Multiple Approaches to the Trophic Role of Mesopelagic Fish around the Iberian Peninsula
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling
2.3. Stomach Content Analyses
2.4. Statistical Analyses
3. Results
3.1. Prey Type
3.1.1. Intra-Specific Variability
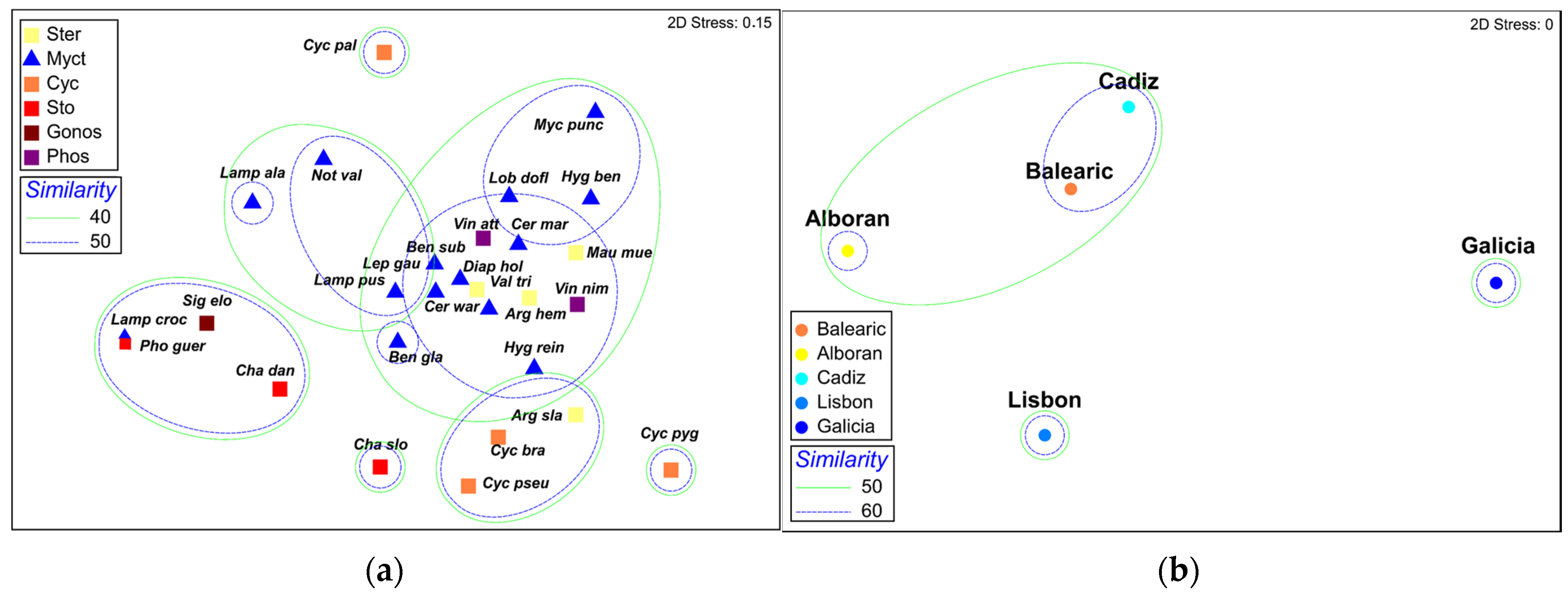
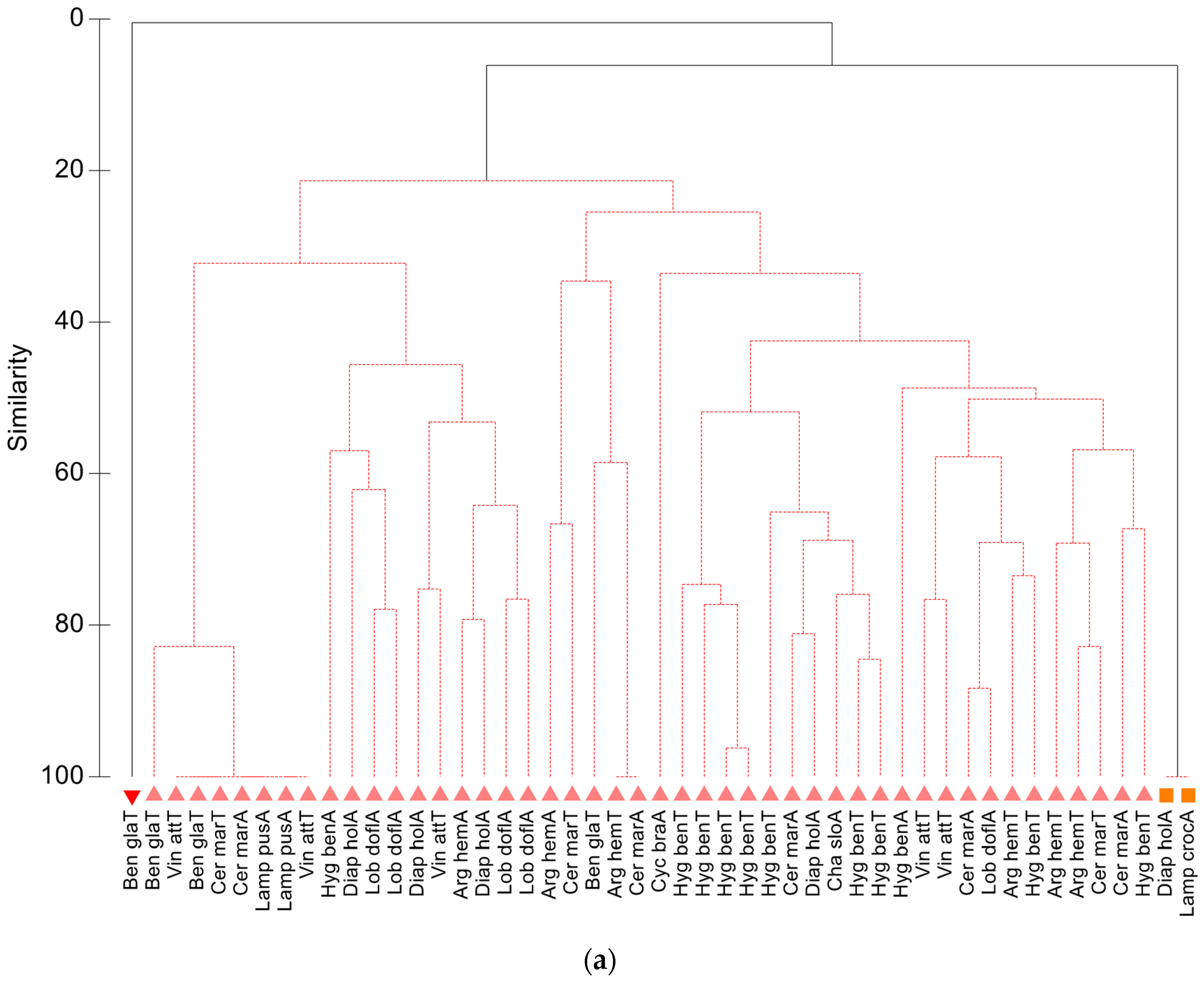
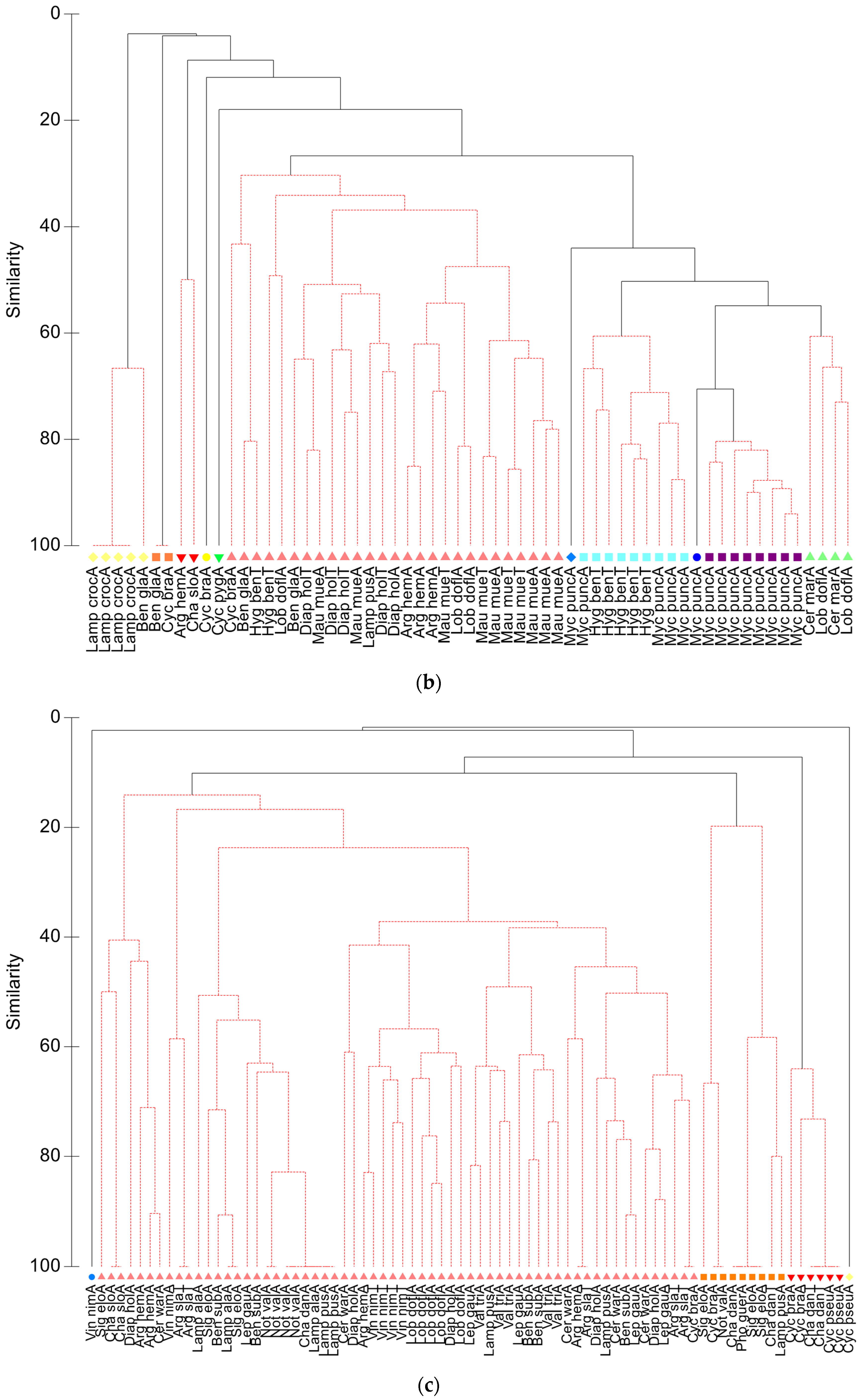
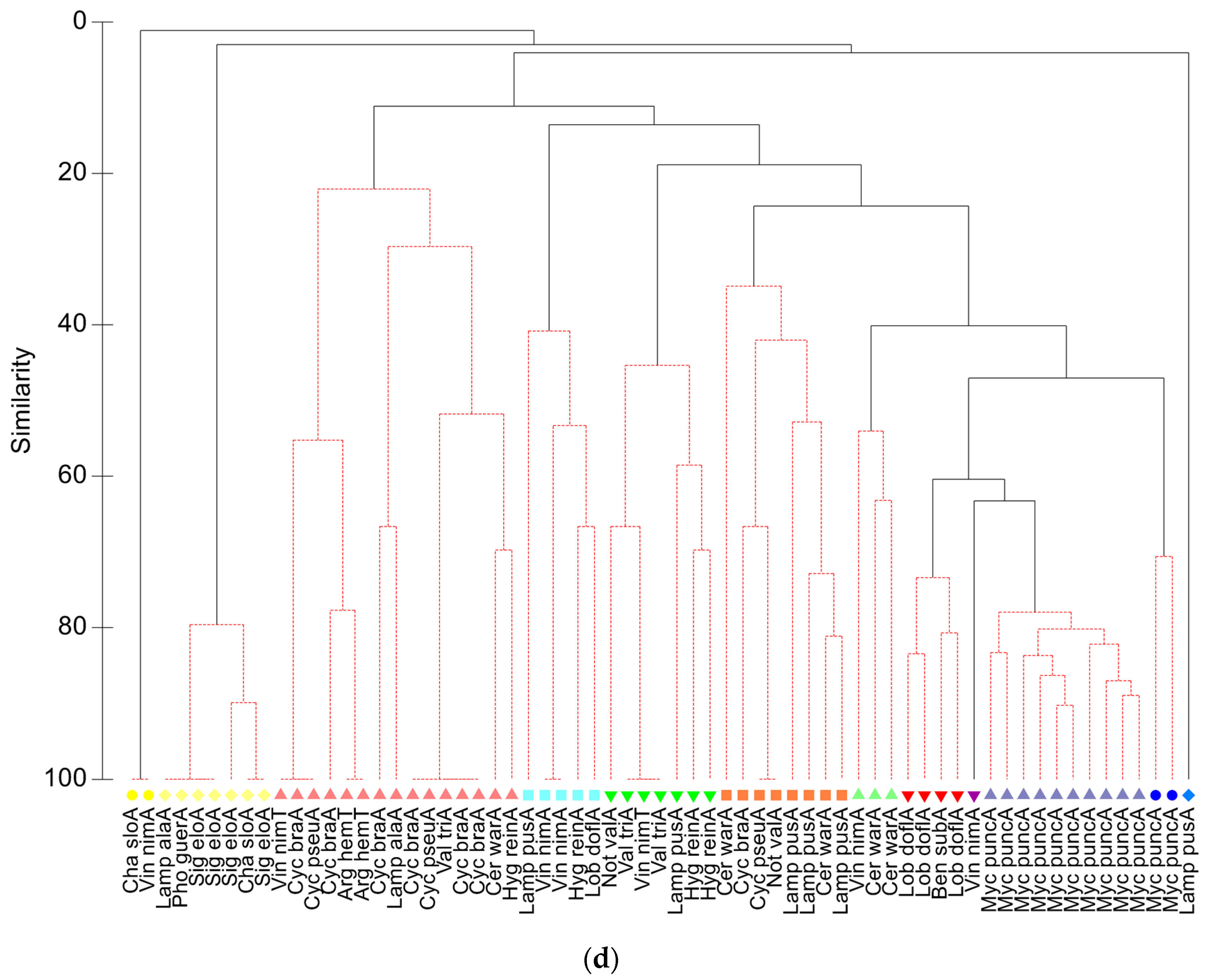
3.1.2. Community Analyses
3.1.3. Environmental Influence on Diets
3.2. Prey Number
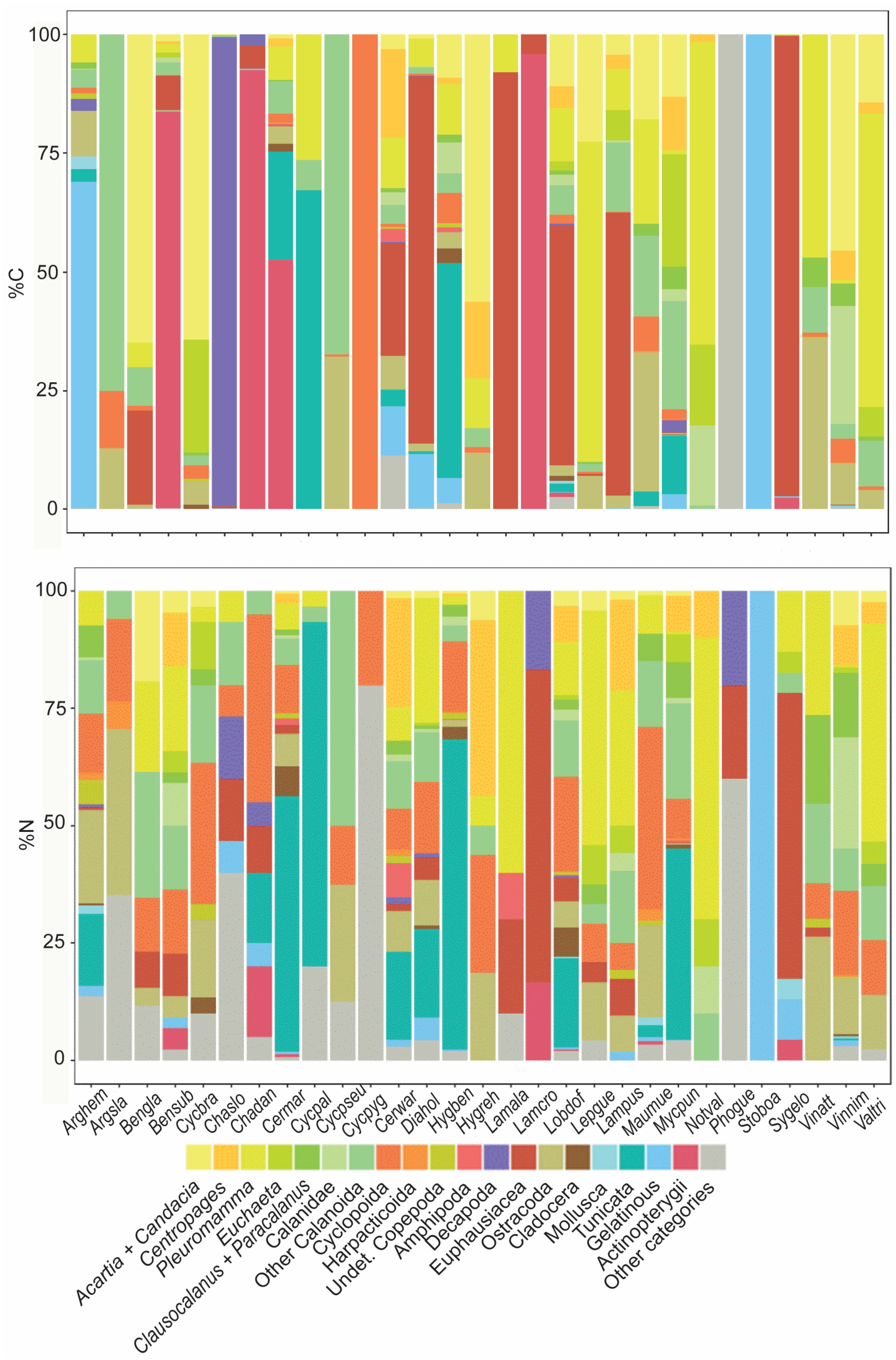
3.3. Diet Composition
3.3.1. Geographic Analysis
3.3.2. Species Diet Composition in Terms of Carbon Content
4. Discussion
4.1. Study Constraints
4.2. Feeding Guilds
4.3. Comparison of the Diet Composition of Mesopelagic Species among Zones
4.4. Influence of Zone Patterns
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gibbs, R.H., Jr.; Krueger, W.H. Biology of Midwater Fishes of the Bermuda Ocean Acre; Smithsonian Institution Press: Washington, DC, USA, 1987. [Google Scholar] [CrossRef]
- Paxton, J.R. Nominal Genera and Species of Lanternfishes (Family Myctophidae). Contrib. Sci. 1979, 322, 1–28. [Google Scholar] [CrossRef]
- Gjøsaeter, J.; Kawaguchi, K. A Review of the World Resources of Mesopelagic Fish; Food and Agriculture Organization of the United Nations: Geneva, Switzerland, 1980. [Google Scholar]
- Hulley, P.A. Results of the Research Cruises of FRV ‘Walther Herwig’ to South America. 58. Family Myctophidae (Osteichthyes, Myctophiformes). Arch. Für Fischereiwissenschaft 1981, 31, 1–300. [Google Scholar]
- Olivar, M.P.; Bernal, A.; Molí, B.; Peña, M.; Balbín, R.; Castellón, A.; Miquel, J.; Massutí, E. Vertical Distribution, Diversity and Assemblages of Mesopelagic Fishes in the Western Mediterranean. Deep Sea Res. I Oceanogr. Res. Pap. 2012, 62, 53–69. [Google Scholar] [CrossRef]
- Nelson, J.S.; Grande, T.C.; Wilson, M.V.H. Fishes of the World; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016. [Google Scholar] [CrossRef]
- Irigoien, X.; Klevjer, T.A.; Røstad, A.; Martinez, U.; Boyra, G.; Acuña, J.L.; Bode, A.; Echevarria, F.; Gonzalez-Gordillo, J.I.; Hernandez-Leon, S.; et al. Large Mesopelagic Fishes Biomass and Trophic Efficiency in the Open Ocean. Nat. Commun. 2014, 5, 3271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Proud, R.; Handegard, N.O.; Kloser, R.J.; Cox, M.J.; Brierley, A.S. From Siphonophores to Deep Scattering Layers: Uncertainty Ranges for the Estimation of Global Mesopelagic Fish Biomass. ICES J. Mar. Sci. 2019, 76, 718–733. [Google Scholar] [CrossRef] [Green Version]
- Anderson, T.R.; Martin, A.P.; Lampitt, R.S.; Trueman, C.N.; Henson, S.A.; Mayor, D.J.; Link, J. Quantifying Carbon Fluxes from Primary Production to Mesopelagic Fish Using a Simple Food Web Model. ICES J. Mar. Sci. 2019, 76, 690–701. [Google Scholar] [CrossRef]
- John Jr, M.A.; Borja, A.; Chust, G.; Heath, M.; Grigorov, I.; Mariani, P.; Martin, A.P.; Santos, R.S. A Dark Hole in Our Understanding of Marine Ecosystems and Their Services: Perspectives from the Mesopelagic Community. Front. Mar. Sci. 2016, 3, 31. [Google Scholar]
- Standal, D.; Grimaldo, E. Lost in Translation? Practical- and Scientific Input to the Mesopelagic Fisheries Discourse. Mar. Policy 2021, 134, 104785. [Google Scholar] [CrossRef]
- Olafsdottir, D.; MacKenzie, B.R.; Chosson, P.V.; Ingimundardottir, T. Dietary Evidence of Mesopelagic and Pelagic Foraging by Atlantic Bluefin Tuna (Thunnus Thynnus L) during Autumn Migrations to the Iceland Basin. Front. Mar. Sci. 2016, 3, 108. [Google Scholar] [CrossRef] [Green Version]
- Battaglia, P.; Andaloro, F.; Consoli, P.; Esposito, V.; Malara, D.; Musolino, S.; Pedà, C.; Romeo, T. Feeding Habits of the Atlantic Bluefin Tuna, Thunnus Thynnus (L 1758), in the Central Mediterranean Sea (Strait of Messina). Helgol. Mar. Res. 2013, 67, 97–107. [Google Scholar] [CrossRef] [Green Version]
- Revill, A.T.; Young, J.W.; Lansdell, M. Stable Isotopic Evidence for Trophic Groupings and Bio-Regionalization of Predators and Their Prey in Oceanic Waters off Eastern Australia. Mar. Biol. 2009, 156, 1241–1253. [Google Scholar] [CrossRef]
- Pakhomov, E.A.; Bushula, T.; Kaehler, S.; Watkins, B.P.; Leslie, R.W. Structure and Distribution of the Slope Fish Community in the Vicinity of the Sub-Antarctic Prince Edward Archipelago. J. Fish Biol. 2006, 68, 1834–1866. [Google Scholar] [CrossRef]
- Bosley, K.L.; Lavelle, J.W.; Brodeur, R.D.; Wakefield, W.W.; Emmett, R.L.; Baker, E.T.; Rehmke, K.M. Biological and Physical Processes in and around Astoria Submarine Canyon, Oregon, USA. J. Mar. Syst. 2004, 50, 21–37. [Google Scholar] [CrossRef]
- Valls, M.; Olivar, M.P.; de Puelles, M.L.F.; Molí, B.; Bernal, A.; Sweeting, C.J. Trophic Structure of Mesopelagic Fishes in the Western Mediterranean Based on Stable Isotopes of Carbon and Nitrogen. J. Mar. Syst. 2014, 138, 160–170. [Google Scholar] [CrossRef]
- Sutton, T.T. Vertical Ecology of the Pelagic Ocean: Classical Patterns and New Perspectives. J. Fish Biol. 2013, 83, 1508–1527. [Google Scholar] [CrossRef]
- Brierley, A.S. Diel Vertical Migration. Curr. Biol. 2014, 24, 1074–1076. [Google Scholar] [CrossRef] [Green Version]
- Steinberg, D.K.; Carlson, C.A.; Bates, N.R.; Goldthwait, S.A.; Madin, L.P.; Michaels, A.F. Zooplankton Vertical Migration and the Active Transport of Dissolved Organic and Inorganic Carbon in the Sargasso Sea. Deep Sea Res. I Part Oceanogr. 2000, 47, 137–158. [Google Scholar] [CrossRef]
- Davison, P.C.; Checkley, D.M., Jr.; Koslow, A.; Barlow, J. Carbon Export Mediated by Mesopelagic Fishes in the Northeast Pacific Ocean. Prog. Oceanogr. 2013, 116, 14–30. [Google Scholar] [CrossRef]
- Aksnes, D.L.; Røstad, A.; Kaartvedt, S.; Martinez, U.; Duarte, C.M.; Irigoien, X. Light Penetration Structures the Deep Acoustic Scattering Layers in the Global Ocean. Sci. Adv. 2017, 3, e1602468. [Google Scholar] [CrossRef] [Green Version]
- Klevjer, T.A.; Melle, W.; Knutsen, T.; Aksnes, D.L. Vertical Distribution and Migration of Mesopelagic Scatterers in Four North Atlantic Basins. Deep Sea Res. Part II Top. Stud. Oceanogr. 2020, 180, 104811. [Google Scholar] [CrossRef]
