PDZK1-Interacting Protein 1(PDZKIP1) Inhibits Goat Subcutaneous Preadipocyte Differentiation through Promoting Autophagy
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Tissue Collection
2.2. Cell Isolation and Cell Culture
2.3. Cell Transfection
- Si-PDZK1IP1:
- forward strand: 5′-GAGAAUGCCUAUGAGAACATT−3′.
- reverse strand: 5′-UGUUCUCAUAGGCAUUCUCTT−3′.
- Si-NC:
- forward strand: 5′-CAAUCGCCUUUGCUGUCAATT−3′.
- reverse strand: 5′-ACGUGACACGUUCGGAGAATT−3′.
2.4. Induced Differentiation of Goat Subcutaneous Preadipocytes
2.5. Oil Red O and Bodipy Staining
2.6. AO and MDC Staining
2.7. Western Blotting
2.8. Real-Time Quantitative PCR (qRT-PCR) Analysis
2.9. Statistical Analysis
3. Results
3.1. The Expression Level of PDZKIP1 Was Downregulated during the Differentiation of Goat Subcutaneous Preadipocytes
3.2. PDZK1IP1 Functioned as a Repressor of Goat Subcutaneous Preadipocyte Differentiation
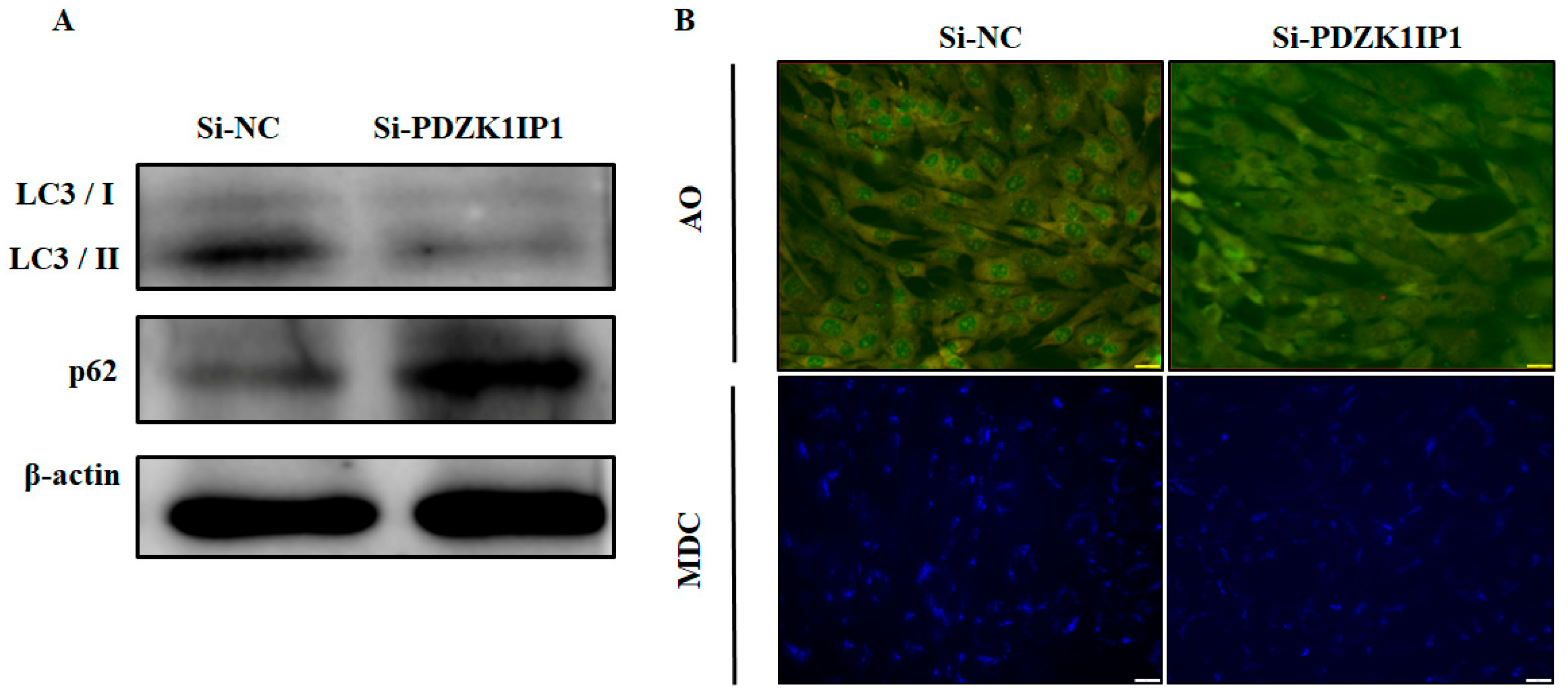
3.3. PDZK1IP1 Positively Modulates Autophagy Activation in Goat Subcutaneous Preadipocytes
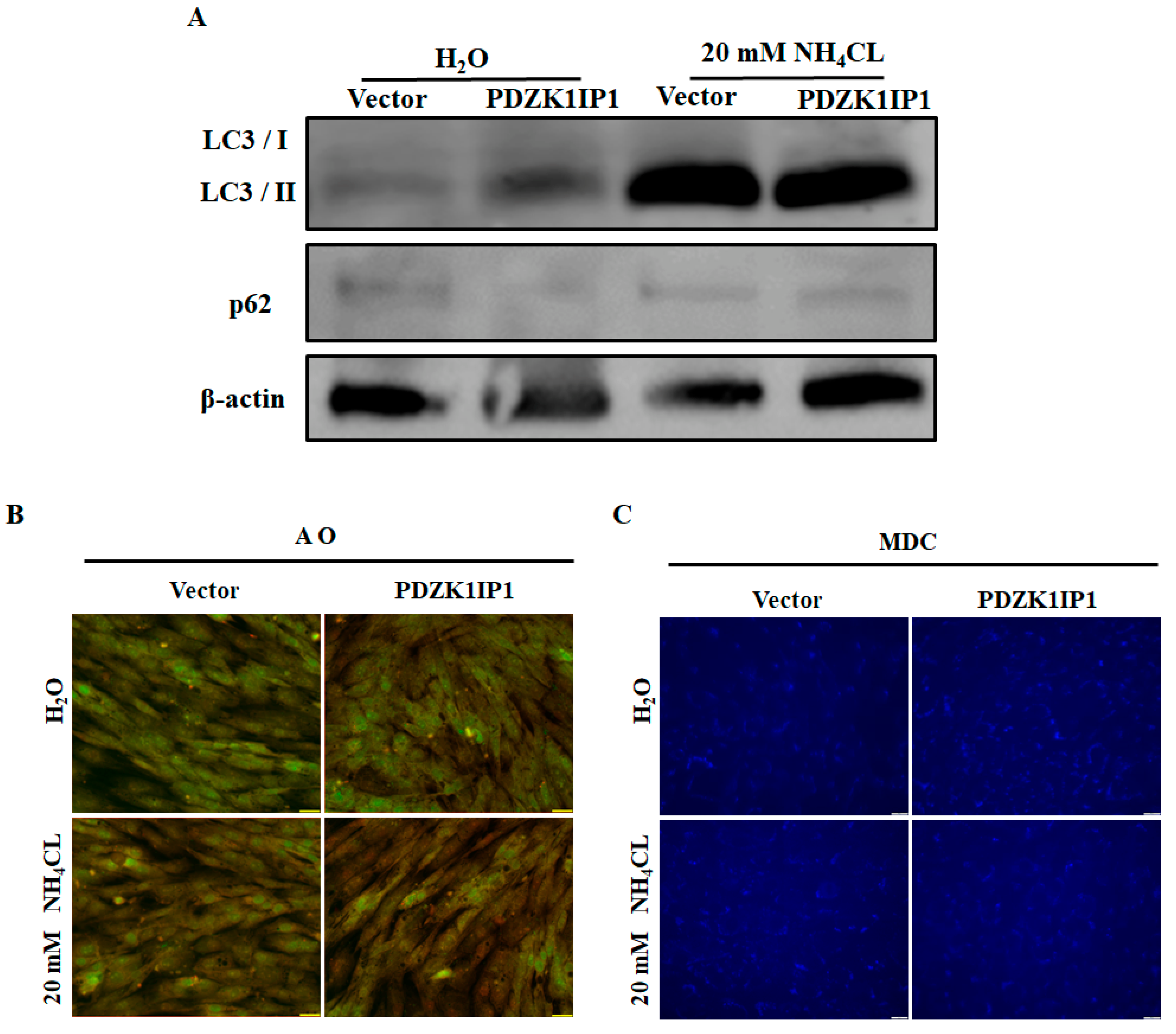
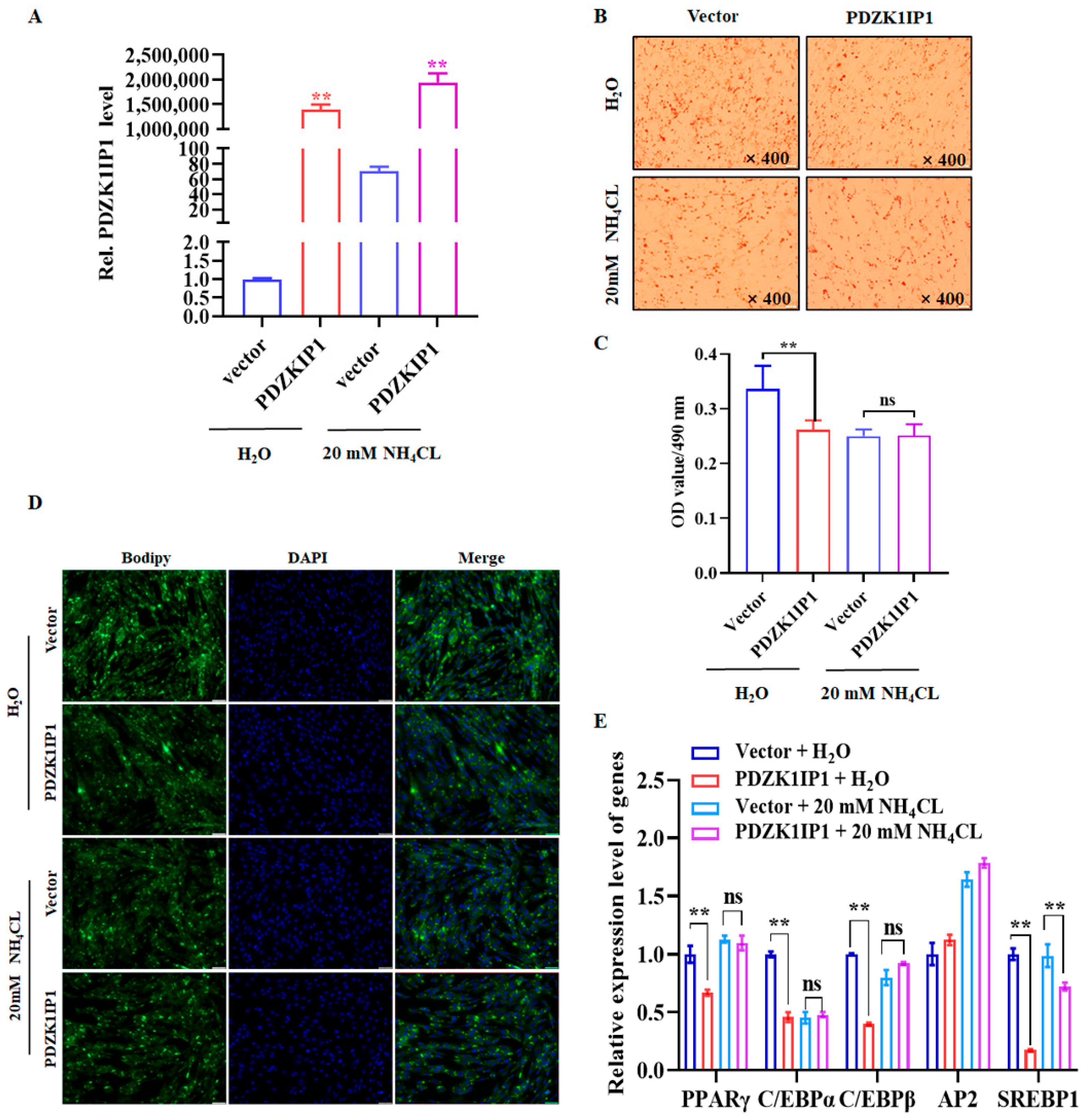
3.4. Inhibition of Autophagy Can Rescue PDZK1IP1-Induced Differentiation Restraint in Goat Subcutaneous Preadipocytes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Trayhurn, P.; Beattie, J.H. Physiological role of adipose tissue: White adipose tissue as an endocrine and secretory organ. Proc. Nutr. Soc. 2001, 60, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Rosen, E.D.; Spiegelman, B.M. What we talk about when we talk about fat. Cell 2014, 156, 20–44. [Google Scholar] [CrossRef] [PubMed]
