrared Thermograms for Measuring Eye and Cheek Temperatures in Calves. Animals 2020, 10, 292. [Google Scholar] [CrossRef]
- Lee, M.; Seo, S. Wearable Wireless Biosensor Technology for Monitoring Cattle: A Review. Animals 2021, 11, 2779. [Google Scholar] [CrossRef]
- Brod, D.L.; Bolsen, K.K.; Brent, B.E. Effect of Water Temperature in Rumen Temperature, Digestion and Rumen Fermentation in Sheep. J. Anim. Sci. 1982, 54, 179–182. [Google Scholar] [CrossRef]
- Bewley, J.M.; Grott, M.W.; Einstein, M.E.; Schutz, M.M. Impact of Intake Water Temperatures on Reticular Temperatures of Lactating Dairy Cows. J. Dairy Sci. 2008, 91, 3880–3887. [Google Scholar] [CrossRef]
- Antanaitis, R.; Anskienė, L.; Rapaliutė, E.; Bilskis, R.; Džermeikaitė, K.; Bačėninaitė, D.; Juškienė, V.; Juška, R.; Meškinytė, E. Relationship between Reticulorumen Parameters Measured in Real Time and Methane Emission and Heat Stress Risk in Dairy Cows. Animals 2022, 12, 3257. [Google Scholar] [CrossRef] [PubMed]
- Cantor, M.C.; Costa, J.H.C.; Bewley, J.M. Impact of Observed and Controlled Water Intake on Reticulorumen Temperature in Lactating Dairy Cattle. Animals 2018, 8, 194. [Google Scholar] [CrossRef] [PubMed]
- Mullins, I.L.; Truman, C.M.; Campler, M.R.; Bewley, J.M.; Costa, J.H.C. Validation of a Commercial Automated Body Condition Scoring System on a Commercial Dairy Farm. Animals 2019, 9, 287. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Kong, H.; Clark, C.; Lomax, S.; Su, D.; Eiffert, S.; Sukkarieh, S. Intelligent perception for cattle monitoring: A review for cattle identification, body condition score evaluation, and weight estimation. Comput. Electron. Agric. 2021, 185, 106143. [Google Scholar] [CrossRef]
- Roche, J.R.; Friggens, N.C.; Kay, J.K.; Fisher, M.W.; Stafford, K.J.; Berry, D.P. Invited review: Body condition score and its association with dairy cow productivity, health, and welfare. J. Dairy Sci. 2009, 92, 5769–5801. [Google Scholar] [CrossRef]
- Albornoz, R.I.; Giri, K.; Hannah, M.C.; Wales, W.J. An Improved Approach to Automated Measurement of Body Condition Score in Dairy Cows Using a Three-Dimensional Camera System. Animals 2022, 12, 72. [Google Scholar] [CrossRef]
- Ruchay, A.; Kober, V.; Dorofeev, K.; Kolpakov, V.; Miroshnikov, S. Accurate body measurement of live cattle using three depth cameras and non-rigid 3-D shape recovery. Comput. Electron. Agric. 2020, 179, 105821. [Google Scholar] [CrossRef]
- Kuzuhara, Y.; Kawamura, K.; Yoshitoshi, R.; Tamaki, T.; Sugai, S.; Ikegami, M.; Kurokawa, Y.; Obitsu, T.; Okita, M.; Sugino, T.; et al. A preliminarily study for predicting body weight and milk properties in lactating Holstein cows using a three-dimensional camera system. Comput. Electron. Agric. 2015, 111, 186–193. [Google Scholar] [CrossRef]
- Eastwood, C.; Ayre, M.; Nettle, R.; Dela Rue, B. Making sense in the cloud: Farm advisory services in a smart farming future. NJAS Wagening. J. Life Sci. 2019, 90, 100298. [Google Scholar] [CrossRef]
