Bone Morphogenetic Protein 15 (BMP-15) Improves In Vitro Mouse Folliculogenesis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Chemicals
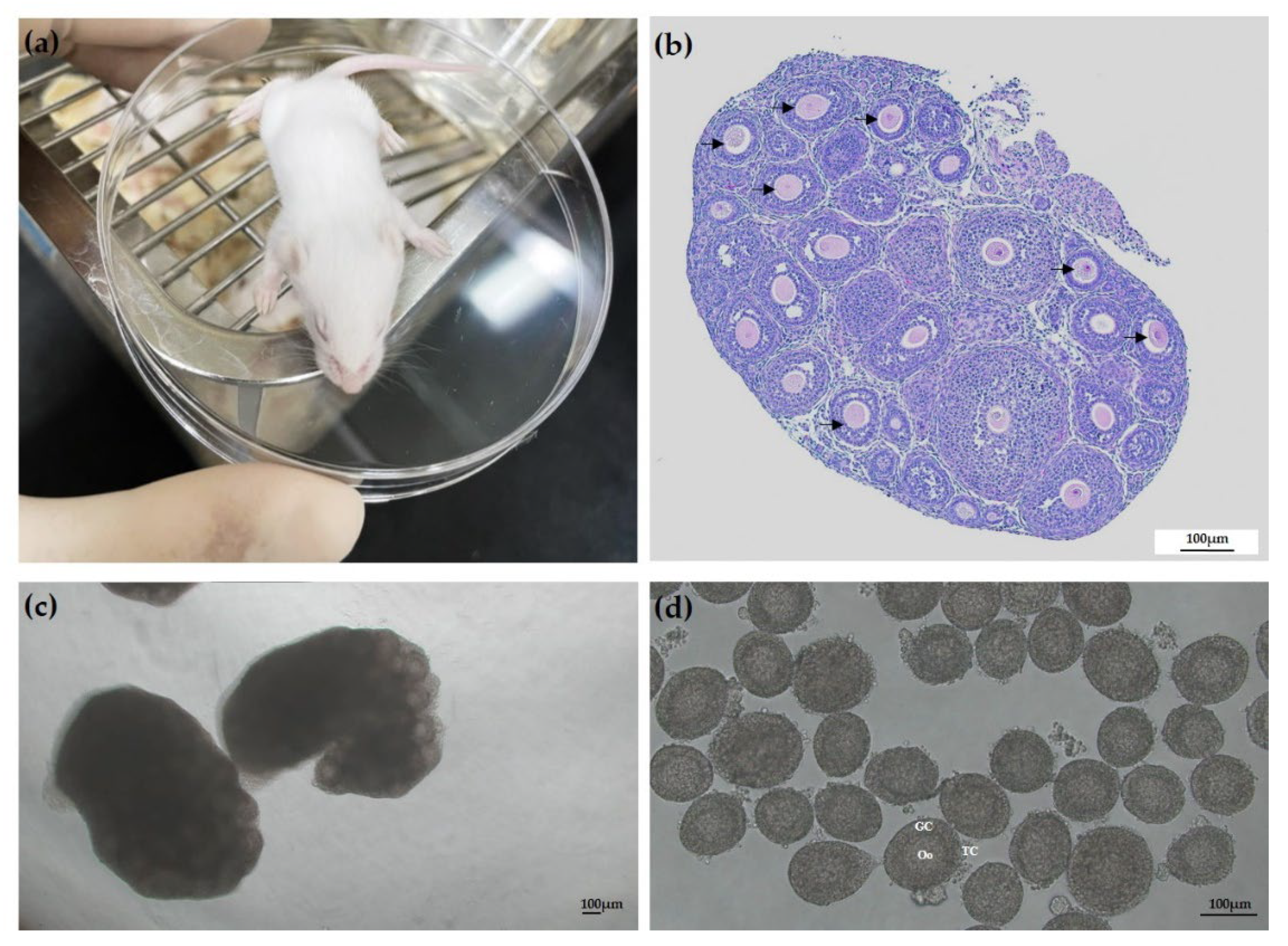
2.2. Follicle Isolation from Ovarian Tissue
2.3. 3D Culture and Alginate Encapsulation of Ovarian Follicles
2.4. Mature Oocytes Retrieved from Alginate Beads
2.5. Percoll Gradient Centrifugation
2.6. In Vitro Fertilization
2.7. Measurement of Steroid Hormones
2.8. Measurement of Reactive Oxygen Species and Glutathione Levels in Matured Oocytes
2.9. Statistical Analysis
3. Results
3.1. Evaluation of Follicle Morphological Characteristics and Growth
3.2. Follicle Degradation and Survival and Antrum Formation
3.3. Fertilization and Embryo Development
3.4. Steroid Hormone Production during In Vitro Culture
3.5. Determination of Intracellular ROS and GSH Level of Mature Oocytes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Woodruff, T.K. The Oncofertility Consortium—Addressing fertility in young people with cancer. Nat. Rev. Clin. Oncol. 2010, 7, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Meirow, D.; Hardan, I.; Dor, J.; Fridman, E.; Elizur, S.; Ra’anani, H.; Slyusarevsky, E.; Amariglio, N.; Schiff, E.; Rechavi, G. Searching for evidence of disease and malignant cell contamination in ovarian tissue stored from hematologic cancer patients. Hum. Reprod. 2008, 23, 1007–1013. [Google Scholar] [CrossRef] [PubMed]
- Lenie, S.; Cortvrindt, R.; Adriaenssens, T.; Smitz, J. A reproducible two-step culture system for isolated primary mouse ovarian follicles as single functional units. Biol. Reprod. 2004, 71, 1730–1738. [Google Scholar] [CrossRef] [PubMed]
- Brito, I.R.; Lima, I.M.; Xu, M.; Shea, L.D.; Woodruff, T.K.; Figueiredo, J.R. Three-dimensional systems for in vitro follicular culture: Overview of alginate-based matrices. Reprod. Fertil. Dev. 2014, 26, 915–930. [Google Scholar] [CrossRef]
- Dolmans, M.-M.; Luyckx, V.; Donnez, J.; Andersen, C.Y.; Greve, T. Risk of transferring malignant cells with transplanted frozen-thawed ovarian tissue. Fertil. Steril. 2013, 99, 1514–1522. [Google Scholar] [CrossRef]
- Kreeger, P.K.; Fernandes, N.N.; Woodruff, T.K.; Shea, L.D. Regulation of mouse follicle development by follicle-stimulating hormone in a three-dimensional in vitro culture system is dependent on follicle stage and dose. Biol. Reprod. 2005, 73, 942–950. [Google Scholar] [CrossRef] [Green Version]
- Filatov, M.; Khramova, Y.V.; Semenova, M. In vitro mouse ovarian follicle growth and maturation in alginate hydrogel: Current state of the art. Acta Nat. (Англoязычная Версия) 2015, 7, 48–56. [Google Scholar] [CrossRef] [Green Version]
- West, E.R.; Shea, L.D.; Woodruff, T.K. Engineering the follicle microenvironment. Semin. Reprod. Med. 2007, 25, 287–299. [Google Scholar] [CrossRef] [Green Version]
- Joo, S.; Oh, S.-H.; Sittadjody, S.; Opara, E.C.; Jackson, J.D.; Lee, S.J.; Yoo, J.J.; Atala, A. The effect of collagen hydrogel on 3D culture of ovarian follicles. Biomed. Mater. 2016, 11, 065009. [Google Scholar] [CrossRef] [Green Version]
