Short-Term Microplastic Exposure Impairs Cognition in Hermit Crabs
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Subjects, Housing, and Treatments
2.3. Shell Selection Testing
2.4. Statistics
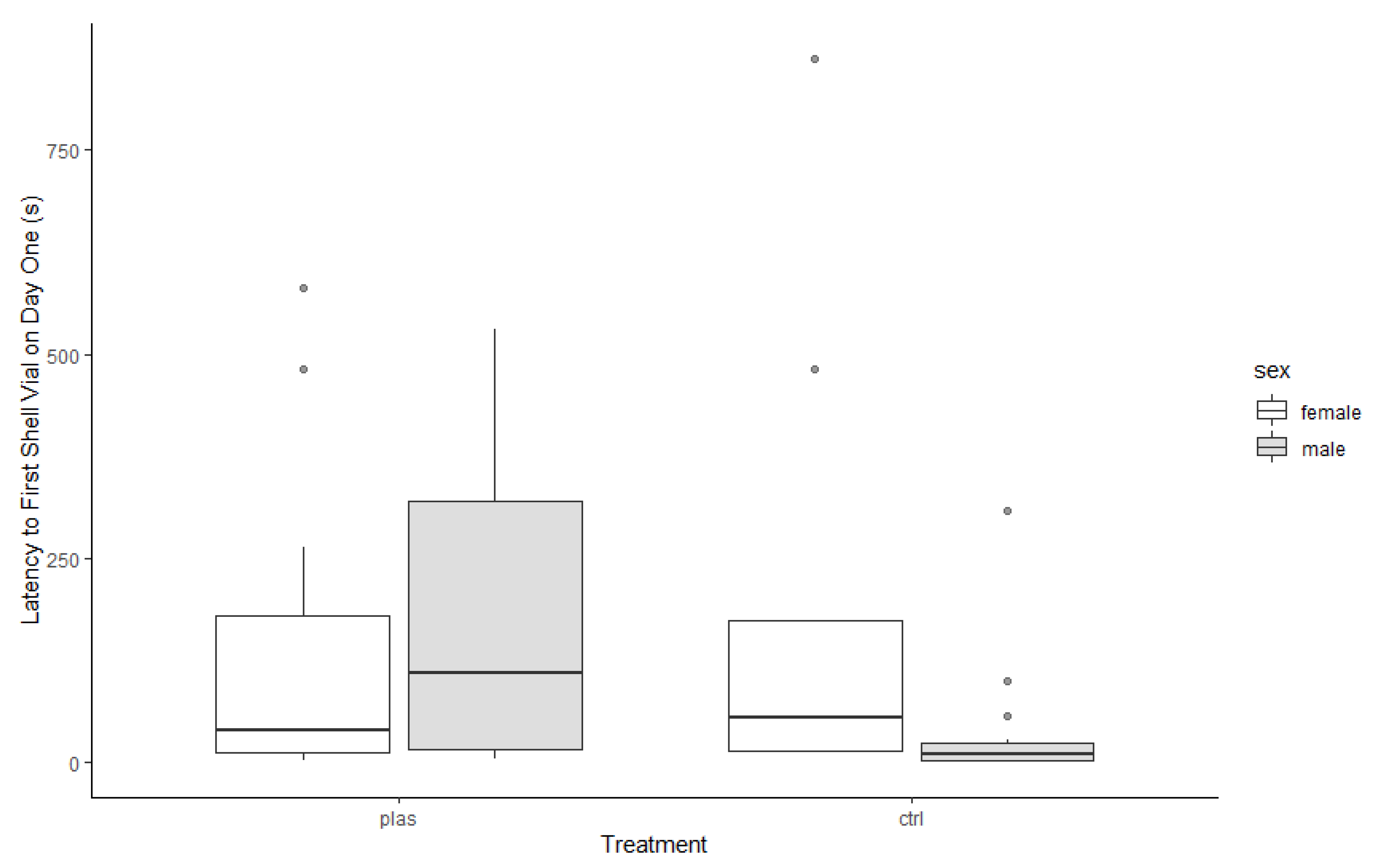
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rebelein, A.; Int-Veen, I.; Kammann, U.; Scharsack, J.P. Microplastic fibers—Underestimated threat to aquatic organisms? Sci. Total Environ. 2021, 777, 146045. [Google Scholar] [CrossRef] [PubMed]
- PlasticsEurope. Plastics—The Facts 2020; Plastics Europe: Brussels, Belgium, 2021. [Google Scholar]
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef] [PubMed]
- Barnes, D.K.; Galgani, F.; Thompson, R.C.; Barlaz, M. Accumulation and fragmentation of plastic debris in global environments. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 1985–1998. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.C.; Olsen, Y.; Mitchell, R.P.; Davis, A.; Rowland, S.J.; John, A.W.; Russell, A.E. Lost at sea: Where is all the plastic? Science 2004, 304, 838. [Google Scholar] [CrossRef]
- Sarkar, D.J.; Sarkar, S.D.; Das, B.K.; Praharaj, J.K.; Mahajan, D.K.; Purokait, B.; Samanta, S. Microplastics removal efficiency of drinking water treatment plant with pulse clarifier. J. Hazard. Mater. 2021, 413, 125347. [Google Scholar] [CrossRef]
- Sarkar, B.; Dissanayake, P.D.; Bolan, N.S.; Dar, J.Y.; Kumar, M.; Haque, M.N.; Ok, Y.S. Challenges and opportunities in sustainable management of microplastics and nanoplastics in the environment. Environ. Res. 2022, 207, 112179. [Google Scholar] [CrossRef]
- Napper, I.E.; Bakir, A.; Rowland, S.J.; Thompson, R.C. Characterisation, quantity and sorptive properties of microplastics extracted from cosmetics. Mar. Pollut. Bull. 2015, 99, 178–185. [Google Scholar] [CrossRef]
- Cole, M.; Lindeque, P.; Halsband, C.; Galloway, T.S. Microplastics as contaminants in the marine environment: A review. Mar. Pollut. Bull. 2011, 62, 2588–2597. [Google Scholar] [CrossRef]
- Eriksen, M.; Lebreton, L.C.; Carson, H.S.; Thiel, M.; Moore, C.J.; Borerro, J.C.; Galgani, F.; Ryan, P.G.; Reisser, J. Plastic pollution in the world's oceans: More than 5 trillion plastic pieces weighing over 250,000 tons afloat at sea. PLoS ONE 2014, 9, e111913. [Google Scholar] [CrossRef]
- Hale, R.C.; Seeley, M.E.; La Guardia, M.J.; Mai, L.; Zeng, E.Y. A global perspective on microplastics. J. Geophys. Res. Ocean. 2020, 125, e2018JC014719. [Google Scholar] [CrossRef]