- Trueman, C.N.; Johnston, G.; O’Hea, B.; MacKenzie, K.M. Trophic Interactions of Fish Communities at Midwater Depths Enhance Long-Term Carbon Storage and Benthic Production on Continental Slopes. Proc. R. Soc. B Biol. Sci. 2014, 281, 20140669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hudson, J.M.; Steinberg, D.K.; Sutton, T.T.; Graves, J.E.; Latour, R.J. Myctophid Feeding Ecology and Carbon Transport along the Northern Mid-Atlantic Ridge. Deep Sea Res. I Oceanogr. Res. Pap. 2014, 93, 104–116. [Google Scholar] [CrossRef] [Green Version]
- Saba, G.K.; Burd, A.B.; Dunne, J.P.; Hernández-León, S.; Martin, A.H.; Rose, K.A.; Salisbury, J.; Steinberg, D.K.; Trueman, C.N.; Wilson, R.W.; et al. Toward a Better Understanding of Fish-based Contribution to Ocean Carbon Flux. Limnol. Oceanogr. 2021, 66, 1639–1664. [Google Scholar] [CrossRef]
- Hernández-León, S.; Olivar, M.P.; de Puelles, M.L.F.; Bode, A.; Castellón, A.; López-Pérez, C.; Tuset, V.M.; González-Gordillo, J.L. Zooplankton and Micronekton Active Flux Across the Tropical and Subtropical Atlantic Ocean. Front. Mar. Sci. 2019, 6, 535. [Google Scholar] [CrossRef] [Green Version]
- Belcher, A.; Saunders, R.; Tarling, G. Respiration Rates and Active Carbon Flux of Mesopelagic Fishes (Family Myctophidae) in the Scotia Sea, Southern Ocean. Mar. Ecol. Prog. Ser. 2019, 610, 149–162. [Google Scholar] [CrossRef] [Green Version]
- Yebra, L.; Almeida, C.; Hernández-León, S. Vertical Distribution of Zooplankton and Active Flux across an Anticyclonic Eddy in the Canary Island Waters. Deep Sea Res. I Oceanogr. Res. Pap. 2005, 52, 69–83. [Google Scholar] [CrossRef]
- Sassa, C.; Kawaguchi, K.; Hirota, Y.; Ishida, M. Distribution Depth of the Transforming Stage Larvae of Myctophid Fishes in the Subtropical–Tropical Waters of the Western North Pacific. Deep Sea Res. I Oceanogr. Res. Pap. 2007, 54, 2181–2193. [Google Scholar] [CrossRef]
- Bowlin, N.M. Ontogenetic Changes in the Distribution and Abundance of Early Life History Stages of Mesopelagic Fishes Off California; University of California: San Diego, CA, USA, 2016. [Google Scholar]
- Olivar, M.P.; Contreras, T.; Hulley, P.A.; Emelianov, M.; López-Pérez, C.; Tuset, V.; Castellón, A. Variation in the Diel Vertical Distributions of Larvae and Transforming Stages of Oceanic Fishes across the Tropical and Equatorial Atlantic. Prog. Oceanogr. 2018, 160, 83–100. [Google Scholar] [CrossRef]
- Contreras, T.; Olivar, M.; González-Gordillo, J.; Hulley, P. Feeding Patterns of Transforming and Juvenile Myctophids that Migrate into Neustonic Layers. Mar. Ecol. Prog. Ser. 2020, 650, 239–252. [Google Scholar] [CrossRef]
- Young, J.W.; Hunt, B.P.V.; Cook, T.R.; Llopiz, J.K.; Hazen, E.L.; Pethybridge, H.R.; Ceccarelli, D.; Lorrain, A.; Olson, R.J.; Allain, V.; et al. The Trophodynamics of Marine Top Predators: Current Knowledge, Recent Advances and Challenges. Deep Sea Res. Part II Top. Stud. Oceanogr. 2015, 113, 170–187. [Google Scholar] [CrossRef]
- Moteki, M.; Tsujimura, E.; Hulley, P.A. Developmental Intervals during the Larval and Juvenile Stages of the Antarctic Myctophid Fish Electrona Antarctica in Relation to Changes in Feeding and Swimming Functions. Polar Sci. 2017, 12, 88–98. [Google Scholar] [CrossRef]
- Czudaj, S.; Giesemann, A.; Hoving, H.J.; Koppelmann, R.; Lüskow, F.; Möllmann, C.; Fock, H.O. Spatial Variation in the Trophic Structure of Micronekton Assemblages from the Eastern Tropical North Atlantic in Two Regions of Differing Productivity and Oxygen Environments. Deep Sea Res. I Oceanogr. Res. Pap. 2020, 163, 103275. [Google Scholar] [CrossRef]
- Eduardo, L.N.; Bertrand, A.; Mincarone, M.M.; Santos, L.V.; Frédou, T.; Assunção, R.V.; Silva, A.; Ménard, F.; Schwamborn, R.; le Loch, F.; et al. Hatchetfishes (Stomiiformes: Sternoptychidae): Biodiversity, Trophic Ecology, Vertical Niche Partitioning and Functional Roles in the Western Tropical Atlantic. Prog. Oceanogr. 2020, 187, 102389. [Google Scholar] [CrossRef]
- De Busserolles, F.; Marshall, N.J. Seeing in the Deep-Sea: Visual Adaptations in Lanternfishes. Philos. Trans. R. Soc. 2017, 372, 20160070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pakhomov, E.A.; Perissinotto, R.; McQuaid, C.D. Prey Composition and Daily Rations of Myctophid Fishes in the Southern Ocean. Mar. Ecol. Prog. Ser. 1996, 134, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Gaskett, A.C.; Bulman, C.; He, X.; Goldsworthy, S.D. Diet Composition and Guild Structure of Mesopelagic and Bathypelagic Fishes near Macquarie Island, Australia. N. Z. J. Mar. Freshw. Res. 2001, 35, 469–476. [Google Scholar] [CrossRef]
- Pusch, C.; Hulley, P.A.; Kock, K.H. Community Structure and Feeding Ecology of Mesopelagic Fishes in the Slope Waters of King George Island (South Shetland Islands, Antarctica). Deep Sea Res. I Oceanogr. Res. Pap. 2004, 51, 1685–1708. [Google Scholar] [CrossRef]
- Shreeve, R.; Collins, M.; Tarling, G.; Main, C.; Ward, P.; Johnston, N. Feeding Ecology of Myctophid Fishes in the Northern Scotia Sea. Mar. Ecol. Prog. Ser. 2009, 386, 221–236. [Google Scholar] [CrossRef] [Green Version]
- Bernal, A.; Olivar, M.P.; Maynou, F.; de Puelles, M.L.F. Diet and Feeding Strategies of Mesopelagic Fishes in the Western Mediterranean. Prog. Oceanogr. 2015, 135, 1–17. [Google Scholar] [CrossRef]
- Contreras, T.; Olivar, M.P.; Bernal, A.; Sabatés, A. Comparative Feeding Patterns of Early Stages of Mesopelagic Fishes with Vertical Habitat Partitioning. Mar. Biol. 2015, 162, 2265–2277. [Google Scholar] [CrossRef]
- Sutton, T.T.; Hopkins, T.L. Trophic Ecology of the Stomiid (Pisces: Stomiidae) Fish Assemblage of the Eastern Gulf of Mexico: Strategies, Selectivity and Impact of a Top Mesopelagic Predator Group. Mar. Biol. 1996, 127, 179–192. [Google Scholar] [CrossRef]
- Riaz, J.; Walters, A.; Trebilco, R.; Bestley, S.; Lea, M.-A. Stomach Content Analysis of Mesopelagic Fish from the Southern Kerguelen Axis. Deep Sea Res. Part II Top. Stud. Oceanogr. 2020, 174, 104659. [Google Scholar] [CrossRef]
- Merret, N.R.; Roe, H.S.J. Patterns and Selectivity in the Feeding of Certain Mesopelagic Fishes. Mar. Biol. 1974, 28, 115–126. [Google Scholar] [CrossRef]
