Supplementary Feed Additives Can Improve Lamb Performance in Terms of Birth Weight, Body Size, and Survival Rate
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Treatments, and Management
2.2. Placental Evaluation
2.3. Lamb Measurements
2.4. Statistical Analysis
3. Results
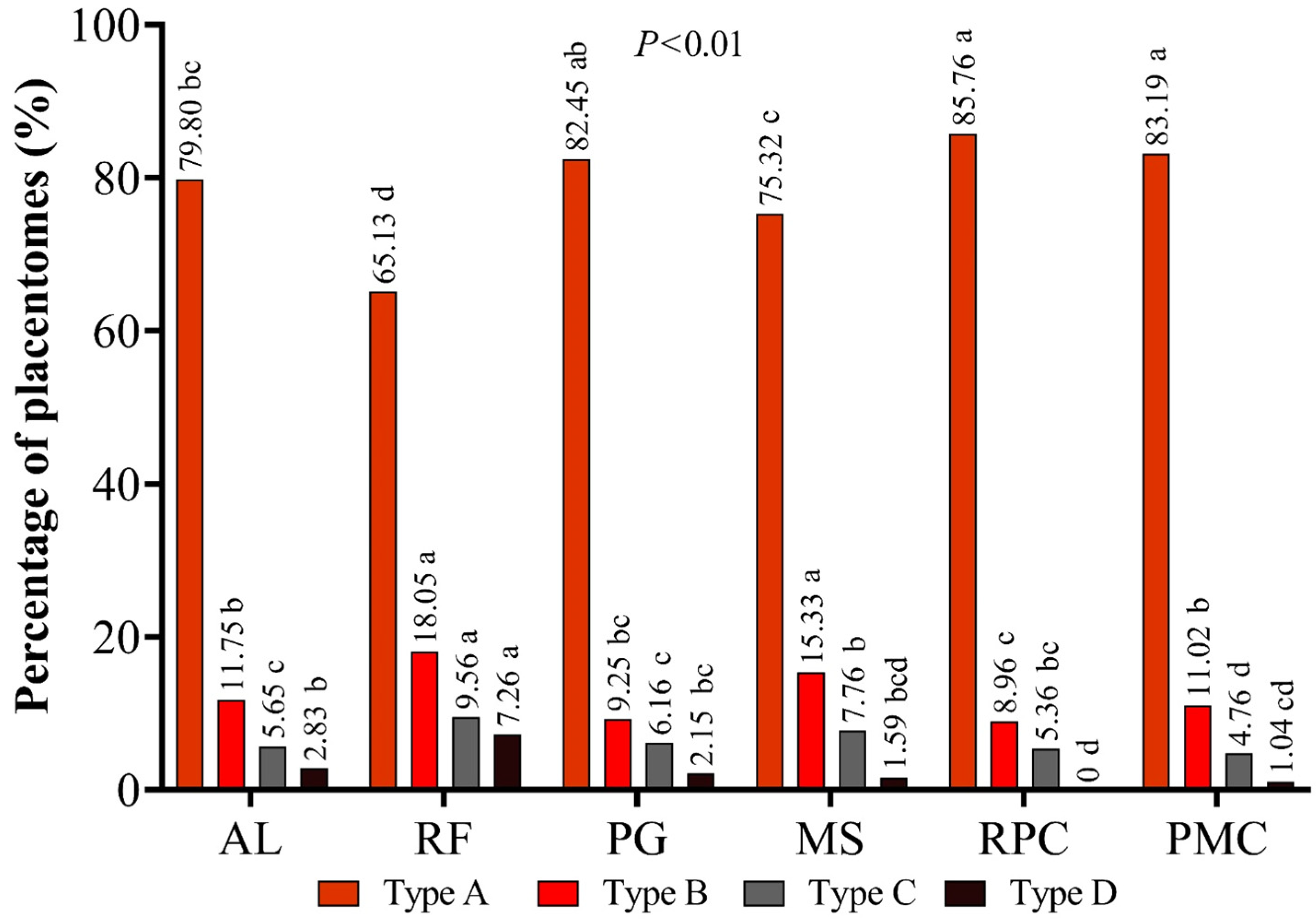
3.1. Placental Characteristics
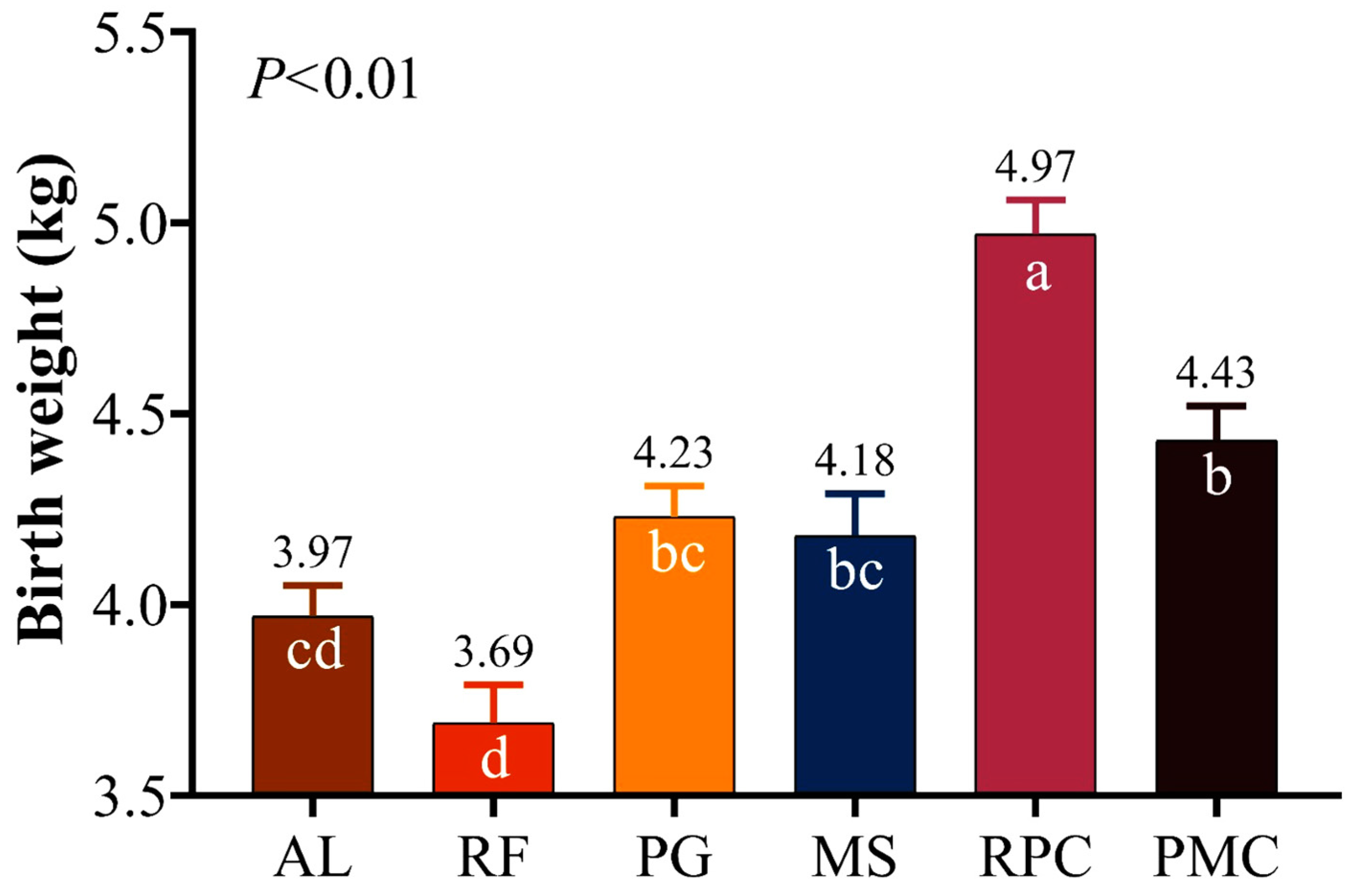
3.2. Lamb Measurements
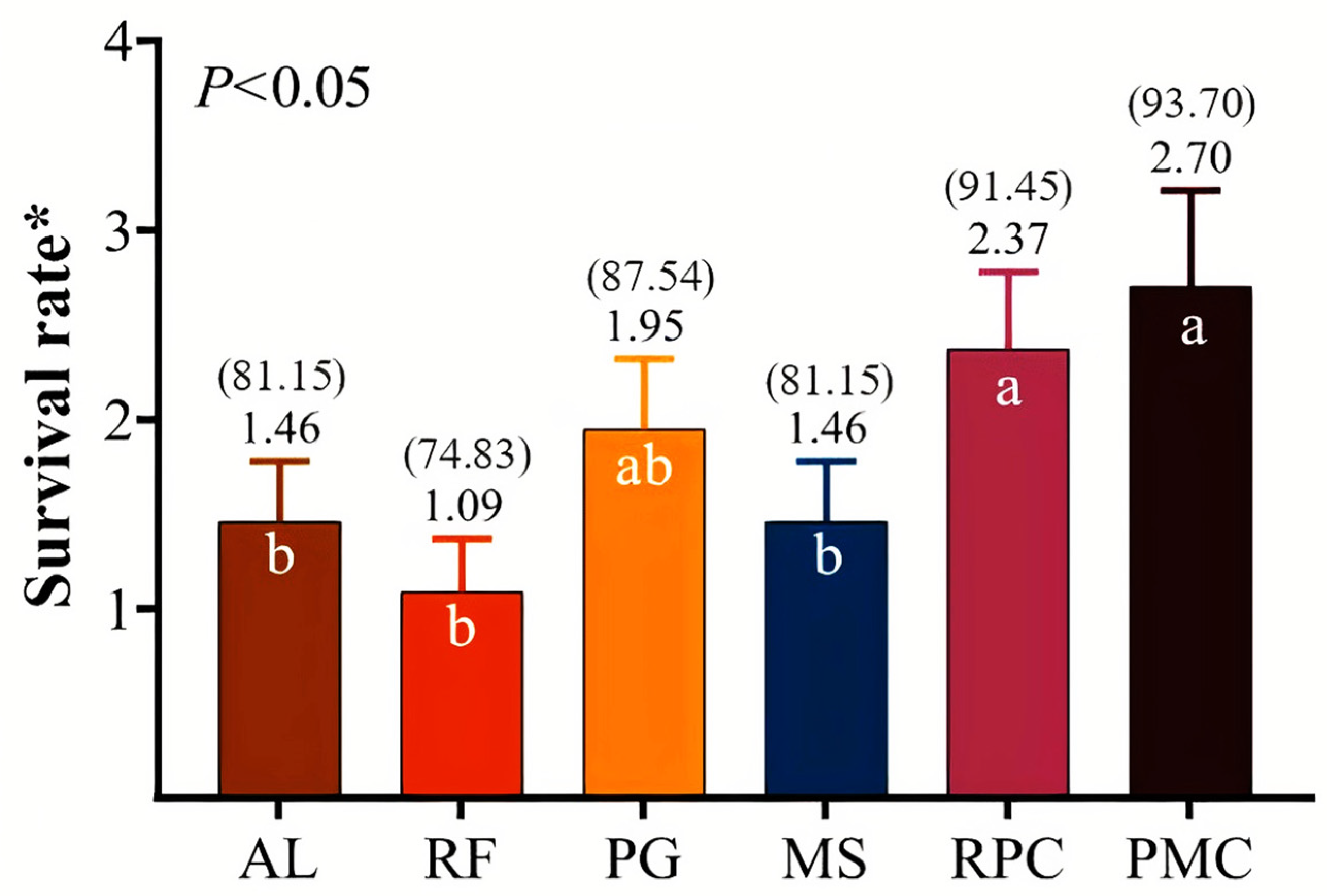
3.3. Offspring Performance
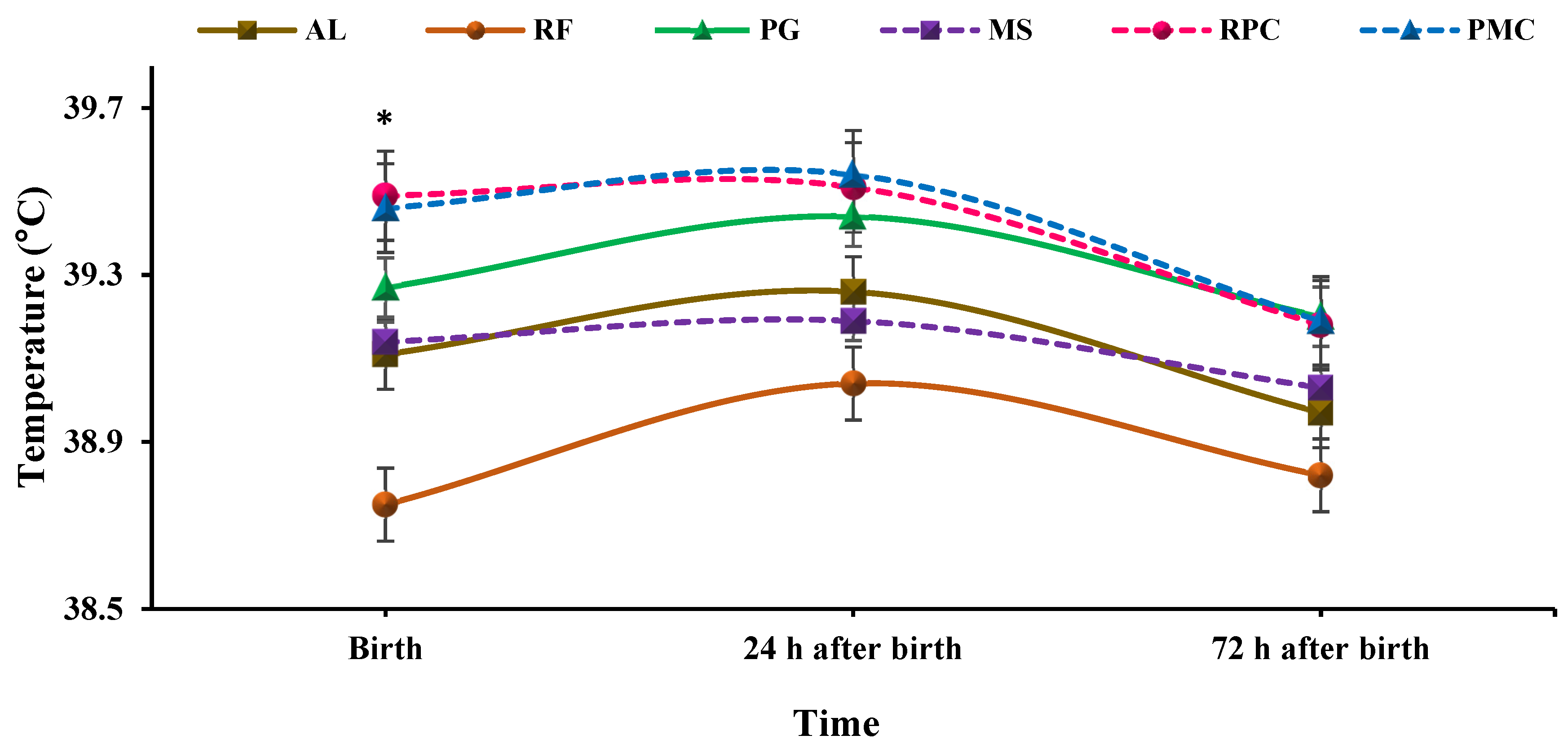
3.4. Rectal Temperature
4. Discussion
4.1. Placental Characteristics
4.2. Lamb Measurements
4.3. Offspring Performance
4.4. Rectal Temperature
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Greenwood, P.L.; Thompson, A.N.; Ford, S.P. Postnatal Consequences of the Maternal Environment and of Growth during Prenatal Life for Productivity of Ruminants. In Managing the Prenatal Environment to Enhance Livestock Productivity; Greenwood, P.L., Bell, A.W., Vercoe, P.E., Viljoen, G.J., Eds.; Springer: Dordrecht, The Netherland, 2009; pp. 3–36. [Google Scholar] [CrossRef]
- Behrendt, R.; van Burgel, A.J.; Bailey, A.; Barber, P.; Curnow, M.; Gordon, D.J.; Edwards, J.H.; Oldham, C.M.; Thompson, A.N. On-farm paddock-scale comparisons across southern Australia confirm that increasing the nutrition of Merino ewes improves their production and the lifetime performance of their progeny. Anim. Prod. Sci. 2011, 51, 805–812. [Google Scholar] [CrossRef]
- Dwyer, C.M. Genetic and physiological determinants of maternal behavior and lamb survival: Implications for low-input sheep management. J. Anim. Sci. 2000, 886, 246–258. [Google Scholar] [CrossRef]
- Blair, H.T.; Van der Linden, D.S.; Jenkinson, C.M.C.; Morris, S.T.; Mackenzie, D.D.S.; Peterson, S.W.; Firth, E.C.; Kenyon, P.R. Do ewe size and nutrition during pregnancy affect foetus and foetal organ weight in twins? Livest. Sci. 2011, 142, 99–107. [Google Scholar] [CrossRef]
