Comparative Analysis of Olfactory Receptor Repertoires Sheds Light on the Diet Adaptation of the Bamboo-Eating Giant Panda Based on the Chromosome-Level Genome
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Genome Data Collection
2.2. OR Gene Identification
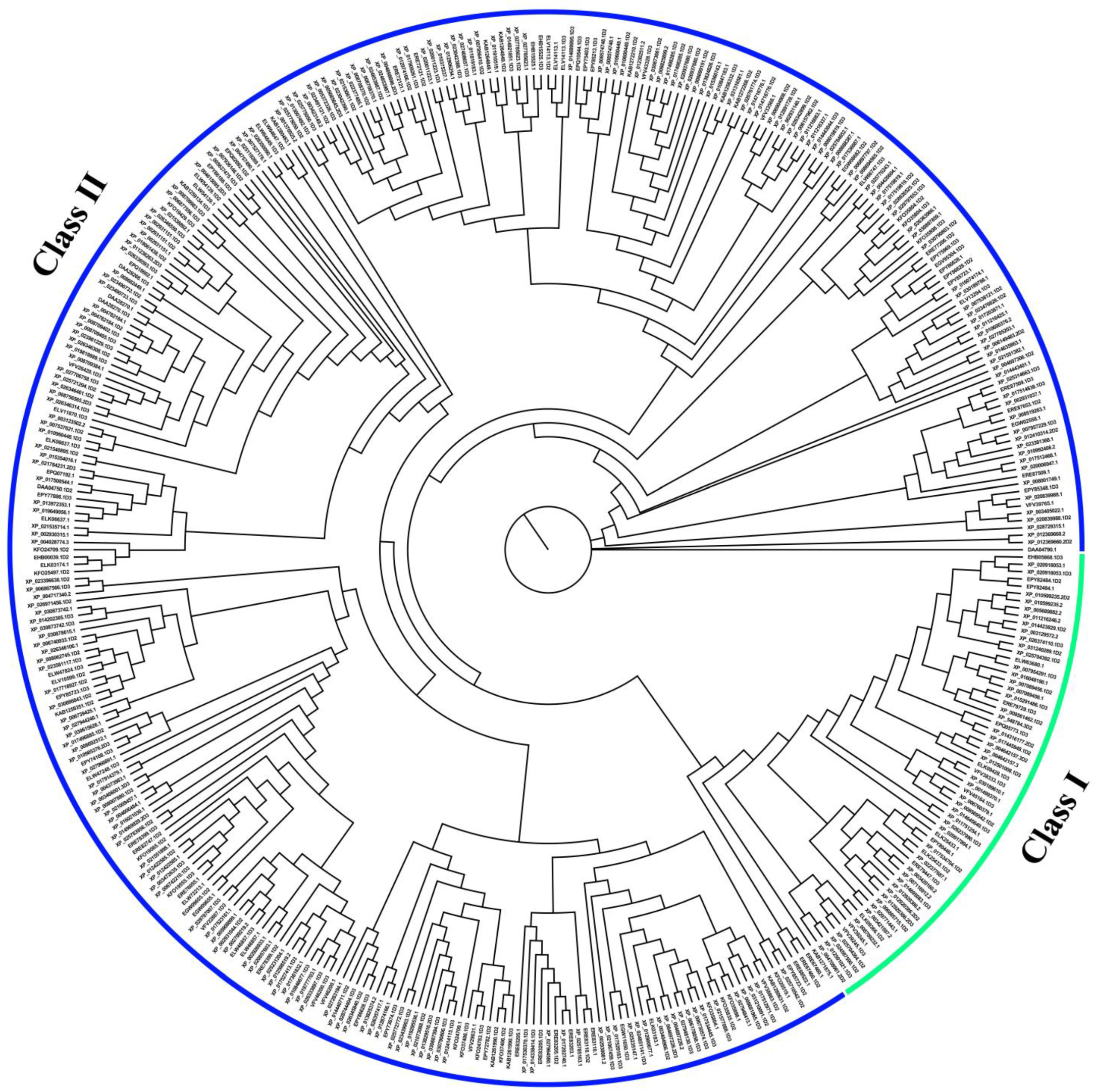
2.3. Phylogenetic Analysis and Classification
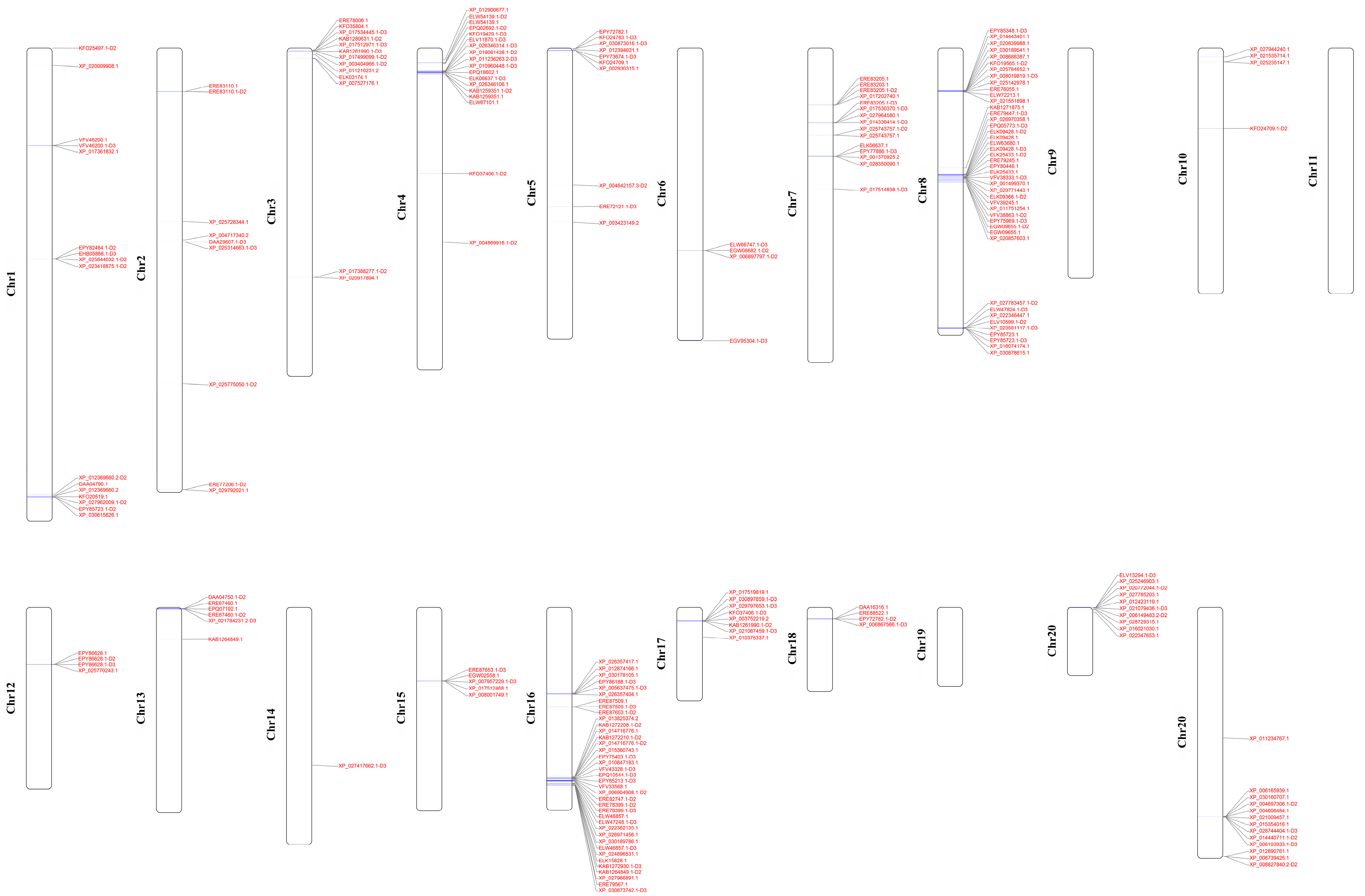
2.4. Chromosomal Distribution and Motifs Analysis
3. Results
3.1. Composition of OR Gene Repertoires
3.2. Classification of OR Gene Repertoires
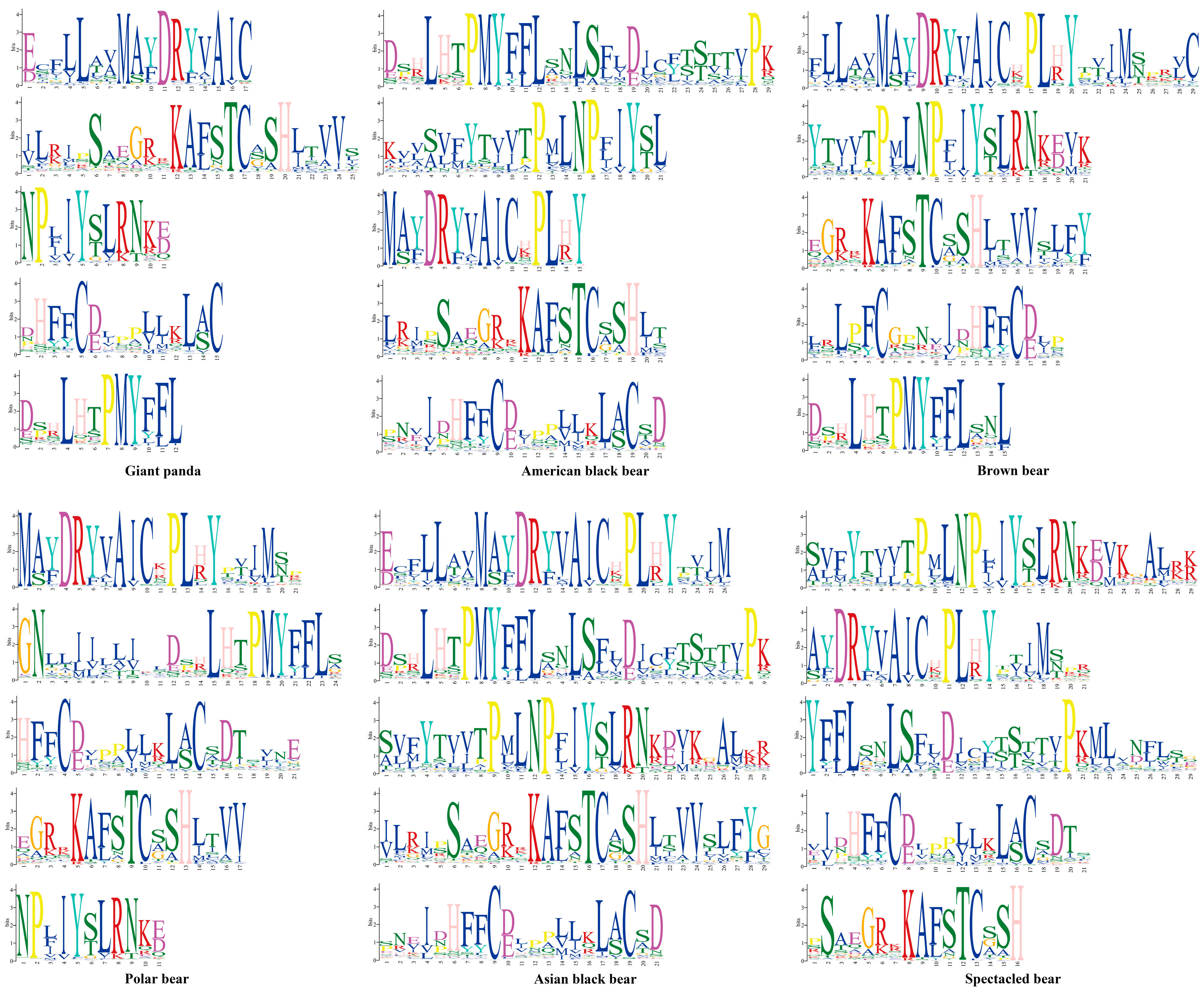
3.3. Patterns of Conserved Motifs for OR Genes
3.4. Potential Odorant Specificity of OR Subfamilies
4. Discussion
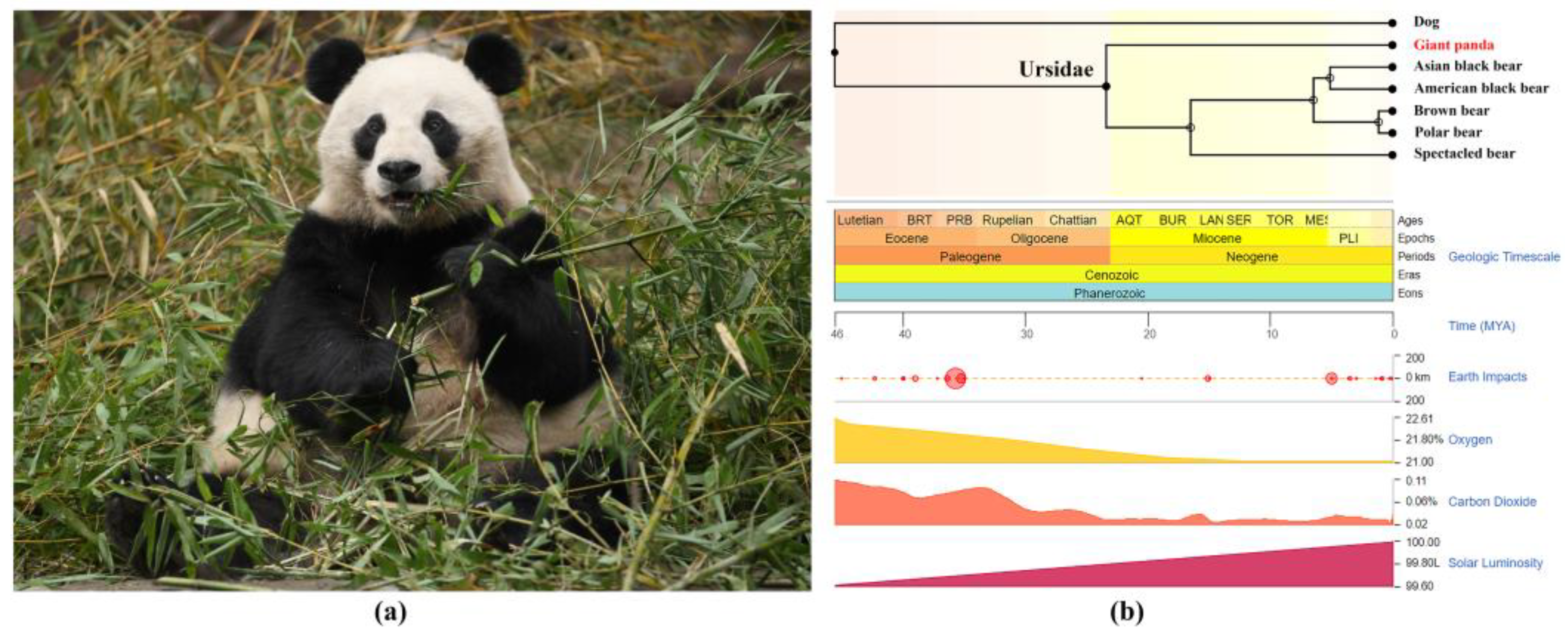
4.1. Olfaction and Transformation of Feeding Habits
4.2. Pseudogenization of OR Genes
4.3. Olfactory and OR Functional Genes
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buck, L.; Axel, R. A novel multigene family may encode odorant receptors: A molecular basis for odor recognition. Cell 1991, 65, 175–187. [Google Scholar] [CrossRef]
- Lv, L.Y.; Liang, X.F.; He, S. Genome-Wide Identification and Characterization of Olfactory Receptor Genes in Chinese Perch, Siniperca chuatsi. Genes 2019, 10, 178. [Google Scholar] [CrossRef] [Green Version]
- Freitag, J.; Krieger, J.; Strotmann, J.; Breer, H. Two classes of olfactory receptors in Xenopus laevis. Neuron 1995, 15, 1383–1392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breer, H.; Strotmann, J. Olfactory receptor gene expression. Semin. Cell Dev. Biol. 1997, 8, 189–195. [Google Scholar] [CrossRef]
- Shirasu, M.; Yoshikawa, K.; Takai, Y.; Nakashima, A.; Takeuchi, H.; Sakano, H.; Touhara, K. Olfactory receptor and neural pathway responsible for highly selective sensing of musk odors. Neuron 2014, 81, 165–178. [Google Scholar] [CrossRef] [Green Version]
- Glusman, G.; Yanai, I.; Rubin, I.; Lancet, D. The complete human olfactory subgenome. Genome Res. 2001, 11, 685–702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kishida, T.; Thewissen, J.; Hayakawa, T.; Imai, H.; Agata, K. Aquatic adaptation and the evolution of smell and taste in whales. Zool. Lett. 2015, 1, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayden, S.; Bekaert, M.; Goodbla, A.; Murphy, W.J.; Dávalos, L.M.; Teeling, E.C. A cluster of olfactory receptor genes linked to frugivory in bats. Mol. Biol. Evol. 2014, 31, 917–927. [Google Scholar] [CrossRef] [Green Version]