- Kane, I.A.; Clare, M.A.; Miramontes, E.; Wogelius, R.; Rothwell, J.J.; Garreau, P.; Pohl, F. Seafloor microplastic hotspots controlled by deep-sea circulation. Science 2020, 368, 1140–1145. [Google Scholar] [CrossRef]
- Henderson, L.; Green, C. Making sense of microplastics? Public understandings of plastic pollution. Mar. Pollut. Bull. 2020, 152, 110908. [Google Scholar] [CrossRef]
- Cunningham, E.M.; Sigwart, J.D. Environmentally accurate microplastic levels and their absence from exposure studies. Integr. Comp. Biol. 2019, 59, 1485–1496. [Google Scholar] [CrossRef]
- Egbeocha, C.O.; Malek, S.; Emenike, C.U.; Milow, P. Feasting on microplastics: Ingestion by and effects on marine organisms. Aquat. Biol. 2018, 27, 93–106. [Google Scholar] [CrossRef]
- Franzellitti, S.; Canesi, L.; Auguste, M.; Wathsala, R.H.; Fabbri, E. Microplastic exposure and effects in aquatic organisms: A physiological perspective. Environ. Toxicol. Pharmacol. 2019, 68, 37–51. [Google Scholar] [CrossRef]
- Foley, C.J.; Feiner, Z.S.; Malinich, T.D.; Höök, T.O. A meta-analysis of the effects of exposure to microplastics on fish and aquatic invertebrates. Sci. Total Environ. 2018, 631, 550–559. [Google Scholar] [CrossRef]
- Chen, Q.; Lackmann, C.; Wang, C.W.; Seiler, T.B.; Hollert, H.; Shi, H. Microsized Microplastics Lead to Hyperactive Swimming Behaviour in Adult Zebrafish. Aquat. Toxicol. 2020, 224, 105521. [Google Scholar] [CrossRef]
- Bringer, A.; Cachot, J.; Prunier, G.; Dubillot, E.; Clérandeau, C.; Thomas, H. Experimental ingestion of fluorescent microplastics by pacific oysters, Crassostrea gigas, and their effects on the behaviour and development at early stages. Chemosphere 2020, 254, 126793. [Google Scholar] [CrossRef]
- Bringer, A.; Thomas, H.; Prunier, G.; Dubillot, E.; Bossut, N.; Churlaud, C.; Cachot, J. High density polyethylene (HDPE) microplastics impair development and swimming activity of Pacific oyster D-larvae, Crassostrea gigas, depending on particle size. Environ. Pollut. 2020, 260, 113978. [Google Scholar] [CrossRef]
- Tosetto, L.; Brown, C.; Williamson, J.E. Microplastics on beaches: Ingestion and behavioural consequences for beachhoppers. Mar. Biol. 2016, 163, 199. [Google Scholar] [CrossRef]
- Suwaki, C.H.; De-La-Cruz, L.T.; Lopes, R.M. Impacts of Microplastics on the Swimming Behavior of the Copepod Temora Turbinata (Dana, 1849). Fluids 2020, 5, 103. [Google Scholar] [CrossRef]