- Wu, G.; Bazer, F.W.; Wallace, J.M.; Spencer, T.E. Board-invited review: Intrauterine growth retardation: Implications for the animal sciences. J. Anim. Sci. 2006, 84, 2316–2337. [Google Scholar] [CrossRef]
- Godfrey, K.M.; Barker, D.J. Fetal programming and adult health. Public. Health Nutr. 2001, 4, 611–624. [Google Scholar] [CrossRef]
- Heasman, L.; Clarke, L.; Stephenson, T.J.; Symonds, M.E. The influence of maternal nutrient restriction in early to mid-pregnancy on placental and fetal development in sheep. Proc. Nutr. Soc. 1999, 58, 283–288. [Google Scholar] [CrossRef]
- Schneider, H. Ontogenic changes in the nutritive function of the placenta. Placenta 1996, 17, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Mellor, D.J. Nutritional and placental determinants of foetal growth rate in sheep and consequences for the newborn lamb. Br. Vet. J. 1983, 139, 307–324. [Google Scholar] [CrossRef] [PubMed]
- Lekatz, L.A.; Caton, J.S.; Taylor, J.B.; Reynolds, L.P.; Redmer, D.A.; Vonnahme, K.A. Maternal selenium supplementation and timing of nutrient restriction in pregnant sheep: Effects on maternal endocrine status and placental characteristics. J. Anim. Sci. 2010, 88, 955–971. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, C.M.; Calvert, S.K.; Farish, M.; Donbavand, J.; Pickup, H.E. Breed, litter and parity effects on placental weight and placentome number, and consequences for the neonatal behaviour of the lamb. Theriogenology 2005, 63, 1092–1110. [Google Scholar] [CrossRef] [PubMed]
- Ahmadzadeh-Gavahan, L.; Hosseinkhani, A.; Taghizadeh, A.; Ghasemi-Panahi, B.; Hamidian, G.; Cheraghi-Saray, S. Impact of supplementing feed restricted ewes’ diet with propylene glycol, monensin sodium and rumen-protected choline chloride during late pregnancy on blood biochemical indices, body condition score and body weight. Anim. Feed Sci. Technol. 2021, 273, 114801. [Google Scholar] [CrossRef]
- Ahmadzadeh-Gavahan, L.; Hosseinkhani, A. Feed restriction and supplementing with propylene glycol, monensin sodium and rumen-protected choline chloride in periparturient Ghezel ewes: Implications on production and performance of ewes and their offspring. Livest. Sci. 2022, 255, 104784. [Google Scholar] [CrossRef]