- Young, J.M.; Friedman, C.; Williams, E.M.; Ross, J.A.; Tonnes-Priddy, L.; Trask, B.J. Different evolutionary processes shaped the mouse and human olfactory receptor gene families. Hum. Mol. Genet. 2002, 11, 535–546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niimura, Y.; Nei, M. Evolution of olfactory receptor genes in the human genome. Proc. Natl. Acad. Sci. USA 2003, 100, 12235–12240. [Google Scholar] [CrossRef] [Green Version]
- Go, Y.; Niimura, Y. Similar numbers but different repertoires of olfactory receptor genes in humans and chimpanzees. Mol. Biol. Evol. 2008, 25, 1897–1907. [Google Scholar] [CrossRef] [Green Version]
- Wei, F.; Costanza, R.; Dai, Q.; Stoeckl, N.; Gu, X.; Farber, S.; Nie, Y.; Kubiszewski, I.; Hu, Y.; Swaisgood, R.; et al. The Value of Ecosystem Services from Giant Panda Reserves. Curr. Biol. 2018, 28, 2174–2180.e2177. [Google Scholar] [CrossRef] [Green Version]
- Wei, F.; Hu, Y.; Zhu, L.; Bruford, M.W.; Zhan, X.; Zhang, L. Black and white and read all over: The past, present and future of giant panda genetics. Mol. Ecol. 2012, 21, 5660–5674. [Google Scholar] [CrossRef]
- He, X.; Hsu, W.H.; Hou, R.; Yao, Y.; Xu, Q.; Jiang, D.; Wang, L.; Wang, H. Comparative genomics reveals bamboo feeding adaptability in the giant panda (Ailuropoda melanoleuca). ZooKeys 2020, 923, 141–156. [Google Scholar] [CrossRef]
- Gilad, O.; Swaisgood, R.R.; Owen, M.A.; Zhou, X. Giant pandas use odor cues to discriminate kin from nonkin. Curr. Zool. 2016, 62, 333–336. [Google Scholar] [CrossRef] [Green Version]
- Hagey, L.; MacDonald, E. Chemical cues identify gender and individuality in Giant pandas (Ailuropoda melanoleuca). J. Chem. Ecol. 2003, 29, 1479–1488. [Google Scholar] [CrossRef]
- Fan, H.; Wu, Q.; Wei, F.; Yang, F.; Ng, B.L.; Hu, Y. Chromosome-level genome assembly for giant panda provides novel insights into Carnivora chromosome evolution. Genome Biol. 2019, 20, 267. [Google Scholar] [CrossRef]
