Fluorescence Microscopy and Flow-Cytometry Assessment of Substructures in European Red Deer Epididymal Spermatozoa after Cryopreservation
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Sampling and Cryopreservation of Spermatozoa
2.3. Sperm Analysis
2.3.1. Sperm Motility
2.3.2. Fluorescence Assay
2.3.3. Flow-Cytometry Analysis
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Comizzoli, P.; Mermillod, P.; Mauget, R. Reproductive biotechnologies for endangered mammalian species. Reprod. Nutr. Dev. 2000, 40, 493–504. [Google Scholar] [CrossRef] [PubMed]
- Garde, J.J.; Martínez-Pastor, F.; Gomendio, M.; Malo, A.F.; Soler, A.J.; Fernández-Santos, M.R.; Esteso, M.C.; García, A.J.; Anel, L.; Roldán, E.R.S. The application of reproductive technologies to natural populations of red deer. Reprod. Domest. Anim. 2006, 41, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Hishinuma, M.; Suzuki, K.; Sekine, J. Recovery and cryopreservation of sika deer (Cervus nippon) spermatozoa from epididymides stored at 4 °C. Theriogenology 2003, 59, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Medina-Chávez, D.A.; Soler, A.J.; Martín-Maestro, A.; Villaverde, S.; Sánchez-Ajofrín, I.; Peris-Frau, P.; Del Olmo, E.; Bisbal, A.; García-Álvarez, O.; Fernández-Santos, M.D.R.; et al. Freezing Protocol Optimization for Iberian Red Deer (Cervus elaphus hispanicus) Epididymal Sperm under Field Conditions. Animals 2022, 12, 869. [Google Scholar] [CrossRef]
- Saragusty, J.; Gacitua, H.; King, R.; Arav, A. Post-mortem semen cryopreservation and characterization in two different endangered gazelle species (Gazella gazella and Gazella dorcas) and one subspecies (Gazella gazelle acaiae). Theriogenology 2006, 66, 775–784. [Google Scholar] [CrossRef]
- Soler, A.J.; Poulin, N.; Fernández-Santos, M.R.; Cognie, Y.; Esteso, M.C.; Garde, J.J.; Mermillod, P. Heterologous in vitro fertility evaluation of cryopreserved Iberian red deer epididymal spermatozoa with zona-intact sheep oocytes and its relationship with the characteristics of thawed spermatozoa. Reprod. Domest. Anim. 2008, 43, 293–298. [Google Scholar] [CrossRef]
- Kaabi, M.; Paz, P.; Alvarez, M.; Anel, E.; Boixo, J.C.; Rouissi, H.; Herraez, P.; Anel, L. Effect of epididymis handling conditions on the quality of ram spermatozoa recovered post-mortem. Theriogenology 2003, 60, 1249–1259. [Google Scholar] [CrossRef]