- Augst, A.D.; Kong, H.J.; Mooney, D.J. Alginate hydrogels as biomaterials. Macromol. Biosci. 2006, 6, 623–633. [Google Scholar] [CrossRef]
- Shikanov, A.; Xu, M.; Woodruff, T.K.; Shea, L.D. Interpenetrating fibrin–alginate matrices for in vitro ovarian follicle development. Biomaterials 2009, 30, 5476–5485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skory, R.M.; Bernabé, B.P.; Galdones, E.; Broadbelt, L.J.; Shea, L.D.; Woodruff, T.K. Microarray analysis identifies COMP as the most differentially regulated transcript throughout in vitro follicle growth. Mol. Reprod. Dev. 2013, 80, 132–144. [Google Scholar] [CrossRef] [Green Version]
- Paulini, F.; Melo, E.O. The role of oocyte-secreted factors GDF9 and BMP15 in follicular development and oogenesis. Reprod. Domest. Anim. 2011, 46, 354–361. [Google Scholar] [CrossRef] [Green Version]
- Peng, J.; Li, Q.; Wigglesworth, K.; Rangarajan, A.; Kattamuri, C.; Peterson, R.T.; Eppig, J.J.; Thompson, T.B.; Matzuk, M.M. Growth differentiation factor 9: Bone morphogenetic protein 15 heterodimers are potent regulators of ovarian functions. Proc. Natl. Acad. Sci. USA 2013, 110, E776–E785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khazaei, M.; Aghaz, F. Reactive oxygen species generation and use of antioxidants during in vitro maturation of oocytes. Int. J. Fertil. Steril. 2017, 11, 63. [Google Scholar]
- Wu, Z.; Hou, Y.; Dai, Z.; Hu, C.-A.A.; Wu, G. Metabolism, nutrition, and redox signaling of hydroxyproline. Antioxid. Redox Signal. 2019, 30, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Si, X.; Ji, Y.; Yang, Q.; Bai, J.; He, Y.; Jia, H.; Song, Z.; Chen, J.; Yang, L. l-Proline improves the cytoplasmic maturation of mouse oocyte by regulating glutathione-related redox homeostasis. Theriogenology 2023, 195, 159–167. [Google Scholar] [CrossRef]
- Hayashi, K.; Hikabe, O.; Obata, Y.; Hirao, Y. Reconstitution of mouse oogenesis in a dish from pluripotent stem cells. Nat. Protoc. 2017, 12, 1733–1744. [Google Scholar] [CrossRef]
- Steel, R.G.D.; Torrie, J.H. Principles and Procedures of Statistics, A Biometrical Approach, 2nd ed.; McGraw-Hill Book Company: New York, NY, USA, 1980. [Google Scholar]
- Atwood, C.S.; Meethal, S.V. The spatiotemporal hormonal orchestration of human folliculogenesis, early embryogenesis and blastocyst implantation. Mol. Cell. Endocrinol. 2016, 430, 33–48. [Google Scholar] [CrossRef]
- Ola, S.I.; Ai, J.S.; Liu, J.H.; Wang, Q.; Wang, Z.B.; Chen, D.Y.; Sun, Q.Y. Effects of gonadotrophins, growth hormone, and activin A on enzymatically isolated follicle growth, oocyte chromatin organization, and steroid secretion. Mol. Reprod. Dev. Inc. Gamete Res. 2008, 75, 89–96. [Google Scholar] [CrossRef]
- Oktem, O.; Oktay, K. The role of extracellular matrix and activin-A in in vitro growth and survival of murine preantral follicles. Reprod. Sci. 2007, 14, 358–366. [Google Scholar] [CrossRef]