- Zhu, J.; Arena, S.; Spinelli, S.; Liu, D.; Zhang, G.; Wei, R.; Cambillau, C.; Scaloni, A.; Wang, G.; Pelosi, P. Reverse chemical ecology: Olfactory proteins from the giant panda and their interactions with putative pheromones and bamboo volatiles. Proc. Natl. Acad. Sci. USA 2017, 114, E9802–E9810. [Google Scholar] [CrossRef] [Green Version]
- Zhou, C.; Wang, L.; Qiao, L.; Lan, Y.; Price, M.; Yang, N.; Yue, B. Characterization of Olfactory Receptor Repertoires in the Endangered Snow Leopard Based on the Chromosome-Level Genome. DNA Cell Biol. 2021, 40, 293–302. [Google Scholar] [CrossRef]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef] [Green Version]
- Zhou, C.; Jin, J.; Peng, C.; Wen, Q.; Wang, G.; Wei, W.; Jiang, X.; Price, M.; Cui, K.; Meng, Y.; et al. Comparative genomics sheds light on the predatory lifestyle of accipitrids and owls. Sci. Rep. 2019, 9, 2249. [Google Scholar] [CrossRef] [Green Version]
- Birney, E.; Clamp, M.; Durbin, R. GeneWise and Genomewise. Genome Res. 2004, 14, 988–995. [Google Scholar] [CrossRef] [Green Version]
- Trifinopoulos, J.; Nguyen, L.T.; von Haeseler, A.; Minh, B.Q. W-IQ-TREE: A fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res. 2016, 44, W232–W235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Godzik, A. Cd-hit: A fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics 2006, 22, 1658–1659. [Google Scholar] [CrossRef] [Green Version]
- Bailey, T.L.; Elkan, C. Fitting a mixture model by expectation maximization to discover motifs in biopolymers. Proc. Int. Conf. Intell. Syst. Mol. Biol. 1994, 2, 28–36. [Google Scholar]
- Gupta, R.; Brunak, S. Prediction of glycosylation across the human proteome and the correlation to protein function. Pac. Symp. Biocomput. 2002, 7, 310–322. [Google Scholar]
- Zhang, X.; De la Cruz, O.; Pinto, J.M.; Nicolae, D.; Firestein, S.; Gilad, Y. Characterizing the expression of the human olfactory receptor gene family using a novel DNA microarray. Genome Biol. 2007, 8, R86. [Google Scholar] [CrossRef] [Green Version]
- Niimura, Y. Olfactory receptor multigene family in vertebrates: From the viewpoint of evolutionary genomics. Curr. Genom. 2012, 13, 103–114. [Google Scholar] [CrossRef] [Green Version]