- Carrasco, A.; Pulgar, J.; Quintanilla-Ahumada, D.; Perez-Venegas, D.; Quijón, P.A.; Duarte, C. The influence of microplastics pollution on the feeding behavior of a prominent sandy beach amphipod, Orchestoidea tuberculata (Nicolet, 1849). Mar. Pollut. Bull. 2019, 145, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Cole, M.; Lindeque, P.; Fileman, E.; Halsband, C.; Galloway, T.S. The impact of polystyrene microplastics on feeding, function and fecundity in the marine copepod Calanus helgolandicus. Environ. Sci. Technol. 2015, 49, 1130–1137. [Google Scholar] [CrossRef] [PubMed]
- Mattsson, K.; Ekvall, M.T.; Hansson, L.A.; Linse, S.; Malmendal, A.; Cedervall, T. Altered behavior, physiology, and metabolism in fish exposed to polystyrene nanoparticles. Environ. Sci. Technol. 2014, 49, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Crump, A.; Mullens, C.; Bethell, E.J.; Cunningham, E.M.; Arnott, G. Microplastics disrupt hermit crab shell selection. Biol. Lett. 2020, 16, 20200030. [Google Scholar] [CrossRef]
- Cunningham, E.M.; Mundye, A.; Kregting, L.; Dick, J.T.; Crump, A.; Riddell, G.; Arnott, G. Animal contests and microplastics: Evidence of disrupted behaviour in hermit crabs Pagurus bernhardus. R. Soc. Open Sci. 2021, 8, 211089. [Google Scholar] [CrossRef]
- Elwood, R.W.; McClean, A.; Webb, L. The development of shell preferences by the hermit crab Pagurus bernhardus. Anim. Behav. 1979, 27, 940–946. [Google Scholar] [CrossRef]
- Lancaster, I. Reproduction and life history strategy of the hermit crab Pagurus bernhardus. J. Mar. Biol. Assoc. UK 1990, 70, 129–142. [Google Scholar] [CrossRef]
- Arnott, G.; Elwood, R.W. Fighting for shells: How private information about resource value changes hermit crab pre-fight displays and escalated fight behaviour. Proc. R. Soc. B Biol. Sci. 2007, 274, 3011–3017. [Google Scholar] [CrossRef]
- Briffa, M.; Elwood, R.W. Use of energy reserves in fighting hermit crabs. Proc. R. Soc. London. Ser. B Biol. Sci. 2004, 271, 373–379. [Google Scholar] [CrossRef]
- Krieger, J.; Hörnig, M.K.; Laidre, M.E. Shells as ‘extended architecture’: To escape isolation, social hermit crabs choose shells with the right external architecture. Anim. Cogn. 2020, 23, 1177–1187. [Google Scholar] [CrossRef]
- Elwood, R.W. Hermit crabs, shells, and sentience. Anim. Cogn. 2022, 25, 1–17. [Google Scholar] [CrossRef]
- Elwood, R.W.; Neil, S.J. Assessments and Decisions: A Study of Information Gathering by Hermit Crabs, 1st ed.; Chapman & Hall: London, UK, 1992. [Google Scholar]
- Shettleworth, S.J. Cognition, Evolution, and Behavior; Oxford University Press: New York, NY, USA, 2009. [Google Scholar]