- Duffield, T.F.; Bagg, R.N. Use of ionophores in lactating dairy cattle: A review. Can. Vet. J. 2000, 41, 388–394. [Google Scholar]
- Novilla, M.N. The veterinary importance of the toxic syndrome induced by ionophores. Vet. Hum. Toxicol. 1992, 34, 66–70. [Google Scholar]
- Roder, J.D. Ionophore toxicity and tolerance. Vet. Clin. N. Am. Food Anim. Pract. 2011, 27, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Zavala, G.; Anderson, D.A.; Davis, J.F.; Dufour-Zavala, L. Acute monensin toxicosis in broiler breeder chickens. Avian Dis. 2011, 55, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Bautista, A.C.; Tahara, J.; Mete, A.; Gaskill, C.L.; Bryant, U.K.; Puschner, B. Diagnostic value of tissue monensin concentrations in horses following toxicosis. J. Vet. Diagn. Investig. 2014, 26, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Booth, N.H.; McDonald, L.E. Veterinary Pharmacology and Therapeutics, 5th ed.; Iowa State University Press: Ames Iowa, IA, USA, 1982. [Google Scholar]
- Schweitzer, D.; Kimberling, C.; Spaker, T.; Stener, F.E.; McChensney, A.E. Accidental monensin sodium intoxication of feedlot cattle. Am. J. Vet. Med. 1984, 184, 1273–1276. [Google Scholar]
- Confer, A.W.; Reavis, D.U.; Panciera, R.J. Light and electron microscopic changes in cardiac and skeletal muscle of sheep with experimental monensin toxicosi. Vet. Pathol. 1983, 20, 590–602. [Google Scholar] [CrossRef]
- Federation of American Societies for Experimental Biology, LSRO, FASEB. Evaluation of the Health Aspects of Choline Chloride and Choline Bitartrate as Food Ingredients; Report No.: SCOGS-42; Department of Health, Education and Welfare: Washington, DC, USA, 1975. [Google Scholar]
- Sharma, B.K.; Erdman, R.A. Effect of high amount of dietary choline supplementation on duodenal choline flow and production responses of dairy cows. J. Dairy Sci. 1988, 71, 2670–2676. [Google Scholar] [CrossRef]