- Haidari, K.; Salehnia, M.; Valojerdi, M.R. The effect of leukemia inhibitory factor and coculture on the in vitro maturation and ultrastructure of vitrified and nonvitrified isolated mouse preantral follicles. Fertil. Steril. 2008, 90, 2389–2397. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.S.; Shikanov, A. Follicle development as an orchestrated signaling network in a 3D organoid. J. Biol. Eng. 2019, 13, 2. [Google Scholar] [CrossRef]
- Green, L.J.; Shikanov, A. In vitro culture methods of preantral follicles. Theriogenology 2016, 86, 229–238. [Google Scholar] [CrossRef]
- Jin, S.Y.; Lei, L.; Shikanov, A.; Shea, L.D.; Woodruff, T.K. A novel two-step strategy for in vitro culture of early-stage ovarian follicles in the mouse. Fertil. Steril. 2010, 93, 2633–2639. [Google Scholar] [CrossRef] [Green Version]
- Jalili, C.; Hemmatabadi, F.K.; Mansouri, K.; Bakhtiyari, M. Effects of sodium alginate capsules as 3D scaffolds on hormones and genes expression in preantral follicles of mice compared to 2D medium: An experimental study. Int. J. Reprod. BioMedicine 2020, 18, 517. [Google Scholar] [CrossRef]
- Peretz, J.; Flaws, J.A. Bisphenol A down-regulates rate-limiting Cyp11a1 to acutely inhibit steroidogenesis in cultured mouse antral follicles. Toxicol. Appl. Pharmacol. 2013, 271, 249–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borman, S.M.; Chaffin, C.L.; Schwinof, K.M.; Stouffer, R.L.; Zelinski-Wooten, M.B. Progesterone promotes oocyte maturation, but not ovulation, in nonhuman primate follicles without a gonadotropin surge. Biol. Reprod. 2004, 71, 366–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, M.; Barrett, S.L.; West-Farrell, E.; Kondapalli, L.A.; Kiesewetter, S.E.; Shea, L.D.; Woodruff, T.K. In vitro grown human ovarian follicles from cancer patients support oocyte growth. Hum. Reprod. 2009, 24, 2531–2540. [Google Scholar] [CrossRef]
- Salehnia, M.; Pajokh, M.; Ghorbanmehr, N. Short term organ culture of mouse ovary in the medium supplemented with bone morphogenetic protein 15 and follicle stimulating hormone: A morphological, hormonal and molecular study. J. Reprod. Infertil. 2016, 17, 199. [Google Scholar]
- Saad, M.; Sarwar, Z.; Saleem, M.; Arshad, U.; Shahzad, M.; Mushtaq, M.H.; Husnain, A.; Riaz, A.; Ahmad, N. Effect of plasma progesterone on oocyte recovery, oocyte quality, and early in-vitro developmental competence of embryos in Bos indicus dairy cows. Anim. Reprod. Sci. 2019, 202, 80–86. [Google Scholar] [CrossRef]
- Komatsu, K.; Masubuchi, S. The concentration-dependent effect of progesterone on follicle growth in the mouse ovary. J. Reprod. Dev. 2017, 63, 271–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossi, R.O.; Costa, J.J.; Silva, A.W.; Saraiva, M.V.; Van den Hurk, R.; Silva, J.R. The bone morphogenetic protein system and the regulation of ovarian follicle development in mammals. Zygote 2016, 24, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Lima, I.M.T.; Brito, I.R.; Rossetto, R.; Duarte, A.B.G.; Rodrigues, G.Q.; Saraiva, M.V.A.; Costa, J.J.N.; Donato, M.A.M.; Peixoto, C.A.; Silva, J.R.V. BMPRIB and BMPRII mRNA expression levels in goat ovarian follicles and the in vitro effects of BMP-15 on preantral follicle development. Cell Tissue Res. 2012, 348, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Gode, F.; Gulekli, B.; Dogan, E.; Korhan, P.; Dogan, S.; Bige, O.; Cimrin, D.; Atabey, N. Influence of follicular fluid GDF9 and BMP15 on embryo quality. Fertil. Steril. 