- Rosen, E.D.; Spiegelman, B.M. Adipocytes as regulators of energy balance and glucose homeostasis. Nature 2006, 444, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Dodson, M.V.; Mir, P.S.; Hausman, G.J.; Guan, L.L.; Du, M.; Jiang, Z.; Fernyhough, M.E.; Bergen, W.G. Obesity, metabolic syndrome, and adipocytes. J. Lipids 2011, 2011, 721686. [Google Scholar] [CrossRef]
- Nicklas, B.J.; Penninx, B.W.; Cesari, M.; Kritchevsky, S.B.; Newman, A.B.; Kanaya, A.M.; Pahor, M.; Jingzhong, D.; Harris, T.B. Association of visceral adipose tissue with incident myocardial infarction in older men and women: The Health, Aging and Body Composition Study. Am. J. Epidemiol 2004, 160, 741–749. [Google Scholar] [CrossRef]
- Schlecht, I.; Gronwald, W.; Behrens, G.; Baumeister, S.E.; Hertel, J.; Hochrein, J.; Zacharias, H.U.; Fischer, B.; Oefner, P.J.; Leitzmann, M.F. Visceral adipose tissue but not subcutaneous adipose tissue is associated with urine and serum metabolites. PLoS ONE 2017, 12, e0175133. [Google Scholar] [CrossRef]
- Sandeep, S.; Gokulakrishnan, K.; Velmurugan, K.; Deepa, M.; Mohan, V. Visceral & subcutaneous abdominal fat in relation to insulin resistance & metabolic syndrome in non-diabetic south Indians. Indian J. Med. Res. 2010, 131, 629–635. [Google Scholar]