- Antanaitis, R.; Malašauskienė, D.; Televičius, M.; Urbutis, M.; Rutkauskas, A.; Šertvytytė, G.; Anskienė, L.; Baumgartner, W. Associations of Automatically Recorded Body Condition Scores with Measures of Production, Health, and Reproduction. Agriculture 2022, 12, 1834. [Google Scholar] [CrossRef]
- Xu, B.; Wang, W.; Guo, L.; Chen, G.; Li, Y.; Cao, Z.; Wu, S. CattleFaceNet: A cattle face identification approach based on RetinaFace and ArcFace loss. Comput. Electron. Agric. 2022, 193, 106675. [Google Scholar] [CrossRef]
- Li, W.; Ji, Z.; Wang, L.; Sun, C.; Yang, X. Automatic individual identification of Holstein dairy cows using tailhead images. Comput. Electron. Agric. 2017, 142, 622–631. [Google Scholar] [CrossRef]
- Gaber, T.; Tharwat, A.; Hassanien, A.E.; Snasel, V. Biometric cattle identification approach based on Weber’s Local Descriptor and AdaBoost classifier. Comput. Electron. Agric. 2016, 122, 55–66. [Google Scholar] [CrossRef]
- Girshick, R. Fast R-CNN. In Proceedings of the 2015 IEEE International Conference on Computer Vision (ICCV), Santiago, Chile, 11–18 December 2015; pp. 1440–1448. [Google Scholar]
- Ren, S.; He, K.; Girshick, R.; Sun, J. Faster R-CNN: Towards Real-Time Object Detection with Region Proposal Networks. Adv. Neural Inf. Process. Syst. 2015, 28, 69. Available online: http://arxiv.org/abs/1506.01497 (accessed on 3 December 2022). [CrossRef] [PubMed]
- Redmon, J.; Divvala, S.; Girshick, R.; Farhadi, A. You Only Look Once: Unified, Real-Time Object Detection. In Proceedings of the 2016 IEEE Conference on Computer Vision and Pattern Recognition (CVPR), Las Vegas, NV, USA, 27–30 June 2016; pp. 779–788. [Google Scholar]
- Liu, W.; Anguelov, D.; Erhan, D.; Szegedy, C.; Reed, S.; Fu, C.-Y.; Berg, A.C. SSD: Single Shot MultiBox Detector. In Proceedings of the Computer Vision–ECCV 2016: 14th European Conference, Amsterdam, The Netherlands, 11–14 October 2016; Volume 9905, pp. 21–37. [Google Scholar]
- Mask R-CNN | IEEE Conference Publication | IEEE Xplore. Available online: https://ieeexplore.ieee.org/document/8237584 (accessed on 3 December 2022).
- Krizhevsky, A.; Sutskever, I.; Hinton, G.E. ImageNet Classification with Deep Convolutional Neural Networks. In Proceedings of the Advances in Neural Information Processing Systems; Curran Associates, Inc.: Red Hook, NY, USA, 2012; Volume 25. [Google Scholar]
- Simonyan, K.; Zisserman, A. Very Deep Convolutional Networks for Large-Scale Image Recognition. arXiv 2015, arXiv:1409.1556. [Google Scholar] [CrossRef]
- He, K.; Zhang, X.; Ren, S.; Sun, J. Deep Residual Learning for Image Recognition. In Proceedings of the 2016 IEEE Conference on Computer Vision and Pattern Recognition (CVPR), Las Vegas, NV, USA, 27–30 June 2016; pp. 770–778. [Google Scholar]
- Zhang, L.; Gray, H.; Ye, X.; Collins, L.; Allinson, N. Automatic individual pig detection and tracking in surveillance videos. arXiv 2018, arXiv:1812.04901. [Google Scholar] [CrossRef]