- Young, J.M.; Shykind, B.M.; Lane, R.P.; Tonnes-Priddy, L.; Ross, J.A.; Walker, M.; Williams, E.M.; Trask, B.J. Odorant receptor expressed sequence tags demonstrate olfactory expression of over 400 genes, extensive alternate splicing and unequal expression levels. Genome Biol. 2003, 4, R71. [Google Scholar] [CrossRef] [Green Version]
- Silva Teixeira, C.S.; Cerqueira, N.M.; Silva Ferreira, A.C. Unravelling the Olfactory Sense: From the Gene to Odor Perception. Chem. Senses 2016, 41, 105–121. [Google Scholar] [CrossRef] [Green Version]
- Ben Khemis, I.; Ben Lamine, A. Physico-chemical investigations of human olfactory receptors OR10G4 and OR2B11 activated by vanillin, ethyl vanillin, coumarin and quinoline molecules using statistical physics method. Int. J. Biol. Macromol. 2021, 193, 915–922. [Google Scholar] [CrossRef]
- Lake, B. Synthesis & pharmacological investigation of 4-hydroxy coumarin derivatives & shown as anti-coagulant. Food Chem. Tox. 1999, 37, 423–453. [Google Scholar]
- Roberts, D.D.; Acree, T.E. Effects of Heating and Cream Addition on Fresh Raspberry Aroma Using a Retronasal Aroma Simulator and Gas Chromatography Olfactometry. J. Agric. Food Chem. 1996, 44, 3919–3925. [Google Scholar] [CrossRef]
- Ong, P.K.C.; Acree, T.E. Gas Chromatography/Olfactory Analysis of Lychee (Litchi chinesis Sonn.). J. Agric. Food Chem. 1998, 46, 2282–2286. [Google Scholar] [CrossRef]
- Menashe, I.; Abaffy, T.; Hasin, Y.; Goshen, S.; Yahalom, V.; Luetje, C.W.; Lancet, D. Genetic elucidation of human hyperosmia to isovaleric acid. PLoS Biol. 2007, 5, e284. [Google Scholar] [CrossRef]
- Guedes, C.M.; Pinto, A.B.; Moreira, R.F.A.; De Maria, C.A.B. Study of the aroma compounds of rose apple (Syzygium jambos Alston) fruit from Brazil. Eur. Food Res. Technol. 2004, 219, 460–464. [Google Scholar] [CrossRef]
- Li, R.; Fan, W.; Tian, G.; Zhu, H.; He, L.; Cai, J.; Huang, Q.; Cai, Q.; Li, B.; Bai, Y.; et al. The sequence and de novo assembly of the giant panda genome. Nature 2010, 463, 311–317. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Wu, Q.; Ma, S.; Ma, T.; Shan, L.; Wang, X.; Nie, Y.; Ning, Z.; Yan, L.; Xiu, Y.; et al. Comparative genomics reveals convergent evolution between the bamboo-eating giant and red pandas. Proc. Natl. Acad. Sci. USA 2017, 114, 1081–1086. [Google Scholar] [CrossRef] [Green Version]