- Ahmadi-Noorbakhsh, S.; Mirabzadeh Ardakani, E.; Sadighi, J.; Aldavood, S.J.; Farajli Abbasi, M.; Farzad-Mohajeri, S.; Ghasemi, A.; Sharif-Paghaleh, E.; Hatami, Z.; Nikravanfard, N.; et al. Guideline for the care and use of laboratory animals in Iran. Lab. Anim. 2021, 50, 303–305. [Google Scholar] [CrossRef]
- Laporte-Broux, B.; Roussel, S.; Ponter, A.A.; Perault, J.; Chavatte-Palmer, P.; Duvaux-Ponter, C. Short-term effects of maternal feed restriction during pregnancy on goat kid morphology, metabolism, and behavior. J. Anim. Sci. 2011, 89, 2154–2163. [Google Scholar] [CrossRef]
- Pinotti, L.; Campagnoli, A.; D’ambrosio, F.; Susca, F.; Innocenti, M.; Rebucci, R.; Fusi, E.; Cheli, F.; Savoini, G.; Dell’Orto, V.; et al. Rumen-protected choline and vitamin E supplementation in periparturient dairy goats: Effects on milk production and folate, vitamin B12 and vitamin E status. Animal 2008, 2, 1019–1027. [Google Scholar] [CrossRef] [PubMed]
- Hajikhajehlou, M.F.; Moghaddam, G.; Shoja, J.; Pirani, N. Effect of propylene glycol on fetal growth rate and its relation with pregnancy toxemia in ewes. Anim. Sci. Res. 2010, 20, 67–76. [Google Scholar]
- Ahmadzadeh, L.; Hosseinkhani, A.; Kia, H.D. Effect of supplementing a diet with monensin sodium and Saccharomyces Cerevisiae on reproductive performance of Ghezel ewes. Anim. Reprod. Sci. 2018, 188, 93–100. [Google Scholar] [CrossRef] [PubMed]
- National Research Council (NRC). Nutrient Requirements of Small Ruminants: Sheep, Goats, Cervids and New Camelids; National Academy Press: Washington, DC, USA, 2007; p. 384. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 16th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 1995. [Google Scholar]
- Vatnick, I.; Ignotz, G.; McBride, B.W.; Bell, A.W. Effect of heat stress on ovine placental growth in early pregnancy. J. Dev. Physiol. 1991, 16, 163–166. [Google Scholar]
- Wilson, M.E.; Ford, S.P. Comparative aspects of placental efficiency. Reprod. Suppl. 2001, 58, 223–232. [Google Scholar] [CrossRef]