- Li, X.; Fu, X.; Yang, G.; Du, M. Review: Enhancing intramuscular fat development via targeting fibro-adipogenic progenitor cells in meat animals. Animal 2020, 14, 312–321. [Google Scholar] [CrossRef]
- Du, M.; Huang, Y.; Das, A.K.; Yang, Q.; Duarte, M.S.; Dodson, M.V.; Zhu, M.J. Meat Science and Muscle Biology Symposium: Manipulating mesenchymal progenitor cell differentiation to optimize performance and carcass value of beef cattle. J. Anim. Sci. 2013, 91, 1419–1427. [Google Scholar] [CrossRef]
- Schumacher, M.; DelCurto-Wyffels, H.; Thomson, J.; Boles, J. Fat Deposition and Fat Effects on Meat Quality-A Review. Animals 2022, 12, 1550. [Google Scholar] [CrossRef]
- Galluzzi, L.; Green, D.R. Autophagy-Independent Functions of the Autophagy Machinery. Cell 2019, 177, 1682–1699. [Google Scholar] [CrossRef]
- Singh, R.; Xiang, Y.; Wang, Y.; Baikati, K.; Cuervo, A.M.; Luu, Y.K.; Tang, Y.; Pessin, J.E.; Schwartz, G.J.; Czaja, M.J. Autophagy regulates adipose mass and differentiation in mice. J. Clin. Investig. 2009, 119, 3329–3339. [Google Scholar] [CrossRef]
- Zhang, Y.; Goldman, S.; Baerga, R.; Zhao, Y.; Komatsu, M.; Jin, S. Adipose-specific deletion of autophagy-related gene 7 (atg7) in mice reveals a role in adipogenesis. Proc. Natl. Acad. Sci. USA 2009, 106, 19860–19865. [Google Scholar] [CrossRef]
- Kovsan, J.; Bluher, M.; Tarnovscki, T.; Kloting, N.; Kirshtein, B.; Madar, L.; Shai, I.; Golan, R.; Harman-Boehm, I.; Schon, M.R.; et al. Altered autophagy in human adipose tissues in obesity. J. Clin. Endocrinol. Metab. 2011, 96, E268–E277. [Google Scholar] [CrossRef]