- Walsh, E.P.; Arnott, G.; Kunc, H.P. Noise affects resource assessment in an invertebrate. Biol. Lett. 2017, 13, 20170098. [Google Scholar] [CrossRef]
- De la Haye, K.L.; Spicer, J.I.; Widdicombe, S.; Briffa, M. Reduced sea water pH disrupts resource assessment and decision making in the hermit crab Pagurus bernhardus. Anim. Behav. 2011, 82, 495–501. [Google Scholar] [CrossRef]
- Gilliand, S.; Pechenik, J.A.; Clark, D. The effects of changes in temperature and salinity on the quality of shells selected by the hermit crab Pagurus longicarpus. Invertebr. Biol. 2021, 140, e12345. [Google Scholar] [CrossRef]
- D’Costa, A.H. Microplastics in decapod crustaceans: Accumulation, toxicity and impacts, a review. Sci. Total Environ. 2022, 832, 154963. [Google Scholar] [CrossRef]
- Nanninga, G.B.; Horswill, C.; Lane, S.M.; Manica, A.; Briffa, M. Microplastic exposure increases predictability of predator avoidance strategies in hermit crabs. J. Hazard. Mater. Lett. 2020, 1, 100005. [Google Scholar] [CrossRef]
- Liu, X.; Yang, H.; Yan, X.; Xu, S.; Fan, Y.; Xu, H.; Zhang, Y. Co-exposure of polystyrene microplastics and iron aggravates cognitive decline in aging mice via ferroptosis induction. Ecotoxicol. Environ. Saf. 2022, 233, 113342. [Google Scholar] [CrossRef]
- McDaid, A.; Cunningham, E.M.; Crump, A.; Hardiman, G.; Arnott, G. Does microplastic exposure and sex influence shell selection and motivation in the common European hermit crab, Pagurus bernhardus? Sci. Total Environ. 2023, 855, 158576. [Google Scholar] [CrossRef]
- Birch, J.; Burn, C.; Schnell, A.; Browning, H.; Crump, A. Review of the Evidence of Sentience in Cephalopod Molluscs and Decapod Crustaceans; Department for Environment, Food, & Rural Affairs (Defra): London, UK, 2021. [Google Scholar]
- Crump, A.; Browning, H.; Schnell, A.; Burn, C.; Birch, J. Sentience in decapod crustaceans: A general framework and review of the evidence. Anim. Sentience 2022, 7, 1. [Google Scholar] [CrossRef]
- Crump, A. Animal sentience science and policy. Anim. Sentience 2022, 6, 15. [Google Scholar] [CrossRef]
- De Sá, L.C.; Oliveira, M.; Ribeiro, F.; Rocha, T.L.; Futter, M.N. Studies of the effects of microplastics on aquatic organisms: What do we know and where should we focus our efforts in the future? Sci. Total Environ. 2018, 645, 1029–1039. [Google Scholar] [CrossRef] [PubMed]
- Champely, S.; Ekstrom, C.; Dalgaard, P.; Gill, J.; Weibelzahl, S.; Anandkumar, A.; De Rosario, H. pwr: Basic Functions for Power Analysis. 2017. Available online: https://cran.r-project.org/web/packages/pwr/ (accessed on 9 March 2023).