- Martínez, A.F.; Martínez-Pastor, F.; Alvarez, M.; Fernández-Santos, M.R.; Esteso, M.C.; de Paz, P.; Garde, J.J.; Anel, L. Sperm parameters on Iberian red deer: Electroejaculation and post-mortem collection. Theriogenology 2008, 70, 216–226. [Google Scholar] [CrossRef]
- Fletcher, T.J. Farmed deer: New domestic animals defined by controlled breeding. Reprod. Fertil. Dev. 2001, 13, 511–516. [Google Scholar] [CrossRef]
- Comizzoli, P.; Mermillod, P.; Cognié, Y.; Chai, N.; Legendre, X.; Mauge, R. Successful in vitro production of embryos in the red deer (Cervus elaphus) and the sika deer (Cervus nippon). Theriogenology 2001, 55, 649–659. [Google Scholar] [CrossRef]
- Dziekońska, A.; Koziorowska-Gilun, M.; Kordan, W.; Neuman, N.M.; Kotlarczyk, A.M.; Korzekwa, A.J. The Quality and Fertilizing Potential of Red Deer (Cervus elaphus L.) Epididymal Spermatozoa Stored in a Liquid State. Int. J. Mol. Sci. 2022, 23, 14591. [Google Scholar] [CrossRef] [PubMed]
- Nichi, M.; Rijsselaere, T.; Losano, J.; Angrimani, D.; Kawai, G.; Goovaerts, I.; Van Soom, A.; Barnabe, V.H.; De Clercq, J.; Bols, P. Evaluation of epididymis storage temperature and cryopreservation conditions for improved mitochondrial membrane potential, membrane integrity, sperm motility and in vitro fertilization in bovine epididymal sperm. Reprod. Domest. Anim. 2017, 52, 257–263. [Google Scholar] [CrossRef] [PubMed]
- An, T.Z.; Wada, S.; Edashige, K.; Sakurai, T.; Kasai, M. Viable spermatozoa can be recovered from refrigerated mice up to 7 days after death. Cryobiology 1999, 38, 27–34. [Google Scholar] [CrossRef]
- Lone, F.A.; Islam, R.; Khan, M.Z.; Sofi, K.A. Effect of transportation temperature on the quality of cauda epididymal spermatozoa of ram. Anim. Reprod. Sci. 2011, 123, 54–59. [Google Scholar] [CrossRef]
- Yu, I.; Leibo, S.P.T. Recovery of motile, membrane-intact spermatozoa from canine epididymides stored for 8 days at 4 degrees C. Theriogenology 2002, 57, 1179–1190. [Google Scholar] [CrossRef] [PubMed]
- Malcotti, V.; Pelufo, V.; Bergamo, N.; Aisen, E. Recovery of epididymal spermatozoa from bull and red deer, stored at different times and temperatures before freezing–thawing. Anim. Prod. Sci. 2012, 52, 741–745. [Google Scholar] [CrossRef]
- Martins, C.F.; Driessen, K.; Costa, P.M.; Carvalho-Neto, J.O.; de Sousa, R.V.; Rumpf, R.; Dode, M.N. Recovery, cryopreservation and fertilization potential of bovine spermatozoa obtained from epididymides stored at 5 degrees C by different periods of time. Anim. Prod. Sci. 2009, 116, 50–57. [Google Scholar] [CrossRef]