- Mizushima, N.; Ohsumi, Y.; Yoshimori, T. Autophagosome formation in mammalian cells. Cell Struct. Funct. 2002, 27, 421–429. [Google Scholar] [CrossRef]
- Kocher, O.; Cheresh, P.; Lee, S.W. Identification and partial characterization of a novel membrane-associated protein (MAP17) up-regulated in human carcinomas and modulating cell replication and tumor growth. Am. J. Pathol. 1996, 149, 493–500. [Google Scholar]
- Jaeger, C.; Schaefer, B.M.; Wallich, R.; Kramer, M.D. The membrane-associated protein pKe#192/MAP17 in human keratinocytes. J. Invest. Derm. 2000, 115, 375–380. [Google Scholar]
- Guijarro, M.V.; Leal, J.F.; Fominaya, J.; Blanco-Aparicio, C.; Alonso, S.; Lleonart, M.; Castellvi, J.; Ruiz, L.; Ramon, Y.C.S.; Carnero, A. MAP17 overexpression is a common characteristic of carcinomas. Carcinogenesis 2007, 28, 1646–1652. [Google Scholar] [CrossRef]
- Guijarro, M.V.; Castro, M.E.; Romero, L.; Moneo, V.; Carnero, A. Large scale genetic screen identifies MAP17 as protein bypassing TNF-induced growth arrest. J. Cell. Biochem. 2007, 101, 112–121. [Google Scholar] [CrossRef]
- Zhang, W.; Zheng, D.; Jin, L.; Hirachan, S.; Bhandari, A.; Li, Y.; Chen, B.; Lu, Y.; Wen, J.; Lin, B.; et al. PDZK1IP1 gene promotes proliferation, migration, and invasion in papillary thyroid carcinoma. Pathol. Res. Pract. 2022, 238, 154091. [Google Scholar] [CrossRef]