2011, 95, 2274–2278. [Google Scholar] [CrossRef]
- Hussein, T.S.; Thompson, J.G.; Gilchrist, R.B. Oocyte-secreted factors enhance oocyte developmental competence. Dev. Biol. 2006, 296, 514–521. [Google Scholar] [CrossRef]
- Hreinsson, J.G.; Scott, J.E.; Rasmussen, C.; Swahn, M.L.; Hsueh, A.J.; Hovatta, O. Growth differentiation factor-9 promotes the growth, development, and survival of human ovarian follicles in organ culture. J. Clin. Endocrinol. Metab. 2002, 87, 316–321. [Google Scholar] [CrossRef]
- Hanrahan, J.P.; Gregan, S.M.; Mulsant, P.; Mullen, M.; Davis, G.H.; Powell, R.; Galloway, S.M. Mutations in the genes for oocyte-derived growth factors GDF9 and BMP15 are associated with both increased ovulation rate and sterility in Cambridge and Belclare sheep (Ovis aries). Biol. Reprod. 2004, 70, 900–909. [Google Scholar] [CrossRef]
- Hussein, T.S.; Froiland, D.A.; Amato, F.; Thompson, J.G.; Gilchrist, R.B. Oocytes prevent cumulus cell apoptosis by maintaining a morphogenic paracrine gradient of bone morphogenetic proteins. J. Cell Sci. 2005, 118, 5257–5268. [Google Scholar] [CrossRef] [Green Version]
- Yoshino, O.; McMahon, H.E.; Sharma, S.; Shimasaki, S. A unique preovulatory expression pattern plays a key role in the physiological functions of BMP-15 in the mouse. Proc. Natl. Acad. Sci. USA 2006, 103, 10678–10683. [Google Scholar] [CrossRef] [Green Version]
- Avella, M.A.; Xiong, B.; Dean, J. The molecular basis of gamete recognition in mice and humans. MHR Basic Sci. Reprod. Med. 2013, 19, 279–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Picut, C.A.; Swanson, C.L.; Scully, K.L.; Roseman, V.C.; Parker, R.F.; Remick, A.K. Ovarian follicle counts using proliferating cell nuclear antigen (PCNA) and semi-automated image analysis in rats. Toxicol. Pathol. 2008, 36, 674–679. [Google Scholar] [CrossRef] [Green Version]
- Oktay, K.; Schenken, R.S.; Nelson, J.F. Proliferating cell nuclear antigen marks the initiation of follicular growth in the rat. Biol. Reprod. 1995, 53, 295–301. [Google Scholar] [CrossRef] [Green Version]
- Rowe, E.; Van Horn, A.; Rockwell, L.C. CYP17 genotype modifies the impact of anthropometric variation on salivary estradiol in healthy women. Am. J. Phys. Anthropol. 2015, 156, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Kempisty, B.; Ziółkowska, A.; Ciesiółka, S.; Piotrowska, H.; Antosik, P.; Bukowska, D.; Nowicki, M.; Brüssow, K.; Zabel, M. Association between the expression of LHR, FSHR and CYP19 genes, cellular distribution of encoded proteins and proliferation of porcine granulosa cells in real-time. J. Biol. Regul. Homeost. Agents 2014, 28, 419–431. [Google Scholar]