- Soler, A.J.; Pérez-Guzmán, M.D.; Garde, J.J. Storage of red deer epididymides for four days at 5 degrees C: Effects on sperm motility, viability, and morphological integrity. J. Exp. Zool. A Comp. Exp. Biol. 2003, 295, 188–199. [Google Scholar] [CrossRef]
- Soler, A.J.; Esteso, M.C.; Fernández-Santos, M.R.; Garde, J.J. Characteristics of Iberian red deer (Cervus elaphus hispanicus) spermatozoa cryopreserved after storage at 5 degrees C in the epididymis for several days. Theriogenology 2005, 64, 1503–1517. [Google Scholar] [CrossRef]
- Fernández-Santos, M.R.; Martínez-Pastor, F.; Matias, D.; Domínguez-Rebolledo, A.E.; Esteso, M.C.; Montoro, V.; Garde, J.J. Effects of long-term chilled storage of red deer epididymides on DNA integrity and motility of thawed spermatozoa. Anim. Reprod. Sci. 2009, 111, 93–104. [Google Scholar] [CrossRef]
- Muratori, M.; Forti, G.; Baldi, E. Comparing flow cytometry and fluorescence microscopy for analyzing human sperm DNA fragmentation by TUNEL labeling. Cytometry 2008, 73, 785–787. [Google Scholar] [CrossRef] [PubMed]
- Brugnon, F.; Ouchchane, L.; Verheyen, G.; Communal, Y.; Van der Elst, J.; Tournaye, H.; Janny, L.; Grizard, G. Fluorescence microscopy and flow cytometry in measuring activated caspases in human spermatozoa. Int. J. Androl. 2009, 32, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Pastor, F.; Mata-Campuzano, M.; Alvarez-Rodríguez, M.; Alvarez, M.; Anel, L.; de Paz, P. Probes and techniques for sperm evaluation by flow cytometry. Reprod. Domest. Anim. 2010, 45, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Christensen, P.; Stenvang, J.P.; Godfrey, W.L. A flow cytometric method for rapid determination of sperm concentration and viability in mammalian and avian semen. J. Androl. 2004, 25, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Perticarari, S.; Ricci, G.; Granzotto, M.; Boscolo, R.; Pozzobon, C.; Guarnieri, S.; Sartore, A.; Presani, G. A new multiparameter flow cytometric method for human semen analysis. Hum. Reprod. 2006, 22, 485–494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dziekońska, A.; Niedźwiecka, E.; Niklewska, M.E.; Koziorowska-Gilun, M.; Kordan, W. Viability longevity and quality of epididymal sperm stored in the liquid state of European red deer (Cervus elaphus elaphus). Anim. Reprod. Sci. 2020, 213, 106269. [Google Scholar] [CrossRef]