- Yu, K.; Lu, H.; Chen, Y.; Xin, Y.; Tan, Z.; Yang, Q. 80MAP17 promotes the tumorigenesis of papillary thyroid carcinoma by reducing the stability of p53. Front. Biosci. 2021, 26, 777–788. [Google Scholar]
- Liang, Q.; Zhang, H. MAP17 contributes to non-small cell lung cancer progression via suppressing miR-27a-3p expression and p38 signaling pathway. Cancer Biol. Ther. 2021, 22, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Wang, R.; Sheng, X.; Zhao, N.; Lin, Y.; Wang, Y.; Zhu, J.; Li, Y. PDZK1-interacting protein 1(PDZK1IP1) promotes subcutaneous preadipocyte proliferation in goats. Anim. Biotechnol. 2022, 33, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Galvan, S.; Gutierrez, G.; Perez, M.; Carnero, A. MAP17 (PDZKIP1) Expression Determines Sensitivity to the Proteasomal Inhibitor Bortezomib by Preventing Cytoprotective Autophagy and NFkappaB Activation in Breast Cancer. Mol. Cancer Ther. 2015, 14, 1454–1465. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, H.; Wang, Y.; Li, Y.; He, C.; Zhu, J.; Xiong, Y.; Lin, Y. RNA-seq analysis reveals the positive role of KLF5 in the differentiation of subcutaneous adipocyte in goats. Gene 2022, 808, 145969. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Heckmann, B.L.; Green, D.R. LC3-associated phagocytosis at a glance. J. Cell. Sci. 2019, 132, jcs222984. [Google Scholar] [CrossRef]
- Yoshii, S.R.; Mizushima, N. Monitoring and Measuring Autophagy. Int. J. Mol. Sci. 2017, 18, 1865. [Google Scholar] [CrossRef]