- Ocak, S.; Ogun, S.; Gunduz, Z.; Onder, H. Goat placental efficiency determination by comparing litter weight to the surface area of the cotyledons. Anim. Reprod. Sci. 2015, 12, 920–926. [Google Scholar] [CrossRef]
- Kelly, R.W. Nutrition and placental development. Proc. Nutr. Soc. Aust. 1992, 17, 203–211. [Google Scholar]
- Clarke, L.; Heasman, L.; Juniper, D.T.; Symonds, M.E. Maternal nutrition in early-mid gestation and placental size in sheep. Br. J. Nutr. 1998, 79, 359–364. [Google Scholar] [CrossRef]
- Cristofolini, A.L.; Turiello, M.P.; Sanchis, E.G.; Cufré, G.; Merkis, C.I. Effect of feed restriction and realimentation with monensin supplementation on placental structure and ultrastructure in an-glo-nubian goats. ISRN Vet. Sci. 2012, 2012, 490530. [Google Scholar] [CrossRef]
- Jiang, X.; Jones, S.; Andrew, B.Y.; Ganti, A.; Malysheva, O.V.; Giallourou, N.; Brannon, P.M.; Roberson, M.S.; Caudill, M.A. Choline inadequacy impairs trophoblast function and vascularization in cultured human placental trophoblasts. J. Cell Physiol. 2014, 229, 1016–1027. [Google Scholar] [CrossRef]
- Lever, M.; Slow, S. The clinical significance of betaine, an osmolyte with a key role in methyl group metabolism. Clin. Biochem. 2010, 43, 732–744. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, L.P.; Borowicz, P.P.; Vonnahme, K.A.; Johnson, M.L.; Grazul-Bilska, A.T.; Redmer, D.A.; Caton, J.S. Placental angiogenesis in sheep models of compromised pregnancy. J. Physiol. 2005, 565, 43–58. [Google Scholar] [CrossRef] [PubMed]
- Osgerby, J.C.; Wathes, D.C.; Howard, D.; Gadd, T.S. The effect of maternal undernutrition on the placental growth trajectory and the uterine insulin-like growth factor axis in the pregnant ewe. J. Endocrinol. 2004, 182, 89–104. [Google Scholar] [CrossRef] [PubMed]
- Heasman, L.; Clarke, L.; Firth, K.; Stephenson, T.; Symonds, M.E. Influence of restricted maternal nutrition in early to mid-gestation on placental and fetal development at term in sheep. Pediatr. Res. 1998, 44, 546–551. [Google Scholar] [CrossRef]