- Guo, Y.; He, D.; Song, H. Region detection of lesion area of knee based on colour edge detection and bilateral projection. Biosyst. Eng. 2018, 173, 19–31. [Google Scholar] [CrossRef]
- Yao, L.; Hu, Z.; Liu, C.; Liu, H.; Kuang, Y.; Gao, Y. Cow face detection and recognition based on automatic feature extraction algorithm. In Proceedings the ACM Turing Celebration Conference—China; ACM: Chengdu, China, 2019; pp. 1–5. [Google Scholar]
- Xue, H.; Qin, J.; Quan, C.; Ren, W.; Gao, T.; Zhao, J. Open Set Sheep Face Recognition Based on Euclidean Space Metric. Math. Probl. Eng. 2021, 2021, 1–15. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, S.K.; Abidi, A.I.; Datta, D.; Sangaiah, A.K. Group Sparse Representation Approach for Recognition of Cattle on Muzzle Point Images. Int. J. Parallel Program. 2018, 46, 812–837. [Google Scholar] [CrossRef]
- Su, Q.; Tang, J.; Zhai, J.; Sun, Y.; He, D. Automatic tracking of the dairy goat in the surveillance video. Comput. Electron. Agric. 2021, 187, 106254. [Google Scholar] [CrossRef]
- Manzoli, A.; Steffens, C.; Paschoalin, R.T.; Graboski, A.M.; De Mello Brandão, H.; de Carvalho, B.C.; Bellini, J.L.; de Paula Herrmann, P.S. Volatile compounds monitoring as indicative of female cattle fertile period using electronic nose. Sens. Actuators B Chem. 2019, 282, 609–616. [Google Scholar] [CrossRef]
- Nogami, H.; Arai, S.; Okada, H.; Zhan, L.; Itoh, T. Minimized Bolus-Type Wireless Sensor Node with a Built-In Three-Axis Acceleration Meter for Monitoring a Cow’s Rumen Conditions. Sensors 2017, 17, 687. [Google Scholar] [CrossRef]
- Neitzel, A.-C.; Stamer, E.; Junge, W.; Thaller, G. Calibration of an automated California mastitis test with focus on the device-dependent variation. SpringerPlus 2014, 3, 760. [Google Scholar] [CrossRef] [PubMed]
- Ospina, P.A.; Nydam, D.V.; Stokol, T.; Overton, T.R. Associations of elevated nonesterified fatty acids and beta-hydroxybutyrate concentrations with early lactation reproductive performance and milk production in transition dairy cattle in the northeastern United States. J. Dairy Sci. 2010, 93, 1596–1603. [Google Scholar] [CrossRef] [PubMed]
- Hogeveen, H.; Kamphuis, C.; Steeneveld, W.; Mollenhorst, H. Sensors and Clinical Mastitis—The Quest for the Perfect Alert. Sensors 2010, 10, 7991–8009. [Google Scholar] [CrossRef] [PubMed]
- Rutter, S.M. Graze: A program to analyze recordings of the jaw movements of ruminants. Behav. Res. Methods Instrum. Comput. 2000, 32, 86–92. [Google Scholar] [CrossRef]
- Siivonen, J.; Taponen, S.S.; Hovinen, M.; Pastell, M.; Lensink, J.; Pyörälä, S.; Hänninen, L. Impact of clinical acute mastitis on cow behaviour. Appl. Anim. Behav. Sci. 2011, 132, 101–106. [Google Scholar] [CrossRef]
- Cyples, J.A.; Fitzpatrick, C.E.; Leslie, K.E.; DeVries, T.J.; Haley, D.B.; Chapinal, N. Short communication: The effects of experimentally induced Escherichia coli clinical mastitis on lying behavior of dairy cows. J. Dairy Sci. 2012, 95, 2571–2575. [Google Scholar] [CrossRef]