- Rivero, M.; Peinado-Serrano, J.; Munoz-Galvan, S.; Espinosa-Sanchez, A.; Suarez-Martinez, E.; Felipe-Abrio, B.; Fernandez-Fernandez, M.C.; Ortiz, M.J.; Carnero, A. MAP17 (PDZK1IP1) and pH2AX are potential predictive biomarkers for rectal cancer treatment efficacy. Oncotarget 2018, 9, 32958–32971. [Google Scholar] [CrossRef]
- Garcia-Heredia, J.M.; Carnero, A. Dr. Jekyll and Mr. Hyde: MAP17’s up-regulation, a crosspoint in cancer and inflammatory diseases. Mol. Cancer 2018, 17, 80. [Google Scholar] [CrossRef]
- Furukawa, K.; Innokentev, A.; Kanki, T. Mitophagy regulation mediated by the Far complex in yeast. Autophagy 2021, 17, 1042–1043. [Google Scholar] [CrossRef]
- Jansen, H.J.; van Essen, P.; Koenen, T.; Joosten, L.A.; Netea, M.G.; Tack, C.J.; Stienstra, R. Autophagy activity is up-regulated in adipose tissue of obese individuals and modulates proinflammatory cytokine expression. Endocrinology 2012, 153, 5866–5874. [Google Scholar] [CrossRef]
- Baerga, R.; Zhang, Y.; Chen, P.H.; Goldman, S.; Jin, S. Targeted deletion of autophagy-related 5 (atg5) impairs adipogenesis in a cellular model and in mice. Autophagy 2009, 5, 1118–1130. [Google Scholar] [CrossRef]
- Yeh, W.C.; Bierer, B.E.; McKnight, S.L. Rapamycin inhibits clonal expansion and adipogenic differentiation of 3T3-L1 cells. Proc. Natl. Acad. Sci. USA 1995, 92, 11086–11090. [Google Scholar] [CrossRef]