- Martínez-Pastor, F.; Martínez, F.; Alvarez, M.; Maroto-Morales, A.; García-Alvarez, O.; Soler, A.J.; Garde, J.J.; de Paz, P.; Anel, L. Cryopreservation of Iberian red deer (Cervus elaphus hispanicus) spermatozoa obtained by electroejaculation. Theriogenology 2009, 71, 628–638. [Google Scholar] [CrossRef]
- Dziekońska, A.; Neuman, N.M.; Burdal, K.K.; Wiszniewska-Łaszczych, A.; Bogdaszewski, M. The Effect of Different Extenders on the Quality Characteristics of European Red Deer Epididymal Sperm Stored at 5 °C. Animals 2022, 12, 2669. [Google Scholar] [CrossRef]
- Partyka, A.; Nizański, W.; Łukaszewicz, E. Evaluation of fresh and frozen-thawed fowl semen by flow cytometry. Theriogenology 2010, 74, 1019–1027. [Google Scholar] [CrossRef]
- Partyka, A.; Nizański, W.; Bajzert, J.; Łukaszewicz, E.; Ochota, M. The effect of cysteine and superoxide dismutase on the quality of post-thawed chicken sperm. Cryobiology 2013, 67, 132–136. [Google Scholar] [CrossRef]
- Peña, F.J.; Johannisson, A.; Wallgren, M.; Rodriguez Martinez, H. Antioxidant supplementation of boar spermatozoa from different fractions of the ejaculate improves cryopreservation: Changes in sperm membrane lipid architecture. Zygote 2004, 12, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Santos, M.R.; Martínez-Pastor, F.; García-Macías, V.; Esteso, M.C.; Soler, A.J.; Paz, P.; Anel, L.; Garde, J.J. Sperm characteristics and DNA integrity of Iberian red deer (Cervus elaphus hispanicus) epididymal spermatozoa frozen in the presence of enzymatic and nonenzymatic antioxidants. J. Androl. 2007, 28, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Pastor, F.; Johannisson, A.; Gil, J.; Kaabi, M.; Anel, L.; Paz, P.; Rodriguez-Martinez, H. Use of chromatin stability assay, mitochondrial stain JC-1, and fluorometric assessment of plasma membrane to evaluate frozen-thawed ram semen. Anim. Reprod. Sci. 2004, 84, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Watson, P.F. The causes of reduced fertility with cryopreserved semen. Anim. Reprod. Sci. 2000, 60, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Martin, G.; Sabido, O.; Durand, P.; Levy, R. Cryopreservation induces an apoptosis-like mechanism in bull sperm. Biol. Reprod. 2004, 71, 28–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, B.; Wang, Y.; Wu, C.; Qiu, S.; Chen, X.; Cai, B.; Xie, H. Freeze-thawing impairs the motility, plasma membrane integrity and mitochondria function of boar spermatozoa through generating excessive ROS. BMC Vet. Res. 2021, 17, 127. [Google Scholar] [CrossRef]