- Medrano-Galarza, C.; Gibbons, J.; Wagner, S.; de Passillé, A.M.; Rushen, J. Behavioral changes in dairy cows with mastitis. J. Dairy Sci. 2012, 95, 6994–7002. [Google Scholar] [CrossRef]
- Yeiser, E.E.; Leslie, K.E.; McGilliard, M.L.; Petersson-Wolfe, C.S. The effects of experimentally induced Escherichia coli mastitis and flunixin meglumine administration on activity measures, feed intake, and milk parameters. J. Dairy Sci. 2012, 95, 4939–4949. [Google Scholar] [CrossRef]
- Bausewein, M.; Mansfeld, R.; Doherr, M.G.; Harms, J.; Sorge, U.S. Sensitivity and Specificity for the Detection of Clinical Mastitis by Automatic Milking Systems in Bavarian Dairy Herds. Animals 2022, 12, 2131. [Google Scholar] [CrossRef] [PubMed]
- Urton, G.; von Keyserlingk, M.A.G.; Weary, D.M. Feeding Behavior Identifies Dairy Cows at Risk for Metritis. J. Dairy Sci. 2005, 88, 2843–2849. [Google Scholar] [CrossRef] [PubMed]
- Huzzey, J.M.; Veira, D.M.; Weary, D.M.; Keyserlingk, M.A.G. Von Prepartum Behavior and Dry Matter Intake Identify Dairy Cows at Risk for Metritis. J. Dairy Sci. 2007, 90, 3220–3233. [Google Scholar] [CrossRef] [PubMed]
- Liboreiro, D.N.; Machado, K.S.; Silva, P.R.B.; Maturana, M.M.; Nishimura, T.K.; Brandão, A.P.; Endres, M.I.; Chebel, R.C. Characterization of peripartum rumination and activity of cows diagnosed with metabolic and uterine diseases. J. Dairy Sci. 2015, 98, 6812–6827. [Google Scholar] [CrossRef]
- Li, S.; Wei, X.; Song, J.; Zhang, C.; Zhang, Y.; Sun, Y. Evaluation of Statistical Process Control Techniques in Monitoring Weekly Body Condition Scores as an Early Warning System for Predicting Subclinical Ketosis in Dry Cows. Animals 2021, 11, 3224. [Google Scholar] [CrossRef]
- Sturm, V.; Efrosinin, D.; Öhlschuster, M.; Gusterer, E.; Drillich, M.; Iwersen, M. Combination of Sensor Data and Health Monitoring for Early Detection of Subclinical Ketosis in Dairy Cows. Sensors 2020, 20, 1484. [Google Scholar] [CrossRef]
- Chapinal, N.; de Passillé, A.M.; Weary, D.M.; von Keyserlingk, M.A.G.; Rushen, J. Using gait score, walking speed, and lying behavior to detect hoof lesions in dairy cows. J. Dairy Sci. 2009, 92, 4365–4374. [Google Scholar] [CrossRef]
- Blackie, N.; Bleach, E.; Amory, J.; Scaife, J. Impact of lameness on gait characteristics and lying behaviour of zero grazed dairy cattle in early lactation. Appl. Anim. Behav. Sci. 2011, 129, 67–73. [Google Scholar] [CrossRef]
- Cook, N.B.; Bennett, T.B.; Nordlund, K.V. Effect of Free Stall Surface on Daily Activity Patterns in Dairy Cows with Relevance to Lameness Prevalence. J. Dairy Sci. 2004, 87, 2912–2922. [Google Scholar] [CrossRef]
- DeLaval Herd NavigatorTM—DeLaval. Available online: https://www.delaval.com/en-gb/discover-our-farm-solutions/delaval-delpro/precision-analytics/delaval-herd-navigator/ (accessed on 4 January 2023).