- Clarke, T.A. Diets of Fourteen Species of Vertically Migrating Mesopelagic Fishes in Hawaiian Water. Fish. Bull. 1980, 78, 619–640. [Google Scholar]
- Choy, C.; Portner, E.; Iwane, M.; Drazen, J. Diets of Five Important Predatory Mesopelagic Fishes of the Central North Pacific. Mar. Ecol. Prog. Ser. 2013, 492, 169–184. [Google Scholar] [CrossRef] [Green Version]
- Clarke, T.A. Diel Feeding Patterns of 16 Species of Mesopelagic Fishes from Hawaiian Waters. Fish. Bull. 1978, 76, 495–513. [Google Scholar]
- Hopkins, T.L.; Sutton, T.T.; Lancraft, T.M. The Trophic Structure and Predation Impact of a Low Latitude Midwater Fish Assemblage. Prog. Oceanogr. 1996, 38, 205–239. [Google Scholar] [CrossRef]
- Hopkins, T.L.; Gartner, J.V., Jr. Resource-Partitioning and Predation Impact of a Low-Latitude Myctophid Community. Mar. Biol. 1992, 114, 185–197. [Google Scholar] [CrossRef]
- Gorelova, T.A. A Quantitative Assessment of Consumption of Zooplankton by Epipelagic Lanternfishes (Family Myctophidae) in the Equatorial Pacific Ocean. J. Ichthyol. 1984, 23, 106–113. [Google Scholar]
- Criado-Aldeanueva, F.; García-Lafuente, J.; Navarro, G.; Ruiz, J. Seasonal and Interannual Variability of the Surface Circulation in the Eastern Gulf of Cadiz (SW Iberia). J. Geophys. Res. 2009, 114, C01011. [Google Scholar] [CrossRef] [Green Version]
- Sousa, M.C.; Alvarez, I.; de Castro, M.; Gomez-Gesteira, M.; Dias, J.M. Seasonality of Coastal Upwelling Trends under Future Warming Scenarios along the Southern Limit of the Canary Upwelling System. Prog. Oceanogr. 2017, 153, 16–23. [Google Scholar] [CrossRef]
- Relvas, P.; Barton, E.D.; Dubert, J.; Oliveira, P.B.; Peliz, A.; da Silva, J.C.B.; Santos, A.M.P. Physical Oceanography of the Western Iberia Ecosystem: Latest Views and Challenges. Prog. Oceanogr. 2007, 74, 149–173. [Google Scholar] [CrossRef] [Green Version]
- Alvarez, I.; Gomez-Gesteira, M.; deCastro, M.; Lorenzo, M.N.; Crespo, A.J.C.; Dias, J.M. Comparative Analysis of Upwelling Influence Between the Western and Northern Coast of the Iberian Peninsula. Cont. Shelf Res. 2011, 31, 388–399. [Google Scholar] [CrossRef]
- Fiúza, A.F.G. Upwelling Patterns off Portugal. In Coastal Upwelling Its Sediment Record; Suess, E., Thiede, J., Eds.; NATO Conference Series; Springer: Boston, MA, USA, 1983; Volume 10B. [Google Scholar] [CrossRef]
- Marty, J.C.; Chiavérini, J.; Pizay, M.D.; Avril, B. Seasonal and Interannual Dynamics of Nutrients and Phytoplankton Pigments in the Western Mediterranean Sea at the DYFAMED Time-Series Station (1991–1999). Deep Sea Res. Part II Top. Stud. Oceanogr. 2002, 49, 1965–1985. [Google Scholar] [CrossRef]
- Badcock, J. Gonostomatidae, Sternoptychidae, Photichthyidae. In Fishes of the North-Eastern Atlantic and the Mediterranean; Whitehead, P.J.P., Bauchot, M.L., Hureau, J.C., Nielsen, J., Tortonese, E., Eds.; UNESCO: Paris, France, 1984; Volume 1, pp. 284–324. [Google Scholar]
- Goodyear, R.H.; Zahuranec, B.J.; Pugh, W.L.; Gibbs, R.H. Ecology and Vertical Distribution of Mediterranean Midwater Fishes. In Mediterranean Biological Studies: Final Report; Report to US Navy Office of Naval Research Contract No. N00014-67-A-399-000-7; Smithsonian Institution: Washington, DC, USA, 1972; pp. 91–229. [Google Scholar]
- Hulley, P.A. Myctophidae. In Fishes of the North-Eastern Atlantic and the Mediterranean; Whitehead, P.J.P., Bauchot, M.L., Hureau, J.C., Nielsen, J., Tortonese, E., Eds.; UNESCO: Paris, France, 1984; Volume 1, pp. 429–448. [Google Scholar]
- Sutton, T.T.; Clark, M.R.; Dunn, D.C.; Halpin, P.N.; Rogers, A.D.; Guinotte, J.; Bograd, S.J.; Angel, M.V.; Perez, J.A.A.; Wishner, K.; et al. A Global Biogeographic Classification of the Mesopelagic Zone. Deep Sea Res. I Oceanogr. Res. Pap. 2017, 126, 85–102. [Google Scholar] [CrossRef]
- Casanova, J.P. Similarity of plankton distribution patterns in two nearly land-locked seas: The Mediterranean and the Red Sea. UNESCO Tech. Pap. Mar. Sci. 1986, 49, 42–46. [Google Scholar]
- Olivar, M.P.; Castellón, A.; Sabatés, A.; Sarmiento, A.; Emelianov, M.; Bernal, A.; Yang, Y.; Proud, R.; Brierley, A.S. Variation in Mesopelagic Fish Community Composition and Structure between Mediterranean and Atlantic Waters around the Iberian Peninsula. Front. Mar. Sci. 2022, 9, 1028717. [Google Scholar] [CrossRef]
- Stefanescu, C.; Cartes, J.E. Benthopelagic Habits of Adult Specimens of Lampanyctus Crocodilus (Risso 1810) (Osteichthyes, Myctophidae) in the Western Mediterranean Deep Slope. Sci. Mar. 1992, 56, 69–74. [Google Scholar]
- Bernal, A.; Olivar, M.P.; de Puelles, M.L.F. Feeding Patterns of Lampanyctus Pusillus (Pisces: Myctophidae) throughout Its Ontogenetic Development. Mar. Biol. 2013, 160, 81–95. [Google Scholar] [CrossRef]
- Battaglia, P.; Andaloro, F.; Esposito, V.; Granata, A.; Guglielmo, L.; Guglielmo, R.; Musolino, S.; Romeo, T.; Zagami, G. Diet and Trophic Ecology of the Lanternfish Electrona Risso (Cocco 1829) in the Strait of Messina (Central Mediterranean Sea) and Potential Resource Utilization from the Deep Scattering Layer (DSL). J. Mar. Syst. 2016, 159, 100–108. [Google Scholar] [CrossRef]
- Cherel, Y.; Ducatez, S.; Fontaine, C.; Richard, P.; Guinet, C. Stable Isotopes Reveal the Trophic Position and Mesopelagic Fish Diet of Female Southern Elephant Seals Breeding on the Kerguelen Islands. Mar. Ecol. Prog. Ser. 2008, 370, 239–247. [Google Scholar] [CrossRef] [Green Version]
- Saunders, R.A.; Hill, S.L.; Tarling, G.A.; Murphy, E.J. Myctophid Fish (Family Myctophidae) are Central Consumers in the Food Web of the Scotia Sea (Southern Ocean). Front. Mar. Sci. 2019, 6, 530. [Google Scholar] [CrossRef] [Green Version]
- Sabatés, A.; Olivar, M.P.; Salat, J.; Palomera, I.; Alemany, F. Physical and Biological Processes Controlling the Distribution of Fish Larvae in the NW Mediterranean. Prog. Oceanogr. 2007, 74, 355–376. [Google Scholar] [CrossRef] [Green Version]
- Salat, J.; Garcia, M.A.; Cruzado, A.; Palanques, A.; Arín, L.; Gomis, D.; Guillén, J.; de León, A.; Puigdefàbregas, J.; Sospedra, J.; et al. Seasonal Changes of Water Mass Structure and Shelf Slope Exchanges at the Ebro Shelf (NW Mediterranean). Cont. Shelf Res. 2002, 22, 327–348. [Google Scholar] [CrossRef]