- Merkies, K.; Chenier, T.; Plante, C.; Buhr, M.M. Assessment of stallion spermatozoa viability by flow cytometry and light microscope analysis. Theriogenology 2000, 54, 1215–1224. [Google Scholar] [CrossRef]
- Garner, D.L.; Johnson, L.A. Viability assessment of mammalian sperm using Sybr-14 and propidium iodide. Biol. Reprod. 1995, 53, 276–284. [Google Scholar] [CrossRef]
- Llavanera, M.; Mislei, B.; Blanco-Prieto, O.; Baldassarro, V.A.; Mateo-Otero, Y.; Spinaci, M.; Yeste, M.; Bucci, D. Assessment of sperm mitochondrial activity by flow cytometry and fluorescent microscopy: A comparative study of mitochondrial fluorescent probes in bovine spermatozoa. Reprod. Fertil. Dev. 2022, 34, 679–688. [Google Scholar] [CrossRef]
- Ettinger, A.; Wittmann, T. Fluorescence live cell imaging. Methods Cell Biol. 2014, 123, 77–94. [Google Scholar] [CrossRef] [Green Version]
- Icha, J.; Weber, M.; Waters, J.C.; Norden, C. Phototoxicity in live fluorescence microscopy, and how to avoid it. Bioessays 2017, 39, 1700003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laissue, P.P.; Alghamdi, R.A.; Tomancak, P.; Reynaud, E.G.; Shroff, H. Assessing phototoxicity in live fluorescence imaging. Nat. Methods 2017, 14, 657–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pierzyńska-Mach, A.; Janowski, P.A.; Dobrucki, J.W. Evaluation of acridine orange, LysoTracker Red, and quinacrine as fluorescent probes for long-term tracking of acidic vesicles. Cytometry 2014, 85, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Boe-Hansen, G.B.; Ersboll, A.K.; Christensen, P. Variability and laboratory factors affecting the sperm chromatin structure assay in human semen. J. Androl. 2005, 26, 360–368. [Google Scholar] [CrossRef]
- Mohammed, E.E.; Mosad, E.; Zahran, A.M.; Hameed, D.A.; Taha, E.A.; Mohamed, M.A. Acridine Orange and Flow Cytometry: Which Is Better to Measure the Effect of Varicocele on Sperm DNA Integrity? Adv Urol. 2015, 2015, 814150. [Google Scholar] [CrossRef] [Green Version]
- Gravance, C.G.; Garner, D.L.; Baumber, J.; Ball, B.A. Assessment of equine sperm mitochondrial function using JC-1. Theriogenology 2000, 53, 1691–1703. [Google Scholar] [CrossRef]
- Khan, D.R.; Ahmad, N.; Anzar, M.; Channa, A.A. Apoptosis in fresh and cryopreserved buffalo sperm. Theriogenology 2009, 71, 872–876. [Google Scholar] [CrossRef]

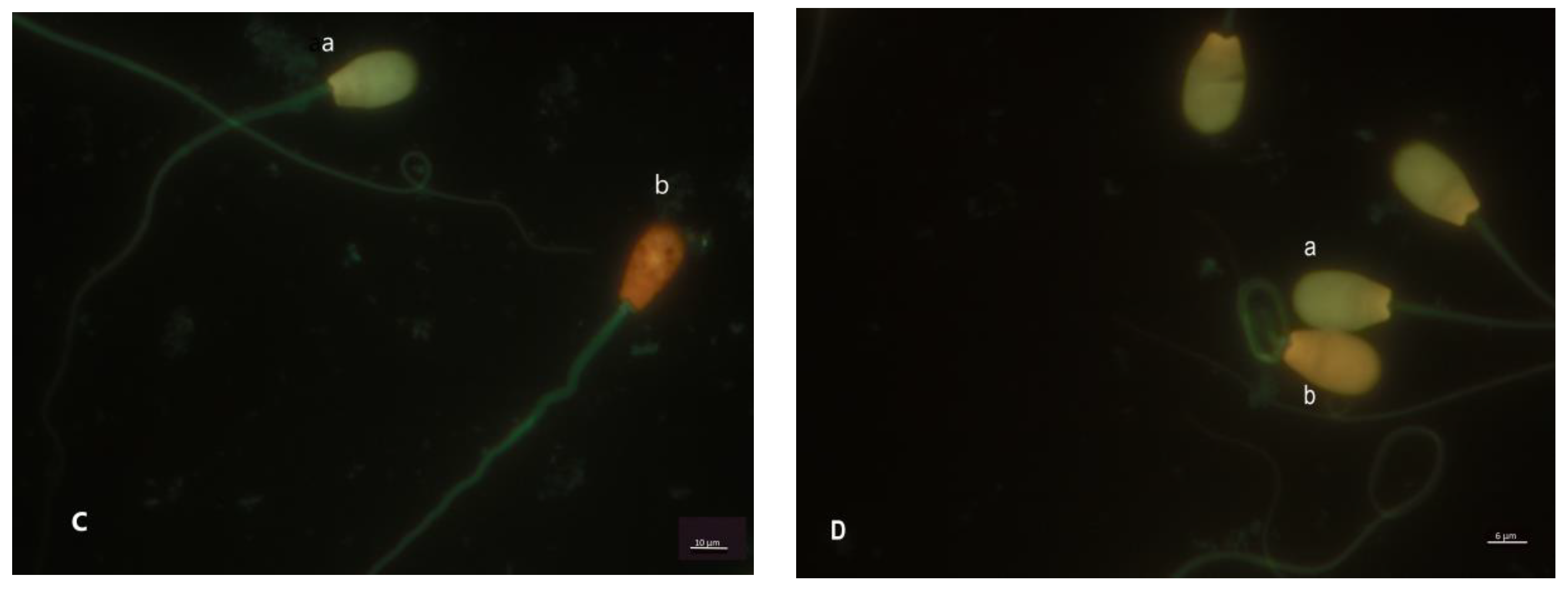
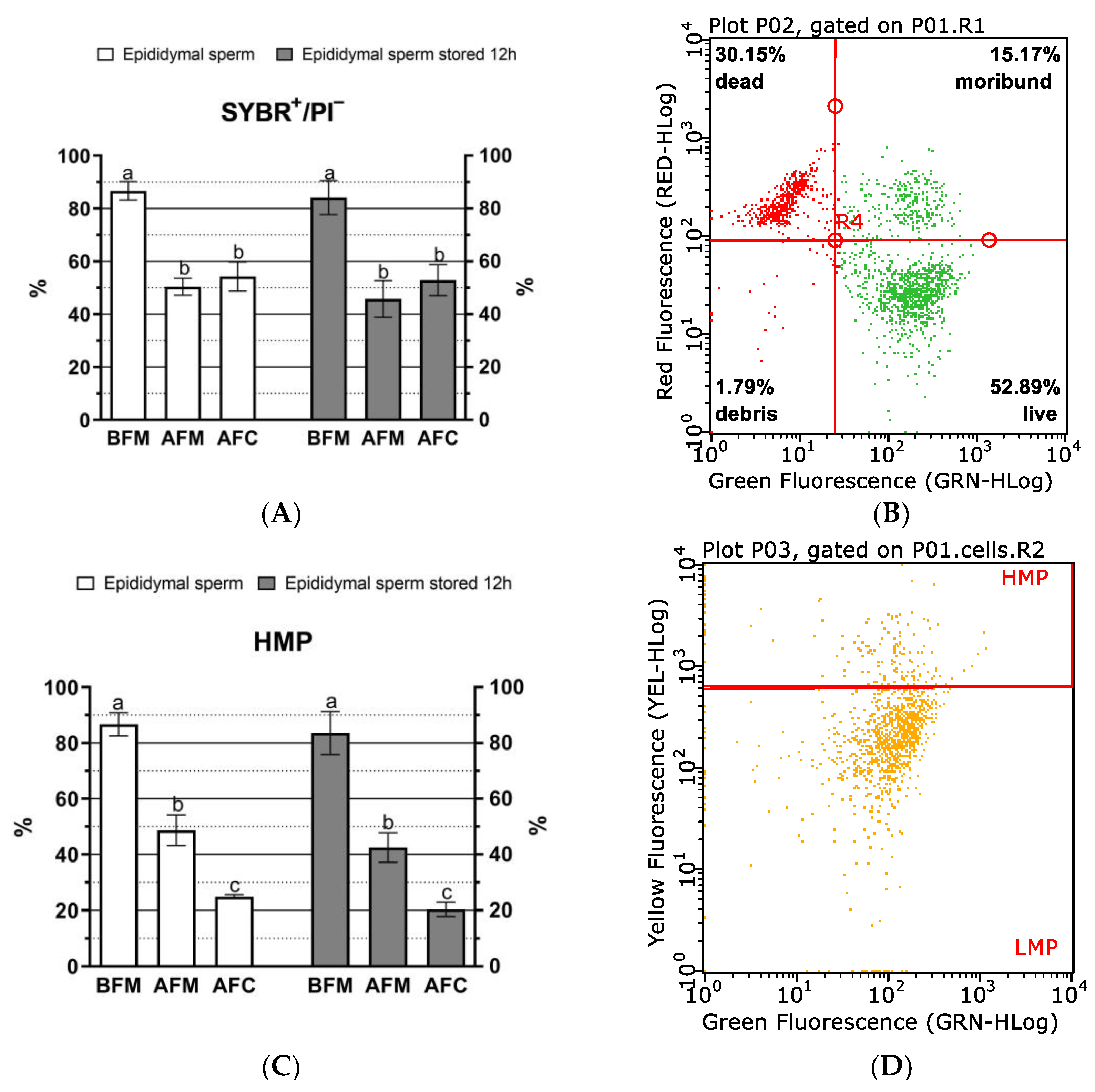