- Nie, Y.; Speakman, J.R.; Wu, Q.; Zhang, C.; Hu, Y.; Xia, M.; Yan, L.; Hambly, C.; Wang, L.; Wei, W.; et al. ANIMAL PHYSIOLOGY. Exceptionally low daily energy expenditure in the bamboo-eating giant panda. Science 2015, 349, 171–174. [Google Scholar] [CrossRef]
- Zhou, C.; Liu, Y.; Zheng, X.; Shang, K.; Cheng, M.; Wang, L.; Yang, N.; Yue, B. Characterization of olfactory receptor repertoires provides insights into the high-altitude adaptation of the yak based on the chromosome-level genome. Int. J. Biol. Macromol. 2022, 209, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Rouquier, S.; Blancher, A.; Giorgi, D. The olfactory receptor gene repertoire in primates and mouse: Evidence for reduction of the functional fraction in primates. Proc. Natl. Acad. Sci. USA 2000, 97, 2870–2874. [Google Scholar] [CrossRef] [Green Version]
- Lai, P.C.; Bahl, G.; Gremigni, M.; Matarazzo, V.; Clot-Faybesse, O.; Ronin, C.; Crasto, C.J. An olfactory receptor pseudogene whose function emerged in humans: A case study in the evolution of structure-function in GPCRs. J. Struct. Funct. Genom. 2008, 9, 29–40. [Google Scholar] [CrossRef] [Green Version]
- Hayden, S.; Bekaert, M.; Crider, T.A.; Mariani, S.; Murphy, W.J.; Teeling, E.C. Ecological adaptation determines functional mammalian olfactory subgenomes. Genome Res. 2010, 20, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Niimura, Y.; Nei, M. Evolutionary dynamics of olfactory receptor genes in fishes and tetrapods. Proc. Natl. Acad. Sci. USA 2005, 102, 6039–6044. [Google Scholar] [CrossRef] [Green Version]
- Laska, M.; Genzel, D.; Wieser, A. The number of functional olfactory receptor genes and the relative size of olfactory brain structures are poor predictors of olfactory discrimination performance with enantiomers. Chem. Senses 2005, 30, 171–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malnic, B.; Godfrey, P.A.; Buck, L.B. The human olfactory receptor gene family. Proc. Natl. Acad. Sci. USA 2004, 101, 2584–2589. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Firestein, S. The olfactory receptor gene superfamily of the mouse. Nat. Neurosci. 2002, 5, 124–133. [Google Scholar] [CrossRef] [PubMed]