- Ford, S.P.; Nijland, M.J.; Miller, M.M.; Hess, B.W.; Nathanielsz, P.W. Maternal undernutrition advanced placentomal type, in association with increased placentomal size, and cotyledonary (COT) blood flow. J. Soc. Gynecol. Investig. 2006, 13, 212a. [Google Scholar]
- Osgerby, J.C.; Wathes, D.C.; Howard, D.; Gadd, T.S. The effect of maternal undernutrition on ovine fetal growth. J. Endocrinol. 2002, 173, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Ford, S.P.; Vonnahme, K.A.; Drumhiller, M.C.; Reynolds, L.P.; Nijland, M.J.; Nathanielsz, P.W. Arteriolar density and capillary volume increase as placentomes advance from type A through type D developmental stages. Biol. Reprod. Suppl. 2004, 71, 509. [Google Scholar]
- Pillai, S.M.; Jones, A.K.; Hoffman, M.L.; McFadden, K.K.; Reed, S.A.; Zinn, S.A.; Govoni, K.E. Fetal and organ development at gestational days 45, 90, 135 and at birth of lambs exposed to under-or over-nutrition during gestation. Transl. Anim. Sci. 2017, 1, 16–25. [Google Scholar] [CrossRef]
- Anthony, R.V.; Scheaffer, A.N.; Wright, C.D.; Regnault, T.R. Ruminant models of prenatal growth restriction. Reprod. Suppl. 2003, 61, 183–194. [Google Scholar] [CrossRef]
- Che, L.; Yang, Z.; Xu, M.; Xu, S.; Che, L.; Lin, Y.; Fang, Z.; Feng, B.; Li, J.; Chen, D.; et al. Maternal nutrition modulates fetal development by inducing placental efficiency changes in gilts. BMC Genom. 2017, 18, 213. [Google Scholar] [CrossRef]
- Martín, N.P.; Kenyon, P.R.; Morel, P.C.H.; Pain, S.J.; Jenkinson, C.M.C.; Hutton, P.G.; Morris, S.T.; Peterson, S.W.; Firth, E.C.; Blair, H.T. Ewe nutrition in early and mid-to late pregnancy has few effects on fetal development. Anim. Prod. Sci. 2012, 52, 533–539. [Google Scholar] [CrossRef]
- Dwyer, C.M.; Lawrence, A.B.; Bishop, S.C.; Lewis, M. Ewe–lamb bonding behaviours at birth are affected by maternal undernutrition in pregnancy. Br. J. Nutr. 2003, 89, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, F.F.; Brochine, L.; Oliveira, M.C.; Ferigato, G.A.; Junior, V.B.; Titto, C.G.; Gallo, S.B. Performance and behavior of the progeny of ewes fed with different sources and energy feed. Livest. Sci. 2022, 260, 104953. [Google Scholar] [CrossRef]