- Yebra, L.; Herrera, I.; Mercado, J.M.; Cortés, D.; Gómez-Jakobsen, F.; Alonso, A.; Sánchez, A.; Salles, S.; Valcárcel-Pérez, N. Zooplankton Production and Carbon Export Flux in the Western Alboran Sea Gyre (SW Mediterranean). Prog. Oceanogr. 2018, 167, 64–77. [Google Scholar] [CrossRef]
- Rodriguez, V.; Blanco, J.M.; Jimenez-Gomez, F.; Rodriguez, J.; Echevarria, F.; Guerrero, F. Spatial Distribution of Phytoplankton Biomass during Summer Stratification in the Alboran Sea (July 1993). Publ. Espec. Inst. Español Oceanogr. 1997, 24, 53–64. [Google Scholar]
- Rodríguez, J.; García, A.; Rodríguez, V. Zooplanktonic Communities of the Divergence Zone in the Northwestern Alboran Sea. Mar. Ecol. 1982, 3, 133–142. [Google Scholar] [CrossRef]
- Cortés, D.R.; Gil, J.; García, A. General Distribution of Chlorophyll, Temperature and Salinity in the North-Western Sector of Alborán Sea. In Proceedings of the Communication from the XXIX Congress-Assemblee Pleniere CIESM, Lucerne, Switzerland, 11–19 October 1985. [Google Scholar]
- Minas, H.J.; Coste, B.; le Corre, P.; Minas, M.; Raimbault, P. Biological and Geochemical Signatures Associated with the Water Circulation through the Strait of Gibraltar and in the Western Alboran Sea. J. Geophys. Res. 1991, 96, 8755. [Google Scholar] [CrossRef]
- Ruiz, J.; Echevarría, F.; Font, J.; Ruiz, S.; García, E.; Blanco, J.M.; Jiménez-Gómez, F.; Prieto, L.; González-Alaminos, A.; García, C.M.; et al. Surface Distribution of Chlorophyll, Particles and Gelbstoff in the Atlantic Jet of the Alborán Sea: From Submesoscale to Subinertial Scales of Variability. J. Mar. Syst. 2001, 29, 277–292. [Google Scholar] [CrossRef]
- Carmo, V.; Santos, M.; Menezes, G.M.; Loureiro, C.M.; Lambardi, P.; Martins, A. Variability of Zooplankton Communities at Condor Seamount and Surrounding Areas, Azores (NE Atlantic). Deep Sea Res. Part II Top. Stud. Oceanogr. 2013, 98, 63–74. [Google Scholar] [CrossRef]
- Fraga, F. Upwelling off the Galician Coast, Northwest Spain. In Coastal Upwelling; Richardson, F.A., Ed.; American Geophysical Union: Washington, DC, USA, 1981; pp. 176–182. [Google Scholar]
- Álvarez, I.; Prego, R.; de Castro, M.; Varela, M. Galicia Upwelling Revisited: Out-of-Season Events in the Rias (1967–2009). Cienc. Mar. 2011, 38, 143–159. [Google Scholar] [CrossRef] [Green Version]
- Olivar, M.P.; Bode, A.; López-Pérez, C.; Hulley, P.A.; Hernández-León, S. Trophic Position of Lanternfishes (Pisces: Myctophidae) of the Tropical and Equatorial Atlantic Estimated Using Stable Isotopes. ICES J. Mar. Sci. 2019, 76, 649–661. [Google Scholar] [CrossRef]
- Olivar, M.P.; Hulley, P.A.; Castellón, A.; Emelianov, M.; López, C.; Tuset, V.M.; Contreras, T.; Molí, B. Mesopelagic Fishes across the Tropical and Equatorial Atlantic: Biogeographical and Vertical Patterns. Prog. Oceanogr. 2017, 151, 116–137. [Google Scholar] [CrossRef]
- Roe, H.S.J.; Badcock, J. The Diel Migrations and Distributions within a Mesopelagic Community in the North East Atlantic. 5. Vertical Migrations and Feeding of Fish. Prog. Oceanogr. 1984, 13, 389–424. [Google Scholar] [CrossRef]
- Pearre, S. Eat and Run? The Hunger/Satiation Hypothesis in Vertical Migration: History, Evidence and Consequences. Biol. Rev. Camb. Philos. Soc. 2003, 78, S146479310200595X. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.C.; Bizzarro, J.J.; Cailliet, G.M.; Ebert, D.A. Breaking with Tradition: Redefining Measures for Diet Description with a Case Study of the Aleutian Skate Bathyraja Aleutica (Gilbert 1896). Environ. Biol. Fishes 2012, 95, 3–20. [Google Scholar] [CrossRef]
- Kashian, D.R.; Zuellig, R.E.; Mitchell, K.A.; Clements, W.H. The Cost of Tolerance: Sensitivity of Stream Benthic Communities to UV-b and Metals. Ecol. Appl. 2007, 17, 365–375. [Google Scholar] [CrossRef] [Green Version]
- Anderson, M.J. PERMANOVA: A FORTRAN Computer Program for Permutational Multivariate Analysis of Variance; Department of Statistics, University of Auckland: Auckland, New Zealand, 2005. [Google Scholar]
- Clarke, K.R.; Gorley, R.N. Primer; PRIMER-e: Plymouth, UK, 2006; Volume 866, Available online: https://www.primer-e.com (accessed on 1 February 2021).
- Patil, I. Visualizations with Statistical Details: The “ggstatsplot” Approach. J. Open Source Softw. 2021, 6, 3167. [Google Scholar] [CrossRef]
- R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: http://www.r-project.org/index.html (accessed on 1 February 2021).
- Ter Braak, C.J.F. Canonical Correspondence Analysis: A New Eigenvector Technique for Multivariate Direct Gradient Analysis. Ecology 1986, 67, 1167–1179. [Google Scholar] [CrossRef] [Green Version]
- Bode, A.; Olivar, M.P.; Hernández-León, S. Trophic Indices for Micronektonic Fishes Reveal Their Dependence on the Microbial System in the North Atlantic. Sci. Rep. 2021, 11, 8488. [Google Scholar] [CrossRef]
- Bordes, F.; Uiblein, F.; Castillo, R.; Barrera, A.; Castro, J.J.; Coca, J.; Gomez, J.; Hansen, K.; Hernandez, V.; Merrett, N.; et al. Epi-and Mesopelagic Fishes, Acoustic Data, and SST Images Collected off Lanzarote, Fuerteventura, and Gran Canaria, Canary Islands, During Cruise “La Bocaina 04-97”. Inf. Técnicos Inst. Canar. Cienc. Mar. 1999, 5, 1–45. [Google Scholar]
- Sutton, T.; Hopkins, T.L. Species Composition, Abundance, and Vertical Distribution of the Stomiid (Pisces: Stomiiformes) Fish Assemblage of the Gulf of Mexico. Bull. Mar. Sci. 1996, 3, 530–542. [Google Scholar]
- Carmo, V.; Sutton, T.; Menezes, G.; Falkenhaug, T.; Bergstad, O.A. Feeding Ecology of the Stomiiformes (Pisces) of the Northern Mid-Atlantic Ridge. 1. The Sternoptychidae and Phosichthyidae. Prog. Oceanogr. 2015, 130, 172–187. [Google Scholar] [CrossRef]
- Calbet, A.; Saiz, E. The Ciliate-Copepod Link in Marine Ecosystems. Aquat. Microb. Ecol. 2005, 38, 157–167. [Google Scholar] [CrossRef] [Green Version]
- Mir-Arguimbau, J.; Olivar, M.P.; Raya, V.; Navarro, J.; Sabatés, A. Trophic Links between Blue Whiting (Micromesistius Poutassou) Larvae and the Winter Planktonic Community in the NW Mediterranean Sea. Mar. Ecol. Prog. Ser. 2021, 670, 185–201. [Google Scholar] [CrossRef]
- Gartner, J.V.; Crabtree, R.E.; Sulak, K.J. Feeding at Depth. In Fish Physiology; Randall, D.J., Farrell, A.P., Eds.; Academic Press: Cambridge, MA, USA, 1997; Volume 16, pp. 115–193. [Google Scholar]