| Species | Functional OR Genes | Partial OR Genes | OR Pseudogenes | Total Number of OR Genes | No. of Subfamilies | |||
|---|---|---|---|---|---|---|---|---|
| Number | Percentage | Number | Percentage | Number | Percentage | |||
| Giant panda (Ailuropoda melanoleuca) | 408 | 63.85% | 94 | 14.71% | 137 | 21.44% | 639 | 248 |
| Spectacled bear (Tremarctos ornatus) | 269 | 60.04% | 106 | 23.66% | 73 | 16.29% | 448 | 205 |
| American black bear (Ursus americanus) | 497 | 80.55% | 17 | 2.76% | 103 | 16.69% | 617 | 249 |
| Brown bear (Ursus arctos) | 423 | 72.68% | 70 | 12.03% | 89 | 15.29% | 582 | 241 |
| Polar bear (Ursus maritimus) | 394 | 75.62% | 33 | 6.33% | 94 | 18.04% | 521 | 252 |
| Asian black bear (Ursus thibetanus) | 608 | 76.77% | 89 | 11.24% | 95 | 11.99% | 792 | 271 |
| Location | Chromosome Size (Mb) | No. of Functional OR Genes | No. of Partial OR Genes | No. of OR Pseudogenes | Total Number of OR Genes | No. of OR Subfamilies |
|---|---|---|---|---|---|---|
| Chr1 | 212.77 | 12 | 2 | 2 | 16 | 10 |
| Chr2 | 199.81 | 6 | 0 | 3 | 9 | 5 |
| Chr3 | 147.63 | 9 | 0 | 4 | 13 | 9 |
| Chr4 | 147.79 | 15 | 0 | 3 | 18 | 14 |
| Chr5 | 130.99 | 6 | 1 | 3 | 10 | 6 |
| Chr6 | 131.59 | 4 | 0 | 0 | 4 | 4 |
| Chr7 | 141.53 | 13 | 0 | 2 | 15 | 11 |
| Chr8 | 129.35 | 36 | 1 | 7 | 44 | 36 |
| Chr9 | 103.69 | 0 | 0 | 0 | 0 | 0 |
| Chr10 | 110.58 | 4 | 0 | 0 | 4 | 3 |
| Chr11 | 110.51 | 0 | 0 | 0 | 0 | 0 |
| Chr12 | 81.78 | 4 | 0 | 0 | 4 | 2 |
| Chr13 | 92.46 | 6 | 0 | 0 | 6 | 4 |
| Chr14 | 106.65 | 0 | 1 | 0 | 1 | 0 |
| Chr15 | 91.61 | 4 | 0 | 1 | 5 | 4 |
| Chr16 | 91.34 | 30 | 1 | 7 | 38 | 25 |
| Chr17 | 42.25 | 6 | 0 | 2 | 8 | 6 |
| Chr18 | 38.12 | 3 | 0 | 1 | 4 | 3 |
| Chr19 | 35.68 | 0 | 0 | 0 | 0 | 0 |
| Chr20 | 30.94 | 5 | 0 | 5 | 10 | 5 |
| ChrX | 112.85 | 7 | 1 | 5 | 13 | 7 |
| Human ORs | Accession Number | No. of Functional OR Genes | Recognized Odorant(s) | |||||
|---|---|---|---|---|---|---|---|---|