- Zhang, W.; Li, P.; Wang, S.; Cheng, G.; Wang, L.; Mi, X.; Su, X.; Wang, Y.; Zan, L. TP53INP2 Promotes Bovine Adipocytes Differentiation Through Autophagy Activation. Animals 2019, 9, 1060. [Google Scholar] [CrossRef]
- Maroni, P.; Bendinelli, P.; Resnati, M.; Matteucci, E.; Milan, E.; Desiderio, M.A. The Autophagic Process Occurs in Human Bone Metastasis and Implicates Molecular Mechanisms Differently Affected by Rab5a in the Early and Late Stages. Int. J. Mol. Sci. 2016, 17, 443. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, L.; Li, K.; Li, J.; Xiang, R.; Zhang, J.; Li, H.; Xu, Y.; Wei, Y.; Gao, J.; et al. ZNF32 inhibits autophagy through the mTOR pathway and protects MCF-7 cells from stimulus-induced cell death. Sci. Rep. 2015, 5, 9288. [Google Scholar] [CrossRef]
- Mesquita, F.S.; Thomas, M.; Sachse, M.; Santos, A.J.; Figueira, R.; Holden, D.W. The Salmonella deubiquitinase SseL inhibits selective autophagy of cytosolic aggregates. PLoS Pathog. 2012, 8, e1002743. [Google Scholar] [CrossRef]
- Kessel, D.H.; Price, M.; Reiners, J.J., Jr. ATG7 deficiency suppresses apoptosis and cell death induced by lysosomal photodamage. Autophagy 2012, 8, 1333–1341. [Google Scholar] [CrossRef]
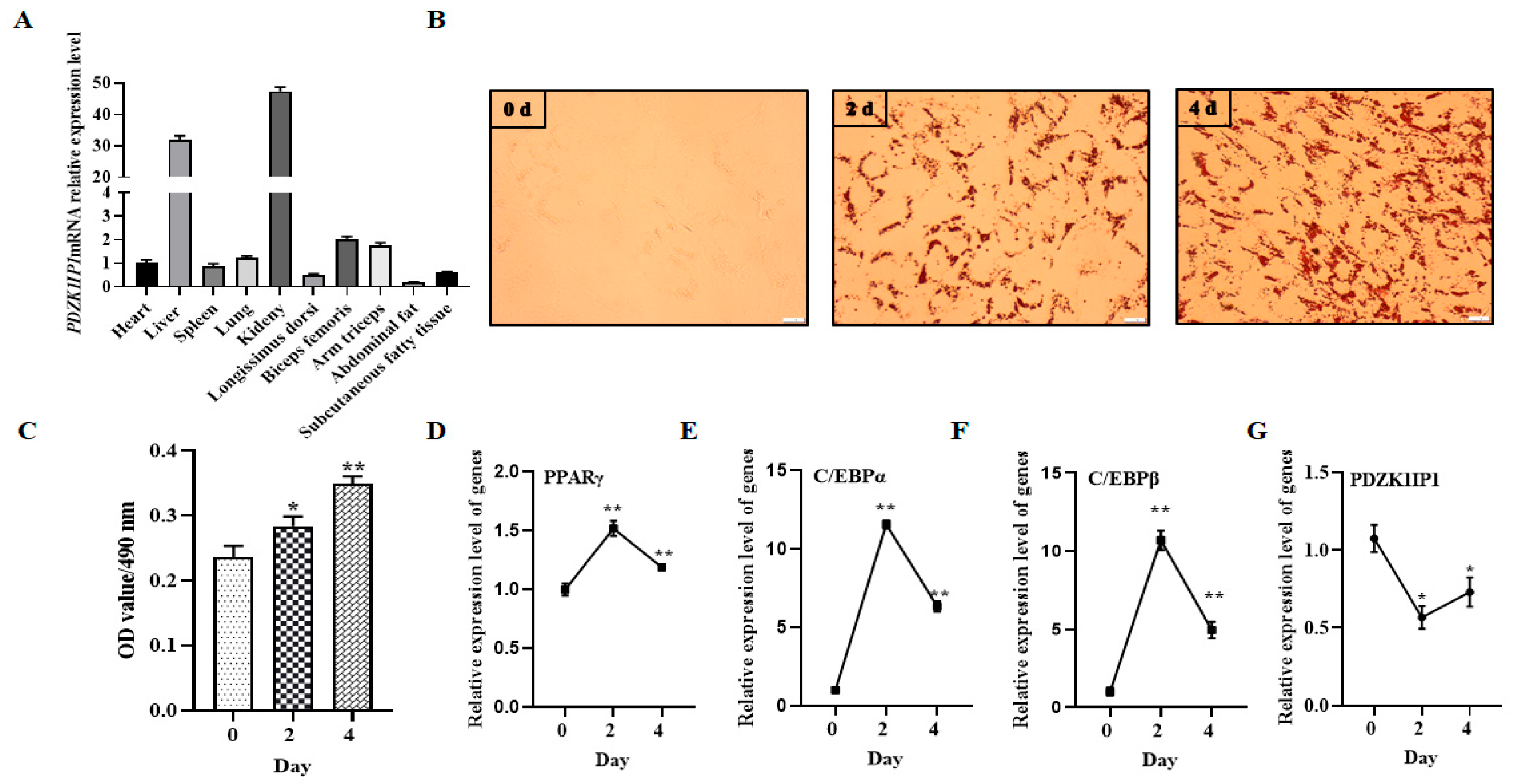
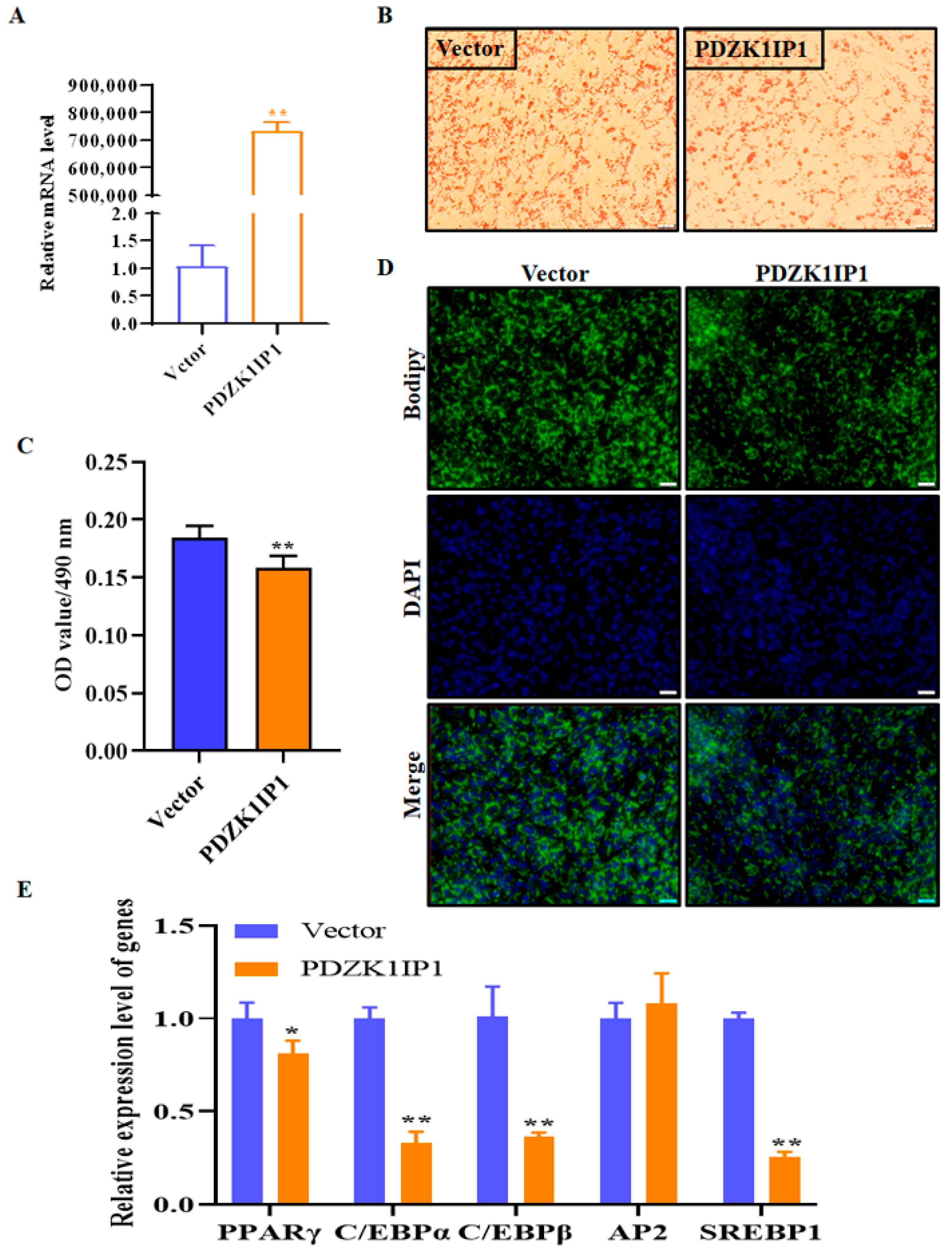
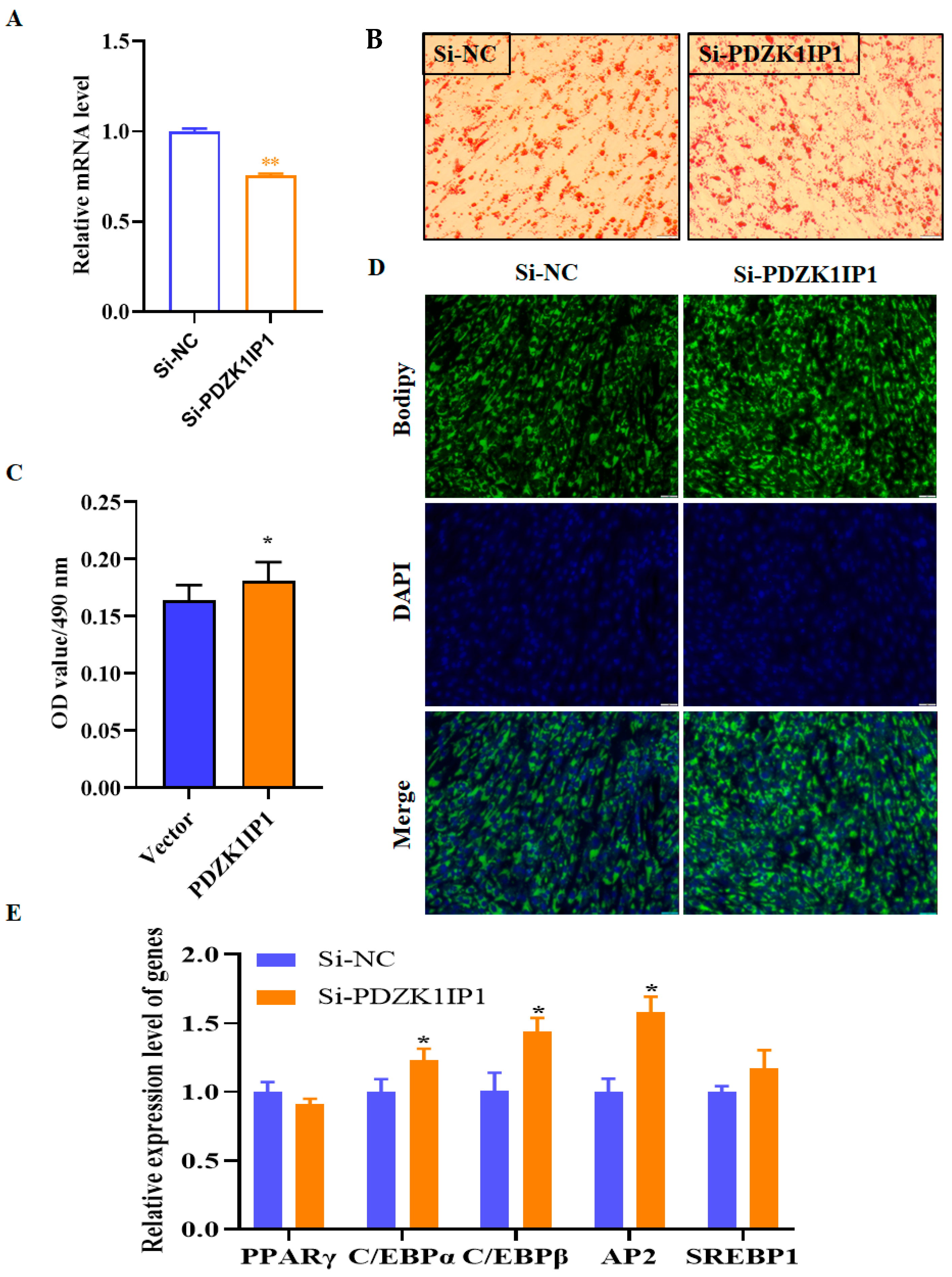
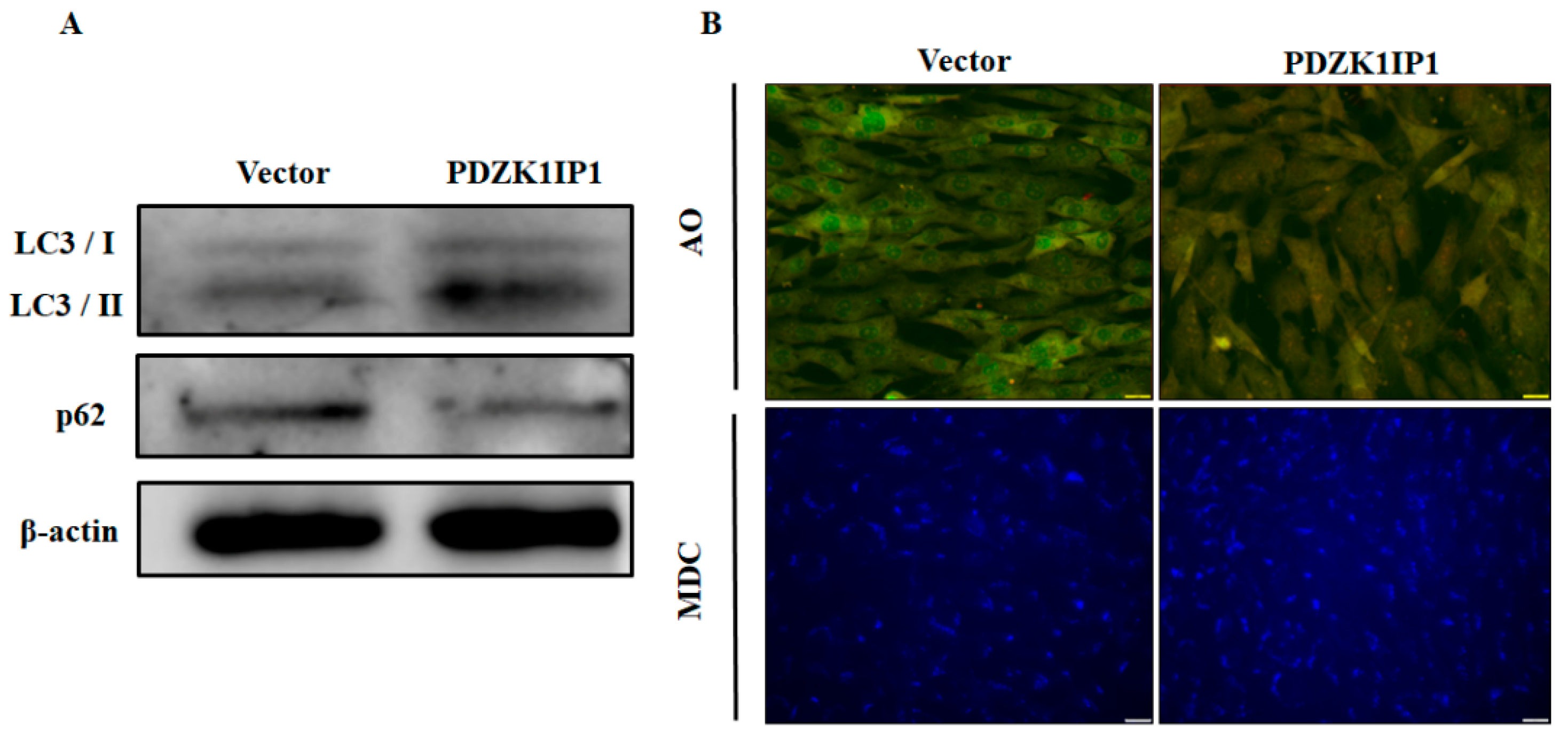
| Gene Name | Forward Sequence (5′–3′) | Reverse Sequence (5′–3′) |
|---|---|---|
| PDZK1IP1 | TGTGTTCCTGGTCCTTGTCG | CCTCCTCCTCTGGGATGTTC |
| C/EBPα | CTCCGGATCTCAAGACTGCC | CCCCTCATCTTAGACGCACC |