- McClain-Counts, J.P.; Demopoulos, A.W.J.; Ross, S.W. Trophic Structure of Mesopelagic Fishes in the Gulf of Mexico Revealed by Gut Content and Stable Isotope Analyses. Mar. Ecol. 2017, 38, e12449. [Google Scholar] [CrossRef]
- Grimaldo, E.; Herrmann, B.; Brčić, J.; Cerbule, K.; Brinkhof, J.; Grimsmo, L.; Jacques, N. Prediction of Potential Net Panel Selectivity in Mesopelagic Trawls. Ocean Eng. 2022, 260, 111964. [Google Scholar] [CrossRef]
- Grimaldo, E.; Grimsmo, L.; Alvarez, P.; Herrmann, B.; Tveit, G.M.; Tiller, R.; Slizyte, R.; Aldanondo, N.; Guldberg, T.; Toldnes, B.; et al. Investigating the Potential for a Commercial Fishery in the Northeast Atlantic Utilizing Mesopelagic Species. ICES J. Mar. Sci. 2020, 77, 2541–2556. [Google Scholar] [CrossRef]
- Czudaj, S.; Koppelmann, R.; Möllmann, C.; Schaber, M.; Fock, H.O. Community Structure of Mesopelagic Fishes Constituting Sound Scattering Layers in the Eastern Tropical North Atlantic. J. Mar. Syst. 2021, 224, 103635. [Google Scholar] [CrossRef]
- Richards, T.M.; Gipson, E.E.; Cook, A.; Sutton, T.T.; Wells, R.J.D. Trophic Ecology of Meso- and Bathypelagic Predatory Fishes in the Gulf of Mexico. ICES J. Mar. Sci. 2019, 76, 662–672. [Google Scholar] [CrossRef] [Green Version]
- Eduardo, L.N.; Lucena-Frédou, F.; Mincarone, M.M.; Soares, A.; le Loch, F.; Frédou, T.; Ménard, F.; Bertrand, A. Trophic Ecology, Habitat, and Migratory Behaviour of the Viperfish Chauliodus Sloani Reveal a Key Mesopelagic Player. Sci. Rep. 2020, 10, 20996. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, P.; Ammendolia, G.; Esposito, V.; Romeo, T.; Andaloro, F. Few but Relatively Large Prey: Trophic Ecology of Chauliodus Sloani (Pisces: Stomiidae) in Deep Waters of the Central Mediterranean Sea. J. Ichthyol. 2018, 58, 8–16. [Google Scholar] [CrossRef]
- Borodulina, O.D. The Feeding of Mesopelagic Predatory Fish in the Open Ocean. J. Ichthyol. 1972, 12, 692–703. [Google Scholar]
- Woods, B.; Walters, A.; Hindell, M.; Trebilco, R. Isotopic Insights into Mesopelagic Niche Space and Energy Pathways on the Southern Kerguelen Plateau. Deep Sea Res. Part II Top. Stud. Oceanogr. 2020, 174, 104657. [Google Scholar] [CrossRef]
- Saunders, R.; Collins, M.; Ward, P.; Stowasser, G.; Hill, S.; Shreeve, R.; Tarling, G. Predatory Impact of the Myctophid Fish Community on Zooplankton in the Scotia Sea (Southern Ocean). Mar. Ecol. Prog. Ser. 2015, 541, 45–64. [Google Scholar] [CrossRef] [Green Version]
- Kozlov, A.N. A Review of the Trophic Role of Mesopelagic Fish of the Family Myctophidae in the Southern Ocean Ecosystem. CCAMLR Sci. 1995, 2, 71–77. [Google Scholar]
- Margalef, R. Producción Secundaria. Alimentación de los Animales. In Ecología; Margalef, R., Ed.; Editorial Omega: Barcelona, Spain, 1974; pp. 473–521. [Google Scholar]
- Rodríguez-Graña, L.; Castro, L.; Loureiro, M.; González, H.; Calliari, D. Feeding Ecology of Dominant Larval Myctophids in an Upwelling Area of the Humboldt Current. Mar. Ecol. Prog. Ser. 2005, 290, 119–134. [Google Scholar] [CrossRef]
- Di Carlo, B.S.; Costanzo, G.; Fresi, E.; Guglielmo, L.; Lanora, A. Feeding Ecology and Stranding Mechanisms in Two Lanternfishes, Hygophum Benoiti and Myctophum Punctatum. Mar. Ecol. Prog. Ser. 1982, 9, 13–24. [Google Scholar] [CrossRef]
- Zobel, M. The Relative of Species Pools in Determining Plant Species Richness: An Alternative Explanation of Species Coexistence? Trends Ecol. Evol. 1997, 12, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Van Noord, J.E.; Olson, R.J.; Redfern, J.V.; Kaufmann, R.S. Diet and Prey Selectivity in Three Surface-Migrating Myctophids in the Eastern Tropical Pacific. Ichthyol. Res. 2013, 60, 287–290. [Google Scholar] [CrossRef]
- Jennings, S.; Maxwell, T.; Schratzberger, M.; Milligan, S. Body-Size Dependent Temporal Variations in Nitrogen Stable Isotope Ratios in Food Webs. Mar. Ecol. Prog. Ser. 2008, 370, 199–206. [Google Scholar] [CrossRef]
- Sarmiento-Lezcano, A.N.; Olivar, M.P.; Peña, M.; Landeira, J.M.; Armengol, L.; Medina-Suárez, I.; Castellón, A.; Hernández-León, S. Carbon Remineralization by Small Mesopelagic and Bathypelagic Stomiiforms in the Northeast Atlantic Ocean. Prog. Oceanogr. 2022, 203, 102787. [Google Scholar] [CrossRef]
- Hopkins, T.L.; Baird, R.C. Feeding Ecology of Four Hatchetfishes (Sternoptychidae) in the Eastern Gulf of Mexico. Bull. Mar. Sci. 1985, 36, 260–277. [Google Scholar]
- Choy, C.A.; Popp, B.N.; Hannides, C.C.S.; Drazen, J.C. Trophic Structure and Food Resources of Epipelagic and Mesopelagic Fishes in the North Pacific Subtropical Gyre Ecosystem Inferred from Nitrogen Isotopic Compositions. Limnol. Oceanogr. 2015, 60, 1156–1171. [Google Scholar] [CrossRef]
- Romero-Romero, S.; Choy, C.A.; Hannides, C.C.S.; Popp, B.N.; Drazen, J.C. Differences in the Trophic Ecology of Micronekton Driven by Diel Vertical Migration. Limnol. Oceanogr. 2019, 64, 1473–1483. [Google Scholar] [CrossRef] [Green Version]
- Fanelli, E.; Cartes, J.E.; Papiol, V. Food web structure of deep-sea macrozooplankton and micronekton off the Catalan slope: Insight from stable isotopes. J. Mar. Syst. 2011, 87, 79–89. [Google Scholar] [CrossRef]
- Kiørboe, T. How zooplankton feed: Mechanisms, traits and trade-offs. Biol. Rev. 2011, 86, 311–339. [Google Scholar] [CrossRef] [PubMed]
- Sardou, J.; Etienne, M.; Andersen, V. Seasonal Abundance and Vertical Distributions of Macroplankton and Micronekton in the Northwestern Mediterranean Sea. Oceanol. Acta 1996, 19, 645–656. [Google Scholar]
- DeWitt, F.A.; Cailliet, G.M. Feeding Habits of Two Bristlemouth Fishes, Cyclothone Acclinidens and C. Signata (Gonostomatidae). Copeia 1972, 868, 868–871. [Google Scholar] [CrossRef]
- Sobrinho-Gonçalves, L.; Moita, M.T.; Garrido, S.; Cunha, M.E. Environmental Forcing on the Interactions of Plankton Communities across a Continental Shelf in the Eastern Atlantic Upwelling System. Hydrobiologia 2013, 713, 167–182. [Google Scholar] [CrossRef]