| AIL | AME | ARC | MAR | THI | TRE | |||
| OR1A1 | Q9P1Q5 | 1 | 1 | 0 | 0 | 1 | 1 | (S)-(−)-citronellol, (S)-(−)-citronellal, (+)-carvone [19] |
| OR1A2 | Q9Y585 | 0 | 1 | 0 | 1 | 0 | 0 | Same as OR1A1 except (S)-(−)-citronellol |
| OR1D2 | P34982 | 2 | 1 | 1 | 1 | 2 | 0 | Bourgeonal |
| OR1E3 | Q8WZA6 | 1 | 1 | 1 | 1 | 0 | 2 | Acetophenone |
| OR1G1 | P47890 | 1 | 1 | 0 | 0 | 1 | 1 | Nonanal, 1-nonanol, 2-ethyl-1-hexanol, γ-decalactone, Ethyl isobutyrate, Isoamyl acetate |
| OR2A25 | A4D2G3 | 1 | 0 | 0 | 0 | 4 | 0 | Geranyl acetate |
| OR2AG1 | Q9H205 | 0 | 1 | 1 | 1 | 0 | 0 | Amylbutyrate |
| OR2B11 | Q5JQS5 | 0 | 2 | 2 | 2 | 4 | 2 | Coumarin |
| OR2C1 | O95371 | 2 | 0 | 2 | 2 | 2 | 3 | Nonanethiol, Octanethiol |
| OR2J2 | O76002 | 0 | 1 | 0 | 1 | 0 | 0 | 1-heptanol, 1-octanol, 1-nonanol, 1-decanol, Coumarin, Ethyl vanilin, cis-3-hexen-1-ol |
| OR2J3 | O76001 | 0 | 1 | 0 | 1 | 0 | 0 | cis-3-hexen-1-ol, Geranyl acetate, Cinnamaldehyde |
| OR2M7 | Q8NG81 | 2 | 5 | 1 | 3 | 2 | 1 | Geraniol (−)-β-citronellol |
| OR2W1 | Q9Y3N9 | 1 | 0 | 0 | 3 | 1 | 1 | allyl phenyl acetate, cis-3-hexen-1-ol, Citral and citronellal [19] |
| OR3A1 | P47881 | 0 | 11 | 0 | 1 | 0 | 0 | Helional, Lilial |
| OR4D6 | Q8NGJ1 | 1 | 1 | 0 | 0 | 1 | 0 | β-ionone |
| OR4D9 | Q8NGE8 | 1 | 0 | 3 | 2 | 0 | 0 | β-ionone |
| OR4Q3 | Q8NH05 | 0 | 2 | 0 | 2 | 0 | 0 | Eugenol |
| OR5A1 | Q8NGJ0 | 0 | 0 | 0 | 1 | 1 | 0 | β-ionone |
| OR5A2 | Q8NGI9 | 0 | 0 | 0 | 1 | 1 | 0 | β-ionone |
| OR5AN1 | Q8NGI8 | 5 | 1 | 2 | 0 | 1 | 0 | Muscone |
| OR5D18 | Q8NGL1 | 1 | 0 | 1 | 0 | 1 | 0 | Eugenol, isoeugenol |
| OR5K1 | Q8NHB7 | 4 | 8 | 5 | 8 | 2 | 2 | Eugenol methyl ether |
| OR5P3 | Q8WZ94 | 1 | 1 | 1 | 1 | 1 | 1 | 1-hexanol, 1-heptanol, (−)-carvone, (+)-carvone, Acetophenone, Coumarin, 1-octanol and celery ketone |
| OR6P1 | Q8NGX9 | 1 | 1 | 1 | 1 | 1 | 1 | Anisaldehyde |
| OR7C1 | O76099 | 3 | 9 | 5 | 5 | 19 | 1 | Androstadienone |
| OR7D4 | Q8NG98 | 1 | 1 | 0 | 1 | 1 | 1 | Androsterone, Androstadienone |
| OR8B3 | Q8NGG8 | 1 | 0 | 1 | 1 | 2 | 0 | (+)-carvone |
| OR8D1 | Q8WZ84 | 1 | 2 | 3 | 1 | 3 | 0 | Caramel furanone |
| OR8K3 | Q8NH51 | 1 | 0 | 1 | 0 | 0 | 1 | (+)-menthol |