- Dougherty, H.C.; Evered, M.; Oltjen, J.W.; Hegarty, R.S.; Neutze, S.A.; Oddy, V.H. Effects of dietary energy density and supplemental rumen undegradable protein on intake, viscera, and carcass composition of lambs recovering from nutritional restriction. J. Anim. Sci. 2022, 158, skac158. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.D.; Kawas, J.R.; Mahgoub, O.G. Fibre digestion and utilization in goats. Small Rumin. Res. 2005, 60, 45–52. [Google Scholar] [CrossRef]
- Shu, P.C. Study on Partitioning of Proteins (Amino acids) in White Cashmere Goats Fed Diets with Different Dietary Metabolizable Glucose Levels. Ph.D. Thesis, Inner Mongolia Agriculture University, Huhhot, China, 2003; p. 630. [Google Scholar]
- Smith, N.A.; McAuliffe, F.M.; Quinn, K.; Lonergan, P.; Evans, A.C.O. Transient high glycaemic intake in the last trimester of pregnancy increases offspring birthweight and postnatal growth rate in sheep: A randomised control trial. BJOG Int. J. Gynecol. Obstet. 2009, 116, 975–983. [Google Scholar] [CrossRef]
- Li, H.; Wang, H.; Yu, L.; Wang, M.; Liu, S.; Sun, L.; Chen, Q. Effects of supplementation of rumen-protected choline on growth performance, meat quality and gene expression in longissimus dorsi muscle of lambs. Arch. Anim. Nutr. 2015, 69, 340–350. [Google Scholar] [CrossRef]
- King, J.H.; Kwan, S.T.C.; Yan, J.; Klatt, K.C.; Jiang, X.; Roberson, M.S.; Caudill, M.A. Maternal choline supplementation alters fetal growth patterns in a mouse model of placental insufficiency. Nutrients 2017, 9, 765. [Google Scholar] [CrossRef]
- Dwyer, C.M.; Morgan, C.A. Maintenance of body temperature in the neonatal lamb: Effects of breed, birth weight, and litter size. J. Anim. Sci. 2006, 84, 1093–1101. [Google Scholar] [CrossRef]
- Hernandez, C.E.; Matthews, L.R.; Oliver, M.H.; Bloomfield, F.H.; Harding, J.E. Effects of sex, litter size and periconceptional ewe nutrition on the ewe–lamb bond. Appl. Anim. Behav. Sci. 2009, 120, 76–83. [Google Scholar] [CrossRef]