- Souza, C.S.; Mafalda Jr., P.; Sallés, S.; Ramirez, T.; Cortés, D.; Garcia, A.; Mercado, J.; Vargas-Yañez, M. Seasonal Changes in the Distribution and Abundance of Marine Cladocerans of the Northwest Alboran Sea (Western Mediterranean), Spain. Braz. Arch. Biol. Technol. 2011, 54, 543–550. [Google Scholar] [CrossRef] [Green Version]
- Yamada, Y.; Ikeda, T. Metabolism and chemical composition of four pelagic amphipods in the Oyashio region, western subarctic Pacific Ocean. Mar. Ecol. Prog. Ser. 2003, 253, 233–241. [Google Scholar] [CrossRef]
- Gorsky, G.; Dallot, S.; Sardou, J.; Fenaux, R.; Carré, C.; Palazzoli, I. C and N composition of some northwestern Mediterranean zooplankton and micronekton species. J. Exp. Mar. Biol. Ecol. 1988, 124, 133–144. [Google Scholar] [CrossRef]
- Uye, S.I. Length-weight relationships of important zooplankton from the Inland Sea of Japan. J. Oceanogr. Soc. Japan 1982, 38, 149–158. [Google Scholar] [CrossRef]
- Chisholm, L.A.; Roff, J.C. Size-weight relationships and biomass of tropical neritic copepods off Kingston, Jamaica. Mar. Biol. 1990, 106, 71–77. [Google Scholar] [CrossRef]
- Van der Lingen, C.D. Diet of sardine Sardinops sagax in the southern Benguela upwelling ecosystem. S. Afr. J. Mar. Sci. 2002, 24, 301–316. [Google Scholar] [CrossRef]
- Breteler, W.K.; Fransz, H.G.; Gonzalez, S.R. Growth and development of four calanoid copepod species under experimental and natural conditions. Nether. J. Sea Res. 1982, 16, 195–207. [Google Scholar] [CrossRef]
- Webber, M.K.; Roff, J.C. Annual biomass and production of the oceanic copepod community off Discovery Bay, Jamaica. Mar. Biol. 1995, 123, 481–495. [Google Scholar] [CrossRef]
- Satapoomin, S. Carbon content of some common tropical Andaman Sea copepods. J. Plankton Res. 1999, 21, 2117–2123. [Google Scholar] [CrossRef]
- Hay, S.J.; Kiørboe, T.; Matthews, A. Zooplankton biomass and production in the North Sea during the Autumn Circulation Experiment, October 1987-March 1988. Cont. Shelf. Res. 1991, 11, 1453–1476. [Google Scholar] [CrossRef]
- Ikeda, T. Respiration and ammonia excretion by marine metazooplankton taxa: Synthesis toward a global-bathymetric model. Mar. Biol. 2014, 161, 2753–2766. [Google Scholar] [CrossRef]
- Nakamura, A.; Matsuno, K.; Abe, Y.; Shimada, H.; Yamaguchi, A. Length-weight relationships and chemical composition of the dominant mesozooplankton taxa/species in the subarctic Pacific, with special reference to the effect of lipid accumulation in Copepoda. Zool. Stud. 2017, 56, 13. [Google Scholar] [CrossRef]
- Ara, K. Length-weight relationships and chemical content of the planktonic copepods in the Cananéia Lagoon estuarine system, São Paulo, Brazil. Plan. Biol. Ecol. 2001, 48, 121–127. [Google Scholar]
- Koski, M.; Swalethorp, R.; Kjellerup, S.; Nielsen, T.G. Aggregate-colonizing copepods in a glacial fjord: Population dynamics, vertical distribution and allometric scaling of growth and mortality rates of Microsetella norvegica and Oncaea spp. Prog. Oceanogr. 2021, 197, 102670. [Google Scholar] [CrossRef]
- Kleppel, G.S.; Holliday, D.V.; Pieper, R.E. Trophic interactions between copepods and microplankton: A question about the role of diatoms. Limnol. Oceanogr. 1991, 36, 172–178. [Google Scholar] [CrossRef]
- Mizdalski, E. Weight and length data of zooplankton in the Weddell Sea in austral spring 1986 (ANT-V/3). In Reports on Polar Research; Alfred Wegener Institute for Polar and Marine Research: Bremerhaven, Germany, 1988; p. 55. [Google Scholar]
- Matthews, J.B.L.; Hestad, L. Ecological studies on the deep-water pelagic community of Korsfjorden, western Norway: Length/weight relationships for some macroplanktonic organisms. Sarsia 1977, 63, 57–63. [Google Scholar] [CrossRef]
- Imao, F. Zooplankton Community Structure and Functional Role in Carbon Cycle in the Oyashio Region, Western North Pacific. Master’s Thesis, Hokkaido University, Hokkaido, Japan, 2005; p. 42. [Google Scholar]
- Bednaršek, N.; Možina, J.; Vogt, M.; O’Brien, C.; Tarling, G.A. The global distribution of pteropods and their contribution to carbonate and carbon biomass in the modern ocean. Earth Sys. Sci. Data 2012, 4, 167–186. [Google Scholar] [CrossRef] [Green Version]
- Vinogradov, A.P. The Elementary Chemical Composition of Marine Organisms; Translated from the Russian by Efron, J. and Setlow, J.K.; Sears Foundation for Marine Research, Yale University: New Haven, CO, USA, 1953; p. 647. [Google Scholar]
- Putt, M.; Stoecker, D.K. An experimentally determined carbon: Volume ratio for marine “oligotrichous” ciliates from estuarine and coastal waters. Limnol. Oceanogr. 1989, 34, 1097–1103. [Google Scholar] [CrossRef]
- Michaels, A.F.; Caron, D.A.; Swanberg, N.R.; Howse, F.A.; Michaels, C.M. Planktonic sarcodines (Acantharia, Radiolaria, Foraminifera) in surface waters near Bermuda: Abundance, biomass and vertical flux. J. Plank. Res. 1995, 17, 131–163. [Google Scholar] [CrossRef]
- Menden-Deuer, S.; Lessard, E.J. Carbon to volume relationships for dinoflagellates, diatoms, and other protist plankton. Limnol. Oceanogr. 2000, 45, 569–579. [Google Scholar] [CrossRef] [Green Version]
- Urban-Rich, J.; Hansell, D.A.; Roman, M.R. Analysis of copepod fecal pellet carbon using a high temperature combustion method. Mar. Ecol. Prog. Ser. 1998, 171, 199–208. [Google Scholar] [CrossRef] [Green Version]
- Deibel, D. Feeding mechanism and house of the appendicularian Oikopleura vanhoeffeni. Mar. Biol. 1986, 93, 429–436. [Google Scholar] [CrossRef]
- Heron, A.C.; McWilliam, P.S.; Pont, G.D. Length–weight relation in the salp Thalia democratica and potential of salps as a source of food. Mar. Ecol. Prog. Ser. 1988, 42, 125–132. [Google Scholar] [CrossRef]
- Bert, D.; Danjon, F. Carbon concentration variations in the roots, stem and crown of mature Pinus pinaster (Ait.). Forest Ecol. Manag. 2006, 222, 279–295. [Google Scholar] [CrossRef]


| Species | Migr | Region | Size Range (SL, mm) | N st | N with Prey |
|---|---|---|---|---|---|
| Stomiiformes | |||||
| Gonostomatidae | |||||
| Cyclothone braueri | NM | Mediterranean | |||
| Bal | A: 23 | 5 | 5 | ||
| Alb | A: 16.50–23.50 | 5 | 4 | ||
| Atlantic | |||||
| Cad | A: 24–25 | 10 | 6 | ||
| Lis | A: 16–33 | 10 | 9 | ||
| Gal | A: 26–27 | 5 | 5 | ||
| Cyclothone pallida | NM | Gal | A: 32–52 | 12 | 8 |