| Sperm Motility Parameters | Epididymal Sperm | Epididymal Sperm Stored for 12 h | ||
|---|---|---|---|---|
| Before Cryopreservation | After Cryopreservation | Before Cryopreservation | After Cryopreservation | |
| TMOT (%) | 79.9 ± 1.2 a | 51.8 ± 7.5 b | 78.5 ± 1.4 a | 47.3 ± 6.2 b |
| PMOT (%) | 41.5 ± 0.8 a | 24.1 ± 4.5 b | 41.9 ± 1.1 a | 18.1 ± 5.1 b |
| VAP (µm/s) | 131.5 ± 4.9 a | 109.5 ± 7.1 b | 126.4 ± 5.2 a | 110.3 ± 7.2 b |
| VSL (µm/s) | 85.7 ± 3.2 a | 72.2 ± 5.6 b | 81.1 ± 2.1 a | 72.5 ± 5.6 b |
| VCL (µm/s) | 253.8 ± 9.3 a | 215.9 ± 14.7 b | 243.1 ± 10.4 a | 220.7 ± 15.7 b |
| ALH (µm) | 9.4 ± 0.3 a | 8.2 ± 0.3 b | 8.9 ± 0.3 a | 8.3 ± 0.4 a |
| BCF (Hz) | 33.1 ± 8.7 a | 33.6 ± 1.7 a | 40.7 ± 5.4 a | 33.6 ± 1.7 a |
| STR (%) | 65.4 ± 2.2 a | 65.3 ± 2.7 ab | 64.8 ± 3.8 a | 65.4 ± 2.8 a |
| LIN (%) | 35.1 ± 1.3 a | 35.2 ± 2.7 a | 35.1 ± 2.3 a | 34.9 ± 2.8 a |
| TMOT | PMOT | SYBR+ /PI− | HMP | PNA−/PI− | YOPRO−/PI− | DFI | |
|---|---|---|---|---|---|---|---|
| TMOT | 0.78 * | 0.59 | 0.73 * | 0.67 * | 0.68 * | −0.04 | |
| PMOT | 0.72 * | 0.38 | 0.17 | 0.15 | 0.41 | ||
| SYBR+/PI− | 0.88 ** | 0.85 ** | 0.72 * | −0.03 | |||
| HMP | 0.83 ** | 0.79 ** | −0.18 | ||||
| PNA−/PI− | 0.87 ** | −0.08 | |||||
| YOPRO−/PI− | 0.28 |
| TMOT | PMOT | SYBR+ /PI− | HMP | PNA−/PI− | YOPRO−/PI− | DFI | |
|---|---|---|---|---|---|---|---|
| TMOT | 0.78 * | 0.60 | −0.23 | 0.67 * | 0.28 | 0.42 | |
| PMOT | 0.68 * | −0.19 | 0.73 * | 0.80 * | −0.34 | ||
| SYBR+/PI− | −0.12 | 0.95 *** | 0.84 ** | 0.20 | |||
| HMP | −0.14 | 0.04 | 0.21 | ||||
| PNA−/PI− | 0.89 ** | 0.12 | |||||
| YOPRO−/PI− | −0.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dziekońska, A.; Lecewicz, M.; Partyka, A.; Niżański, W. Fluorescence Microscopy and Flow-Cytometry Assessment of Substructures in European Red Deer Epididymal Spermatozoa after Cryopreservation. Animals 2023, 13, 990. https://doi.org/10.3390/ani13060990
Dziekońska A, Lecewicz M, Partyka A, Niżański W. Fluorescence Microscopy and Flow-Cytometry Assessment of Substructures in European Red Deer Epididymal Spermatozoa after Cryopreservation. Animals. 2023; 13(6):990. https://doi.org/10.3390/ani13060990
Chicago/Turabian StyleDziekońska, Anna, Marek Lecewicz, Agnieszka Partyka, and Wojciech Niżański. 2023. "Fluorescence Microscopy and Flow-Cytometry Assessment of Substructures in European Red Deer Epididymal Spermatozoa after Cryopreservation" Animals 13, no. 6: 990. https://doi.org/10.3390/ani13060990