- Santos, C.A.; Landim, N.M.; Araújo, H.X.; Paim, T.D. Automated Systems for Estrous and Calving Detection in Dairy Cattle. AgriEngineering 2022, 4, 475–482. [Google Scholar] [CrossRef]
- Bruinjé, T.C.; Ambrose, D.J. Technical note: Validation of an automated in-line milk progesterone analysis system to diagnose pregnancy in dairy cattle. J. Dairy Sci. 2019, 102, 3615–3621. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, H.; Kou, H.; Chen, X.; Lu, Y.; Li, L.; Wang, D. Early pregnancy diagnoses based on physiological indexes of dairy cattle: A review. Trop. Anim. Health Prod. 2020, 52, 2205–2212. [Google Scholar] [CrossRef] [PubMed]
- Muasa, B.S. Monitoring the Reproductive Status of Dairy Cows Using Cow-Side Oestrus Detection Technologies. Ph.D. Thesis, The University of Edinburgh, Edinburgh, Scotland, July 2021. [Google Scholar]
- Zheng, S.; Zhou, C.; Jiang, X.; Huang, J.; Xu, D. Progress on Infrared Imaging Technology in Animal Production: A Review. Sensors 2022, 22, 705. [Google Scholar] [CrossRef] [PubMed]
- Maw, S.Z.; Zin, T.T.; Tin, P.; Kobayashi, I.; Horii, Y. An Absorbing Markov Chain Model to Predict Dairy Cow Calving Time. Sensors 2021, 21, 6490. [Google Scholar] [CrossRef] [PubMed]
| Technology | Benefits of Use | Reference |
|---|---|---|
| Milk progesterone | Milk progesterone is a potential non-invasive indicator of reproductive status in dairy cows | [41] |
| Somatic cell count | SCC has proven to be a useful, non-invasive indicator of subclinical mastitis | [36] |
| Breath, Sweat and Saliva analysis | Biomarkers for metabolic and pathologic processes are examples of metabolites found in the breath. VOCs such as ketone bodies, ethanol, methanol, and exogenous compounds are commonly associated with blood glucose levels. Saliva collection is a non-invasive alternative to blood sampling | [9,44] |
| Sensor | Method | Detected Analytes | Reference |
|---|---|---|---|
| RumiWatch (Itin + Hoch GmbH, Liestal, Switzerland) | The RW system comes with software for controlling the sensor (RW Manager) and studying unprocessed data (RW Converter). The RW sensors, which include a noseband pressure sensor, a three-axis accelerometer to track three-dimensional head motions, and a data logger, are built into a halter that fits the head of each particular animal. The noseband pressure sensor, which is mounted in a belt on the animal’s nose bridge, is connected to a tube filled with propylene glycol to detect jaw movements. As the animal moves its jaw, pressure within the tube varies, and this information is recorded with a 10 Hz resolution. Approximately 100 days of raw data logging were covered by the battery life. | Different pressure signatures of jaw motions, which are then detected and categorized into prehension bites, mastication chews, and rumination chews | [57] |
| Ear tag–based accelerometer system (Smartbow GmbH, Weibern, Austria) | The ear tag has an acceleration sensor, a radio chip, and a temperature sensor for calibration and it can monitor rumination and detect estrus and localization. | Rumination, estrus, and current localization | [63] |
| MoonSyst (Moonsyst International Ltd.: P.O. Box 1329, Kinsale, Co., Cork, Republic of Ireland) | System captures rumen data in real time. The bolus is meant to be readily ingested and will remain in the rumen (particularly the reticulum) throughout the animal’s life. System sends data from the animal to specialized cloud-based servers via a communication gateway. Farmers may use the Mooncloud software application to view information from anywhere, anytime. The bolus can be used on animals weighing more than 350 kg. Once implanted, the bolus interacts with a gateway over a large geographical region. | Heats, monitor health conditions, activity, rumen temperature and movement | [64] |