| Cyclothone pseudopallida | NM | Atlantic | |||
| Cad | A: 20–35 | 5 | 4 | ||
| Lis | A: 18–26 | 5 | 5 | ||
| Gal | A: 28 | 1 | 1 | ||
| Cyclothone pygmaea | NM | Alb | A: 18–35 | 5 | 5 |
| Sigmops elongatus | PM | Atlantic | |||
| Cad | A: 116–140 | 6 | 6 | ||
| Lis | A: 106–125 | 5 | 4 | ||
| Sternoptychidae | |||||
| Argyropelecus hemigymnus | PM | Mediterranean | |||
| Bal | T: 7–16.2 | 5 | 5 | ||
| A: 22–27 | 3 | 3 | |||
| Alb | A: 20–35 | 11 | 11 | ||
| Atlantic | |||||
| Cad | A: 21.5–43 | 4 | 4 | ||
| T: 9–14.5 | 4 | 4 | |||
| Lis | A: 18–31 | 4 | 0 | ||
| Argyropelecus sladeni | PM | Cad | T: 7–11 | 5 | 5 |
| Maurolicus muelleri | PM | Alb | T: 13–15 | 4 | 4 |
| A: 16–26.5 | 6 | 6 | |||
| Valenciennellus tripunctulatus | NM | Atlantic | |||
| Cad | A: 18.5–24.5 | 5 | 5 | ||
| Lis | A: 23–27.5 | 4 | 4 | ||
| Phosichthyidae | |||||
| Vinciguerria attenuata | VM | Bal | T: 16.5–19 | 5 | 5 |
| Vinciguerria nimbaria | VM | Atlantic | |||
| Cad | T: 15–17.5 | 5 | 5 | ||
| A: 26–33 | 5 | 5 | |||
| Lis | T: 16–23 | 5 | 4 | ||
| A: 23.5–35.5 | 6 | 6 | |||
| Stomiidae | |||||
| Chauliodus danae | VM | Atlantic | |||
| Cad | T: 28.5–38 | 5 | 3 | ||
| A: 73–123 | 5 | 5 | |||
| Gal | A: 97–116 | 5 | 5 | ||
| Chauliodus sloani | VM | Mediterranean | |||
| Bal | A: 61–73 | 3 | 2 | ||
| Alb | A: 200 | 1 | 1 | ||
| Atlantic | |||||
| Cad | A: 40–113 | 5 | 5 | ||
| Lis | A: 43–123 | 7 | 7 | ||
| Photostomias guernei | PM | Lis | A: 63–72 | 4 | 4 |
| Stomias boa | VM | Cad | A: 64–123 | 5 | 2 |
| Myctophiformes | |||||
| Myctophidae | |||||
| Benthosema glaciale | VM | Mediterranean | |||
| Bal | T: 17.5–20 | 5 | 5 | ||
| A: 22–29.5 | 5 | 5 | |||
| Alb | A: 31–32.5 | 5 | 5 | ||
| Benthosema suborbitale | VM | Atlantic | |||
| Cad | A: 20–29 | 5 | 5 | ||
| Lis | A: 27 | 1 | 1 | ||
| Ceratoscopelus maderensis | VM | Mediterranean | |||
| Bal | T: 16.5–18.5 | 5 | 5 | ||
| A: 21–44 | 5 | 5 | |||
| Ceratoscopelus warmingii | VM | Atlantic | |||
| Cad | A: 36–42 | 5 | 5 | ||
| Lis | A: 19–29 | 5 | 5 | ||
| Diaphus holti | VM | Mediterranean | |||
| Bal | A: 33–38 | 5 | 5 | ||
| Alb | T: 16–18 | 4 | 4 | ||
| Atlantic | A: 43 | 1 | 1 | ||
| Cad | A: 45–50 | 5 | 5 | ||
| Hygophum benoiti | VM | Mediterranean | |||
| Bal | T: 11.5–18 | 10 | 10 | ||
| A: 19.5–20 | 2 | 2 | |||
| Alb | T: 15–19.4 | 8 | 7 | ||
| Hygophum reinhardtii | VM | Lis | A: 20–39 | 5 | 5 |
| Lampanyctus alatus | VM | Atlantic | |||
| Cad | A: 28–52 | 4 | 4 | ||
| Lis | A: 46–70 | 4 | 4 | ||
| Lampanyctus crocodilus | VM | Mediterranean | |||
| Bal | A: 35.5, 61.5 | 2 | 2 | ||
| Alb | A: 61–84 | 5 | 5 | ||
| Lampanyctus pusillus | VM | Mediterranean | |||
| Bal | T: 10, 18 | 2 | 2 | ||
| A: 10–57.5 | 4 | 4 | |||
| Alb | A: 31 | 1 | 1 | ||
| Atlantic | |||||
| Cad | A: 25.5–33 | 5 | 5 | ||
| Lis | A: 17.5–49 | 6 | 6 | ||
| Lepidophanes gaussi | VM | Cad | A: 18–24 | 5 | 5 |
| Lobianchia dofleini | VM | Mediterranean | |||
| Bal | A: 30–33.5 | 5 | 5 | ||
| Alb | A: 25.5–28.5 | 5 | 5 | ||
| Atlantic | |||||
| Cad | A: 20.5–25 | 5 | 5 | ||
| Lis | A: 25–28 | 4 | 4 | ||
| Myctophum punctatum | VM | Mediterranean | |||
| Alb | A: 32–49 | 14 | 14 | ||
| Atlantic | |||||
| Lis | A: 41.5–60 | 12 | 12 | ||
| Notolychnus valdiviae | VM | Atlantic | |||
| Cad | A: 18.5–20 | 5 | 5 | ||
| Lis | A: 20, 21 | 2 | 2 | ||
| Source | Levels | df | SS | MS | Pseudo-F-Statistic | p (perm) |
|---|---|---|---|---|---|---|
| Fish species | 28 | 25 | 2.56 × 105 | 10,250 | 4.6669 | 0.001 |
| Zone | 5 | 4 | 51,261 | 12,815 | 5.8352 | 0.001 |
| Zone (fish species) | 34 | 2.07 × 105 | 6098.9 | 2.5025 | 0.001 | |
| Dev (fish species) | 2 | 1 | 8572.6 | 8572.6 | 3.9033 | 0.001 |
| Migrator | 3 | 1 | 4143.3 | 4143.3 | 1.8866 | 0.02 |
| Groups | Sim % | t | P | perm | Cum % | Prey Categories (% diss) |
|---|---|---|---|---|---|---|
| A. hemigymnus Alboran, Cadiz | 14.71 | 1.88 | 0.03 | 35 | 57 | Appendicularia (18); Calanoida (13); Clausocalanus + Paracalanus (10); Conchoecia (9) |
| A. hemigymnus Balearic, Alboran | 14.79 | 1.85 | 0.007 | 312 | 58 | Appendicularia (23); Calanoida (14); Conchoecia (11); Clausocalanus + Paracalanus (10) |
| C. braueri Lisbon, Galicia | 0 | 1.93 | 0.046 | 12 | 57 | Oncaea (22); Calanoida (21); POM (14) |
| C. maderensis Balearic, Alboran | 12.55 | 1.77 | 0.016 | 123 | 53 | Appendicularia (21); Copepoda (12); Pleuromamma (11); Conchoecia (9) |
| C. warmingii Cadiz, Lisbon | 8.4 | 2.05 | 0.01 | 126 | 56 | Centropages (17); Conchoecia (12); Appendicularia (10); Calanoida (9); Pleuromamma (6) |
| D. holti Balear, Cadiz | 19.5 | 1.83 | 0.012 | 126 | 52 | Conchoecia (16); Pleuromamma (11), Oncaea (9); Euphausiacea (8); Chromista (8) |
| D. holti Alboran, Cadiz | 35.6 | 1.88 | 0.008 | 126 | 57 | Conchoecia (13); Pleuromamma (12); Oncaea (12); Chromista (10); Appendicularia (10) |
| H. benoiti Balearic, Alboran | 23.59 | 2.05 | 0.001 | 987 | 54 | Appendicularia (36); Oncaea (7); Corycaeidae (6); Evadne + Podon (5) |
| L. dofleini Alboran, Cadiz | 39.38 | 2.02 | 0.005 | 126 | 53 | Appendicularia (12); Centropages (12); Calanoida (8); Corycaeidae (7); Conchoecia (6); Penilia (6) |
| L. dofleini Balearic, Cadiz | 33.78 | 2.23 | 0.008 | 126 | 50 | Oncaea (16); Corycaeidae (14); Euphausiacea (11); Conchoecia (9) |
| L. dofleini Alboran, Lisbon | 37.8 | 1.42 | 0.024 | 126 | 35 | Appendicularia (14); Centropages (8); Calanoida (7); Penilia (6) |
| L. dofleini Balear, Lisbon | 26.38 | 1.84 | 0.006 | 126 | 38 | Calanoida (9); Euphausicea (8); Centropages (8); Calanus (7); Candacia (7); Larval Euphausiacea (5); Candacia (5); Calanus (5) |
| L. dofleini Cadiz, Lisbon | 29.33 | 2.28 | 0.01 | 126 | 57 | Corycaeidae (13); Conchoecia (12); Oncaea (12); Calanoida (7); Centropages (7); Appendicularia (6) |
| L. dofleini Balearic, Lisbon | 39.67 | 1.79 | 0.006 | 126 | 52 | Calanoida (9); Euphausiacea (8); Centropages (8); Calanus (7); Candacia (7); Pleuromamma (7); Conchoecia (6) |
| M. punctatum Alboran, Lisbon | 17.53 | 5.85 | 0.001 | 999 | 51 | Appendicularia (21); Centropages (11); Euchaeta (10); Calanoida (9) |
| V. tripunctulatus Cadiz, Lisbon | 5.04 | 3.4 | 0.009 | 163 | 61 | Pleuromamma (29), Calanoida (12), Conchoecia (11), Centropages (10) |