- Bates, D.M.; Mächler, M.; Bolker, B.; Walker, S. () Fitting linear mixed-effects models using “lme4”. J. Stat. Softw. 2015, 67, 10–48. [Google Scholar] [CrossRef]
- Welden, N.A.; Cowie, P.R. Long-term microplastic retention causes reduced body condition in the langoustine, Nephrops norvegicus. Environ. Pollut. 2016, 218, 895–900. [Google Scholar] [CrossRef] [PubMed]
- Briffa, M.; Rundle, S.D.; Fryer, A. Comparing the strength of behavioural plasticity and consistency across situations: Animal personalities in the hermit crab Pagurus bernhardus. Proc. R. Soc. B Biol. Sci. 2008, 275, 1305–1311. [Google Scholar] [CrossRef]
- Mowles, S.L.; Cotton, P.A.; Briffa, M. Consistent crustaceans: The identification of stable behavioural syndromes in hermit crabs. Behav. Ecol. Sociobiol. 2012, 66, 1087–1094. [Google Scholar] [CrossRef]
- Lee, Y.; Yoon, D.S.; Lee, Y.H.; Kwak, J.I.; An, Y.J.; Lee, J.S.; Park, J.C. Combined exposure to microplastics and zinc produces sex-specific responses in the water flea Daphnia magna. J. Hazard. Mater. 2021, 420, 126652. [Google Scholar] [CrossRef]
- Briffa, M.; Dallaway, D. Inter-sexual contests in the hermit crab Pagurus bernhardus: Females fight harder but males win more encounters. Behav. Ecol. Sociobiol. 2007, 61, 1781–1787. [Google Scholar] [CrossRef]
- Hazlett, B.A.; Rittschof, D. Predation–reproduction conflict resolution in the hermit crab, Clibanarius vittatus. Ethol. Res. Pap. 2000, 106, 811–818. [Google Scholar] [CrossRef]
- Delaeter, C.; Spilmont, N.; Bouchet, V.M.; Seuront, L. Plastic leachates: Bridging the gap between a conspicuous pollution and its pernicious effects on marine life. Sci. Total Environ. 2022, 826, 154091. [Google Scholar] [CrossRef]
- Seuront, L. Microplastic leachates impair behavioural vigilance and predator avoidance in a temperate intertidal gastropod. Biol. Lett. 2018, 14, 20180453. [Google Scholar] [CrossRef]
- Savoca, M.S.; Wohlfeil, M.E.; Ebeler, S.E.; Nevitt, G.A. Marine plastic debris emits a keystone infochemical for olfactory foraging seabirds. Sci. Adv. 2016, 2, e1600395. [Google Scholar] [CrossRef]
- Savoca, M.S.; Tyson, C.W.; McGill, M.; Slager, C.J. Odours from marine plastic debris induce food search behaviours in a forage fish. Proc. R. Soc. B Biol. Sci. 2017, 284, 20171000. [Google Scholar] [CrossRef]
- Greenshields, J.; Schirrmacher, P.; Hardege, J.D. Plastic additive oleamide elicits hyperactivity in hermit crabs. Mar. Pollut. Bull. 2021, 169, 112533. [Google Scholar] [CrossRef]
- De Marco, G.; Conti, G.O.; Giannetto, A.; Cappello, T.; Galati, M.; Iaria, C.; Maisano, M. Embryotoxicity of polystyrene microplastics in zebrafish Danio rerio. Environ. Res. 2022, 208, 112552. [Google Scholar] [CrossRef]