| SmaXtec (SmaXtec animal care GmbH, Graz, Austria) | The rumen bolus accurately monitors direct, informative values inside cows’ reticulum. The boluses are given once and require no further maintenance. The data from the boluses are read out by the readout devices with an integrated Internet connection and promptly transferred to the cloud. The pH and temperature variation data are gathered with an analogue-to-digital converter (A/D converter) and stored in an external memory chip. This indwelling system may be simply orally supplied to an adult cow due to its dimensions (length: 12 cm, width: 3.5 cm, weight: 210 g), and its particular construction makes it shock-proof and resistant to rumen fluid. | pH, ruminal temperature, cow activity, drinking, eating, rumen behavior | [65] |
| Body Condition Score Camera (DeLaval, International AB, Tumba, Sweden) | Body condition score system is based on a 3D camera that records certain areas of the animal: from above, the rear part of the back from the short ribs to the tail end. When a cow moves in front of the camera, the system recognizes the movement and records photographs of the cow; it then selects the best image of the cow from the video clip. The 3D camera employs light coding technology to project a pattern of infrared ray dots on the cow’s back. Following that, the distances between these specific dots are measured; the company claims that a 3D picture of the back is created, and an algorithm translates the image information into a body condition score. | BCS | [10] |
| CattleEye (Cattle Eye Ltd., Belfast, UK) | Camera is above the exit gate of a milking parlor. It records video of each cow as it exits the milking parlor. If a sort of gate or RFID system provides ID information, use it. Artificial intelligence systems in the cloud analyze video to uniquely identify the cows and track their wellness, among other things. System allows tracking the health and performance of cows in real time. It includes a dashboard that monitors and visualizes a variety of vital indicators at the herd and cow levels. | Cow identification | [66] |
| Cainthus (© 2022 Ever.Ag, Frisco, TX, USA) | Smart camera system that monitors animal behavior and farm activities 24 h a day, seven days a week, 365 days a year. It is artificial intelligence that converts visual input from cameras into real-time insights. These insights are provided daily on any farm device, phone, tablet, or computer. The information provided is accurate and unbiased. This technique is easily scalable, does not require any hardware on the cows, and requires extremely minimal maintenance in comparison to other solutions. | Animal behavior | [67] |
| BROLIS Herdline (Vilnius, Lithuania) | The analyzer examines the composition of each cow’s milk during each milking. This “mini-spectroscope” is installed in the milking stalls or milking robot in the milk line and does not use additional reagents and does not require special maintenance. The analysis of protein, fat, lactose, and electrical conductivity provides a proper evaluation of the health, productivity, and economic efficiency of dairy cattle. The data collected during milking are processed in real time and can be viewed using the BROLIS HerdLine application. | Milk fat, protein, lactose, milk electrical conductivity | [32] |
| HeatWatch (HeatWatch® DDx, Inc., Denver, CO, USA) | A tiny radionic transmitter is linked to a pressure sensor in a stiff plastic box implanted in a nylon packaging that is glued to the cow’s tail hair in the sacral region. The device is activated by the weight of the mounting animal for a minimum of 2 seconds, after which the transmitter sends the breeding approval signal to the system along with the animal’s identification. In general, this device’s assessed performance ranges from 37% to 94%. | Heat detection | [68] |
| Technology | Benefits of Use | Reference |
|---|---|---|
| Head/muzzle and noseband sensors | Noseband sensor was designed and validated as a scientific monitoring device for the automated detection of rumination and eating behaviors. It can be executed without contact with the animal. | [59] |
| Motion, movement, and behavior sensors | Accelerometers, pedometers, and GPS tracking all can be used to monitor animal behavior. Active time can predict heat; prolonged laying time can signal diseases such as mastitis, ketosis, and lameness. GPS helps to locate animals on the farm. | [69,72,76] |
| Technology | Benefits of Use | Reference |