| C/EBPβ | CCGCCTTTAAATCCATGGAA | CTCGTGCTCTCCGATGCTAC |
| PPARγ | AAGCGTCAGGGTTCCACTATG | GAACCTGATGGCGTTATGAGAC |
| AP2 | TGAAGTCACTCCAGATGACAGG | TGACACATTCCAGCACCAGC |
| SREBP1 | AACATCTGTTGGAGCGAGCA | TCCAGCCATATCCGAACAGC |
| UXT | GCAAGTGGATTTGGGCTGTAAC | ATGGAGTCCTTGGTGAGGTTGT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, D.; Li, Y.; Hu, T.; Gong, C.; Lu, G.; Ma, X.; Wang, Y.; Wang, Y.; Lin, Y. PDZK1-Interacting Protein 1(PDZKIP1) Inhibits Goat Subcutaneous Preadipocyte Differentiation through Promoting Autophagy. Animals 2023, 13, 1046. https://doi.org/10.3390/ani13061046
Chen D, Li Y, Hu T, Gong C, Lu G, Ma X, Wang Y, Wang Y, Lin Y. PDZK1-Interacting Protein 1(PDZKIP1) Inhibits Goat Subcutaneous Preadipocyte Differentiation through Promoting Autophagy. Animals. 2023; 13(6):1046. https://doi.org/10.3390/ani13061046
Chicago/Turabian StyleChen, Dingshuang, Yanyan Li, Tingting Hu, Chengsi Gong, Guangyu Lu, Xiaotong Ma, Yong Wang, Youli Wang, and Yaqiu Lin. 2023. "PDZK1-Interacting Protein 1(PDZKIP1) Inhibits Goat Subcutaneous Preadipocyte Differentiation through Promoting Autophagy" Animals 13, no. 6: 1046. https://doi.org/10.3390/ani13061046
APA StyleChen, D., Li, Y., Hu, T., Gong, C., Lu, G., Ma, X., Wang, Y., Wang, Y., & Lin, Y. (2023). PDZK1-Interacting Protein 1(PDZKIP1) Inhibits Goat Subcutaneous Preadipocyte Differentiation through Promoting Autophagy. Animals, 13(6), 1046. https://doi.org/10.3390/ani13061046