- Huang, W.; Wang, X.; Chen, D.; Xu, E.G.; Luo, X.; Zeng, J.; Huan, T.; Li, L.; Wang, Y. Toxicity mechanisms of polystyrene microplastics in marine mussels revealed by high-coverage quantitative metabolomics using chemical isotope labeling liquid chromatography mass spectrometry. J. Hazard. Mater. 2021, 417, 126003. [Google Scholar] [CrossRef]
- Hayden, D.; Jennings, A.; Müller, C.; Pascoe, D.; Bublitz, R.; Webb, H.; Hardege, J. Sex-specific mediation of foraging in the shore crab, Carcinus maenas. Horm. Behav. 2007, 52, 162–168. [Google Scholar] [CrossRef]
- Weis, J.S.; Palmquist, K.H. Reality Check: Experimental Studies on Microplastics Lack Realism. Appl. Sci. 2021, 11, 8529. [Google Scholar] [CrossRef]
- Clements, J.C.; Hunt, H.L. Marine animal behaviour in a high CO2 ocean. Mar. Ecol. Prog. Ser. 2015, 536, 259–279. [Google Scholar] [CrossRef]
- Porteus, C.S.; Roggatz, C.C.; Velez, Z.; Hardege, J.D.; Hubbard, P.C. Acidification can directly affect olfaction in marine organisms. J. Exp. Biol. 2021, 224, jeb237941. [Google Scholar] [CrossRef]
- Roggatz, C.C.; Saha, M.; Blanchard, S.; Schirrmacher, P.; Fink, P.; Verheggen, F.; Hardege, J.D. Becoming nose-blind—Climate change impacts on chemical communication. Glob. Chang. Biol. 2022, 28, 4495–4505. [Google Scholar] [CrossRef] [PubMed]
- Feugere, L.; Angell, L.; Fagents, J.; Nightingale, R.; Rowland, K.; Skinner, S.; Wollenberg Valero, K.C. Behavioural stress propagation in benthic invertebrates caused by acute ph drop-induced metabolites. Front. Mar. Sci. 2021, 8, 1788. [Google Scholar] [CrossRef]




| Treatment | Touched 100% First | Touched 25% First |
|---|---|---|
| PLAS | 8 | 17 |
| CTRL | 16 | 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crump, A.; Aiken, C.; Cunningham, E.M.; Arnott, G. Short-Term Microplastic Exposure Impairs Cognition in Hermit Crabs. Animals 2023, 13, 1055. https://doi.org/10.3390/ani13061055
Crump A, Aiken C, Cunningham EM, Arnott G. Short-Term Microplastic Exposure Impairs Cognition in Hermit Crabs. Animals. 2023; 13(6):1055. https://doi.org/10.3390/ani13061055
Chicago/Turabian StyleCrump, Andrew, Catherine Aiken, Eoghan M. Cunningham, and Gareth Arnott. 2023. "Short-Term Microplastic Exposure Impairs Cognition in Hermit Crabs" Animals 13, no. 6: 1055. https://doi.org/10.3390/ani13061055
APA StyleCrump, A., Aiken, C., Cunningham, E. M., & Arnott, G. (2023). Short-Term Microplastic Exposure Impairs Cognition in Hermit Crabs. Animals, 13(6), 1055. https://doi.org/10.3390/ani13061055