|---|---|---|
| Infrared Thermography | Determine thermal abnormalities in animals by identifying a rise or fall in the surface temperature of skin. Infrared thermography is a noninvasive method that monitors infrared radiation emitted from the body. Inflammation, stress, calving, and heat can be evaluated. Thermography can detect physiological changes before they emerge as clinical symptoms. | [79,80,81] |
| Bolus Sensors | Wireless intraruminal boluses without constant contact, can measure and analyze ruminal and eating behavior, examine ruminal pH. | [6,41,118] |
| Body Condition Score Cameras | Tracking BCS can help reduce postpartum disease percent; it helps to notice obese or poor health animals. When it comes to production and reproduction, lower calving BCS is connected with lower rates, while greater calving BCS is associated with an increased risk of metabolic diseases | [92,93,94,96] |
| Cattle Face Recognition | Face analysis can help to identify pain, unwell animals, locate, identify, and select animals on the farm. | [102,113] |
| Electronic Nose for Estrus Detection | Can detect estrus by direct sampling of odor from the perineal headspace. | [117] |
| Disease/Status of Cow | Technology for Diagnosis | Analytes | Reference |
|---|---|---|---|
| Mastitis | Image processing, spectroscopy, electrical conductivity, biosensors, SCC sensors, tri-axial accelerometers, pedometers, spectroscopy | Temperature, lying behavior, eating behavior, milk analytes (fat, protein, electrical conductivity), rumination time, somatic cell count (SCC), milk pH, milk yield | [25,32,34,121,122,123,124,125,126,127] |
| Metritis/Endometritis | Tri-axial accelerometer, electronic feeding system | Eating, drinking time, rumination, activity, laying time, | [2,25,128,129,130] |
| Ketosis | 3D cameras, spectroscopy, milking robots, accelerometers | body condition score, BHB, milk analytes (fat, protein), milk yield, activity, rumination behavior | [10,13,131,132] |
| Acidosis | Three-axis accelerometers, angular velocity sensors, pH meter, milking robots | Milk yield, milk analytes (fat, protein) activity, rumination behavior, walking behavior, feeding behavior | [118] |
| Lameness | Tri-axial accelerometers, pedometers, video observations, accelerometers, rumination sensor | Walking behavior, feeding behavior, rumination, activity, and laying time have been linked to lameness | [25,80,133,134,135] |
| Heat | Tri-axial accelerometers, pedometers, video observations, accelerometers, spectroscopy, chemical analysis, electronic nose, acoustic sensors | Activity, milk analytes (progesterone), odor from the perineal headspace, pression, friction, rumen movement | [20,21,25,117,136,137] |
| Pregnancy | Milking robots, radioimmunoassay, enzyme immunoassay, accelerometers, pedometers | Milk progesterone, activity, temperature | [136,137,138,139,140] |
| Calving, dystocia | Intravaginal thermometer, tri-axial accelerometers, pedometers, video observations, accelerometers, rumination sensor, infrared thermometry (IRT imaging) | Body temperature, activity, rumination time, laying time | [137,141,142] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Džermeikaitė, K.; Bačėninaitė, D.; Antanaitis, R. Innovations in Cattle Farming: Application of Innovative Technologies and Sensors in the Diagnosis of Diseases. Animals 2023, 13, 780. https://doi.org/10.3390/ani13050780
Džermeikaitė K, Bačėninaitė D, Antanaitis R. Innovations in Cattle Farming: Application of Innovative Technologies and Sensors in the Diagnosis of Diseases. Animals. 2023; 13(5):780. https://doi.org/10.3390/ani13050780
Chicago/Turabian StyleDžermeikaitė, Karina, Dovilė Bačėninaitė, and Ramūnas Antanaitis. 2023. "Innovations in Cattle Farming: Application of Innovative Technologies and Sensors in the Diagnosis of Diseases" Animals 13, no. 5: 780. https://doi.org/10.3390/ani13050780
APA StyleDžermeikaitė, K., Bačėninaitė, D., & Antanaitis, R. (2023). Innovations in Cattle Farming: Application of Innovative Technologies and Sensors in the Diagnosis of Diseases. Animals, 13(5), 780. https://doi.org/10.3390/ani13050780