| OR10A6 | Q8NH74 | 1 | 2 | 3 | 1 | 3 | 0 | 3-phenyl propyl propionate |
| OR10G3 | Q8NGC4 | 0 | 4 | 2 | 2 | 2 | 1 | Ethyl vanillin, Vanillin |
| OR10G4 | Q8NGN3 | 1 | 1 | 1 | 0 | 0 | 0 | Guaiacol, Vanillin |
| OR10G7 | Q8NGN6 | 1 | 1 | 1 | 0 | 0 | 0 | Eugenol |
| OR10G9 | Q8NGN4 | 1 | 1 | 1 | 0 | 0 | 0 | Ethyl vanillin |
| OR10J5 | Q8NHC4 | 1 | 2 | 2 | 2 | 2 | 2 | Lyral |
| OR11A1 | Q9GZK7 | 0 | 0 | 0 | 0 | 1 | 1 | 2-ethyl fenchol |
| OR11H4 | Q8NGC9 | 5 | 7 | 3 | 5 | 4 | 2 | Isovaleric acid |
| OR11H6 | Q8NGC7 | 0 | 5 | 1 | 5 | 2 | 2 | Isovaleric acid |
| OR11H7P | Q8NGC8 | 0 | 5 | 1 | 5 | 2 | 2 | Isovaleric acid |
| OR51E1 | Q8TCB6 | 0 | 0 | 0 | 1 | 0 | 0 | Nonanoic acid, Butyl butyryllactate, Butyric acid, Isovaleric acid, Propionic acid |
| OR51E2 | Q9H255 | 0 | 0 | 0 | 1 | 0 | 0 | Propionic acid |
| OR51L1 | Q8NGJ5 | 4 | 1 | 1 | 0 | 0 | 1 | Hexanoic acid, Allyl phenyl acetate |
| OR52D1 | Q9H346 | 2 | 1 | 0 | 1 | 4 | 0 | Ethyl heptanoate, Methyl octanoate, 1-nonanol, 2-nonanol, 3-nonanone, 3-octanone |
| OR56A1 | Q8NGH5 | 1 | 2 | 0 | 2 | 1 | 0 | Undecanal |
| OR56A4 | Q8NGH8 | 1 | 2 | 0 | 2 | 1 | 0 | Decyl adehyde, Undecanal |
| OR56A5 | P0C7T3 | 1 | 2 | 0 | 2 | 1 | 0 | Undecanal |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, C.; Liu, Y.; Zhao, G.; Liu, Z.; Chen, Q.; Yue, B.; Du, C.; Zhang, X. Comparative Analysis of Olfactory Receptor Repertoires Sheds Light on the Diet Adaptation of the Bamboo-Eating Giant Panda Based on the Chromosome-Level Genome. Animals 2023, 13, 979. https://doi.org/10.3390/ani13060979
Zhou C, Liu Y, Zhao G, Liu Z, Chen Q, Yue B, Du C, Zhang X. Comparative Analysis of Olfactory Receptor Repertoires Sheds Light on the Diet Adaptation of the Bamboo-Eating Giant Panda Based on the Chromosome-Level Genome. Animals. 2023; 13(6):979. https://doi.org/10.3390/ani13060979
Chicago/Turabian StyleZhou, Chuang, Yi Liu, Guangqing Zhao, Zhengwei Liu, Qian Chen, Bisong Yue, Chao Du, and Xiuyue Zhang. 2023. "Comparative Analysis of Olfactory Receptor Repertoires Sheds Light on the Diet Adaptation of the Bamboo-Eating Giant Panda Based on the Chromosome-Level Genome" Animals 13, no. 6: 979. https://doi.org/10.3390/ani13060979