| Fish Species | Groups | t | df | p (perm) | Sim % | Prey Cat (% Diss) | |
|---|---|---|---|---|---|---|---|
| A. hemigymnus | T, A | 1.61 | 11 | 0.045 | T: 63.9; A: 30.9 T, A: 34.1 | Transformation | |
| Ostracoda | 67.5 | ||||||
| Non-Calanoida | 30.1 | ||||||
| Adult | |||||||
| Ostracoda | 53.5 | ||||||
| Appendicularia | 19.1 | ||||||
| H. benoiti A | T, A | 1.75 | 15 | 0.01 | T: 40.9; A: 38.2 T, A: 30.0 | Transformation | |
| Oncaea | 53.8 | ||||||
| Calanoida | 22.2 | ||||||
| Appendicularia | 15.1 | ||||||
| Adult | |||||||
| Pleuromamma | 55.1 | ||||||
| Oncaea | 45.1 | ||||||
| Species | M | NM | Av Diss | SD | Contr (%) |
|---|---|---|---|---|---|
| Pleuromamma | 0.78 | 0.33 | 12.55 | 0.85 | 14.75 |
| Non-Calanoida | 1.14 | 0.34 | 12.39 | 0.91 | 14.56 |
| Other Calanoida | 1.23 | 0.32 | 11.16 | 0.90 | 13.11 |
| Eucarida (euphausiids and decapods) | 0.36 | 0.27 | 8.70 | 0.61 | 10.22 |
| Ostracoda | 0.44 | 0.24 | 7.61 | 0.66 | 8.94 |
| Appendicularia | 1.16 | 0.00 | 7.18 | 0.50 | 8.44 |
| Centropages | 0.63 | 0.05 | 5.24 | 0.52 | 6.16 |
| Euchaeta | 0.44 | 0.14 | 4.70 | 0.50 | 5.52 |
| Candacia spp. and Acartia spp. | 0.36 | 0.05 | 4.24 | 0.46 | 4.98 |
| Gelatinous | 0.14 | 0.16 | 3.48 | 0.35 | 4.08 |
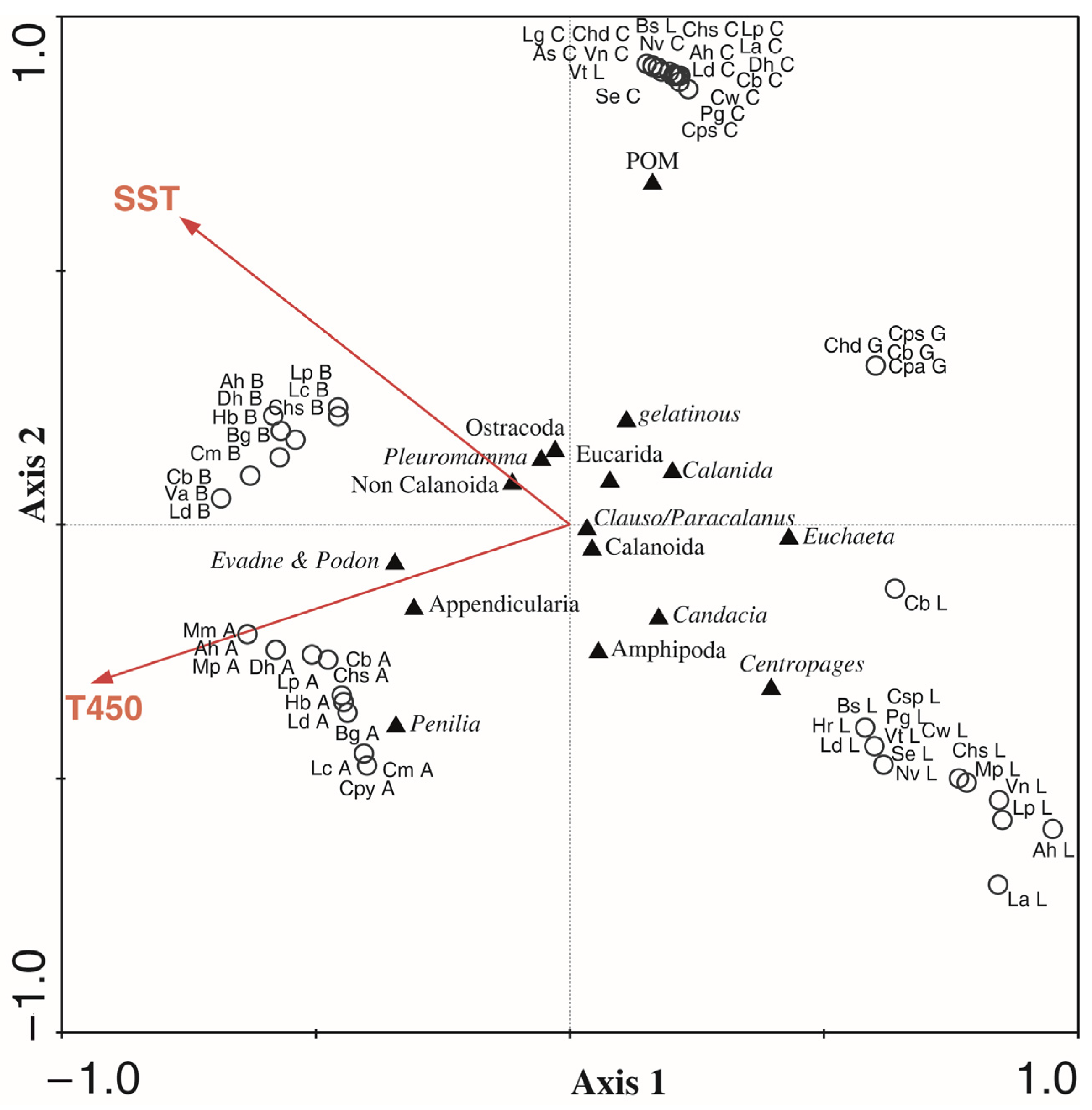
| Species | Conditional Effects | Inter-Set Correlations | ||||
|---|---|---|---|---|---|---|
| Variable | Var (%) | p | Perms | F | Axis 1 | Axis 2 |
| T450 | 12.6 | 0.001 | 999 | 3.82 | −0.6839 | −0.1812 |
| SST | 11.3 | 0.003 | 999 | 2.96 | −0.5571 | 0.3512 |
| Fl | 11.2 | 0.15 | 999 | 1.41 | 0.5537 | 0.3547 |
| Species | Trophic-Migratory Guilds | Feeding Patterns Based on | Hopkins, Sutton, Lancraft (1996) [51] | Czujaz et al. (2020) [36] | |
|---|---|---|---|---|---|
| Prey Carbon Weight | Count of Prey Items | ||||
| Argyropelecus hemygimnus | Zooplanktivore PM | Gelatinous, crustaceans | Mixed crustacean feeder | Not analyzed | Not analyzed |
| Argyropelecus sladeni | Zooplanktivore PM | Mixed crustacean feeder | Mixed crustacean feeder | Not analyzed | Not analyzed |
| Maurolicus muelleri | Zooplanktivore M | Mixed crustacean feeder | Copepod feeder 71% * | Small crustaceans * | Not analyzed |
| Valenciennellus tripunctulatus | Zooplanktivore PM | Mixed crustacean feeder | Copepod feeder 77% * | Small crustaceans * | Not analyzed |
| Chauliodus danae | Micronektivore M | Decapod feeder | Mixed crustacean, fish, micronekton, POM | Piscivorous | Not analyzed |
| Chauliodus sloani | Micronektivore M | Piscivorous | Micronektivore | Piscivorous | Not analyzed |
| Photostomias guernei | Micronektivore PM | Other minor categories | Euphausiida, Decapoda | Decapod feeder | Not analyzed |
| Stomias boa | [Two guts with prey] | Gelatinous | Gelatinous | Not analyzed | Not analyzed |
| Cyclothone braueri | Zooplanktivore NM | Copepods (Pleuromamma, Euchaeta) * | Copepod feeder 60% * | Copepod 72% * | Not analyzed |
| Cyclothone pallida | Gelatinous feeder NM | Gelatinous and copepods | Gelatinous (Tunicata) | Copepod 72% * | Not analyzed |
| Cyclothone pseudopallida | Zooplanktivore NM | Mixed crustacean feeder | Copepod feeder | Copepod 72% * | Not analyzed |
| Cyclothone pygmaea | Zooplanktivore NM | Small copepod feeder | Non-defined | Not analyzed | Not analyzed |
| Sigmops elongatus | Micronektivore PM | Euphausiid feeder | Euphausiid 61% * | Euphausiids 40% * | Mixed crustacean NM |
| Vinciguerria attenuata | Zooplanktivore M | Mixed crustacean feeder | Mixed crustacean feeder | Small crustaceans | Not analyzed |
| Vinciguerria nimbaria | Zooplanktivore M | Mixed crustacean feeder | Mixed crustacean feeder | Crustaceans, fish | Copepod feeders PM |
| Benthosema glaciale | Zooplanktivore M | Mixed crustacean feeder | Mixed crustacean feeder | Not analyzed | Not analyzed |
| Benthosema suborbitale | Zooplanktivore M | Mixed crustacean feeder | Micronektivorous | Small crustaceans | Not analyzed |
| Ceratoscopelus maderensis | Generalist M | Crustaceans, gelatinous, fish | Crustaceans and gelatinous 55% * | Not analyzed | Copepod feeder M |
| Ceratoscopelus warmingii (Atl) | Generalist M | Mixed crustacean feeder | Crustaceans and gelatinous 20% * | Gelatinous 37% * | Copepod feeder M |
| Diaphus holti | Generalist M | Euphausiid feeder | Mixed crustacean and gelatinous feeder | Not analyzed | Not analyzed |
| Hygophum benoiti (Med) | Generalist M | Mixed crustacean and gelatinous feeder | Mixed crustacean and gelatinous feeder | Small crustaceans * | Not analyzed |
| Hygophum reindhardtii | Zooplanktivore M | Mixed crustacean feeder | Mixed crustacean feeder | Mixed crustacean * | Not analyzed |
| Lampanyctus alatus | Micronektivore M | Euphausiid | Mixed crustacean feeder | Mixed crustacean | Not analyzed |
| Lampanyctus crocodilus | Micronektivore M | Piscivorous | Micronektivore | Not analyzed | Not analyzed |
| Lampanyctus pusillus | Micronektivore M | Euphausiid feeder 60% | Copepod feeder >80% | Not analyzed | Not analyzed |
| Lobianchia dofleini | Generalist M | Mixed crustacean feeder (copepods, euphausiids) | Mixed crustacean and gelatinous feeder | Not analyzed | Not analyzed |
| Lepidophanes gaussi (Atl) | Copepod feeder M | Copepod feeder (Pleuromamma, Candacia) | Mixed crustacean feeder | Mixed crustacean feeder | Copepod feeder M ** |
| Myctophum punctatum | Zooplanktivore M | Mixed crustacean, gelatinous feeder | Gelatinous and copepod feeder | Not analyzed | Not analyzed |
| Notolychnus valdiviae (Atl) | Copepod feeder M | Copepod feeder | Copepod feeder | Small crustaceans * | Not analyzed |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernal, A.; Tuset, V.M.; Olivar, M.P. Multiple Approaches to the Trophic Role of Mesopelagic Fish around the Iberian Peninsula. Animals 2023, 13, 886. https://doi.org/10.3390/ani13050886
Bernal A, Tuset VM, Olivar MP. Multiple Approaches to the Trophic Role of Mesopelagic Fish around the Iberian Peninsula. Animals. 2023; 13(5):886. https://doi.org/10.3390/ani13050886
Chicago/Turabian StyleBernal, Ainhoa, Víctor Manuel Tuset, and María Pilar Olivar. 2023. "Multiple Approaches to the Trophic Role of Mesopelagic Fish around the Iberian Peninsula" Animals 13, no. 5: 886. https://doi.org/10.3390/ani13050886