- Scarlet, D.; Walter, I.; Hlavaty, J.; Aurich, C. Expression and immunolocalisation of follicle-stimulating hormone receptors in gonads of newborn and adult female horses. Reprod. Fertil. Dev. 2016, 28, 1340–1348. [Google Scholar] [CrossRef]
- Gasperin, B.G.; Ferreira, R.; Rovani, M.T.; Bordignon, V.; Duggavathi, R.; Buratini, J.; Oliveira, J.F.; Gonçalves, P.B. Expression of receptors for BMP15 is differentially regulated in dominant and subordinate follicles during follicle deviation in cattle. Anim. Reprod. Sci. 2014, 144, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, F.; Yao, Z.; Lee, T.-h.; Yamamoto, S.; Erickson, G.F.; Shimasaki, S. Bone morphogenetic protein-15: Identification of target cells and biological functions. J. Biol. Chem. 2000, 275, 39523–39528. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, A.; Gupta, S.; Sharma, R. Oxidative stress and its implications in female infertility—A clinician’s perspective. Reprod. Biomed. Online 2005, 11, 641–650. [Google Scholar] [CrossRef]
- Tripathi, A.; Khatun, S.; Pandey, A.; Mishra, S.; Chaube, R.; Shrivastav, T.; Chaube, S. Intracellular levels of hydrogen peroxide and nitric oxide in oocytes at various stages of meiotic cell cycle and apoptosis. Free Radic. Res. 2009, 43, 287–294. [Google Scholar] [CrossRef]
- Van Blerkom, J. Mitochondrial function in the human oocyte and embryo and their role in developmental competence. Mitochondrion 2011, 11, 797–813. [Google Scholar] [CrossRef] [PubMed]
- Harada, Y.; Kinutani, M.; Horiuchi, T. Improved developmental potential of mouse vitrified-warmed oocytes achieved by culturing in recovery medium with glutathione ethyl ester (GSH-OEt). Reprod. Med. Biol. 2021, 20, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Suski, J.M.; Lebiedzinska, M.; Bonora, M.; Pinton, P.; Duszynski, J.; Wieckowski, M.R. Relation between mitochondrial membrane potential and ROS formation. In Mitochondrial Bioenergetics; Palmeira, C., Moreno, A., Eds.; Humana Press: Totowa, NJ, USA, 2012; pp. 183–205. [Google Scholar]
- Orrenius, S. Reactive oxygen species in mitochondria-mediated cell death. Drug Metab. Rev. 2007, 39, 443–455. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jitjumnong, J.; Tang, P.-C. Bone Morphogenetic Protein 15 (BMP-15) Improves In Vitro Mouse Folliculogenesis. Animals 2023, 13, 980. https://doi.org/10.3390/ani13060980
Jitjumnong J, Tang P-C. Bone Morphogenetic Protein 15 (BMP-15) Improves In Vitro Mouse Folliculogenesis. Animals. 2023; 13(6):980. https://doi.org/10.3390/ani13060980
Chicago/Turabian StyleJitjumnong, Jakree, and Pin-Chi Tang. 2023. "Bone Morphogenetic Protein 15 (BMP-15) Improves In Vitro Mouse Folliculogenesis" Animals 13, no. 6: 980. https://doi.org/10.3390/ani13060980
APA StyleJitjumnong, J., & Tang, P.-C. (2023). Bone Morphogenetic Protein 15 (BMP-15) Improves In Vitro Mouse Folliculogenesis. Animals, 13(6), 980. https://doi.org/10.3390/ani13060980