- Budge, H.; Dandrea, J.; Mostyn, A.; Evens, Y.; Watkins, R.; Sullivan, C.; Ingleton, P.; Stephenson, T.; Symonds, M.E. Differential effects of fetal number and maternal nutrition in late gestation on prolactin receptor abundance and adipose tissue development in the neonatal lamb. Pediatr. Res. 2003, 53, 302–308. [Google Scholar] [CrossRef]
- Ekpe, E.D.; Christopherson, R.J. Metabolic and endocrine responses to cold and feed restriction in ruminants. Can. J. Anim. Sci. 2000, 80, 87–95. [Google Scholar] [CrossRef]
- Moore, R.W.; Millar, C.M.; Lynch, P.R.; Dow, B.W. The effect of prenatal nutrition and type of birth and rearing of lambs on vigour, temperature and weight at birth, and weight and survival at weaning. Proc. N. Z. Soc. Anim. Prod. 1986, 46, 259–262. [Google Scholar]
- Clarke, L.; Yakubu, D.P.; Symonds, M.E. Influence of maternal bodyweight on size, conformation and survival of newborn lambs. Reprod. Fertil. Dev. 1997, 9, 509–514. [Google Scholar] [CrossRef] [PubMed]
| Item | Diet Composition | |
|---|---|---|
| Prepartum | Postpartum | |
| Ingredients (g/kg DM) | ||
| Corn silage | 330 | 200 |
| Alfalfa hay | 190 | 290 |
| Wheat straw | 140 | 110 |
| Ground barley grain | 160 | 132 |
| Wheat bran | 122 | 150 |
| Molasses | 50 | 60 |
| Soybean meal | 0 | 50 |
| Salt | 3 | 3 |
| Mineral–vitamin premix 2 | 5 | 5 |
| Chemical composition | ||
| Dry matter (% of fresh weight) | 73 | 78.90 |
| Metabolizable energy 3 (MJ/kg DM) | 9.37 | 9.66 |
| Crude protein (g/kg of DM) | 100.6 | 127.0 |
| Calcium (g/kg of DM) | 4.37 | 5.48 |
| Phosphorus (g/kg of DM) | 3.5 | 4.1 |
| Dry matter intake (kg/day) | 2.4 | 1.9 |
| Item | AL | RF | PG | MS | RPC | PMC | p Value |
|---|---|---|---|---|---|---|---|
| Placental weight (g) | 781.6 ± 23.2 a | 587.5 ± 22.2 b | 592.7 ± 24.5 b | 477.3 ± 25.0 c | 638.1 ± 23.8 b | 591.7 ± 20.0 b | <0.01 |
| Placental efficiency | 10.5 ± 0.5 b | 8.2 ± 0.5 c | 11.3 ± 0.6 ab | 10.7 ± 0.5 b | 12.8 ± 0.5 a | 10.9 ± 0.4 ab | <0.05 |
| Placentome number | 75.9 ± 2.1 a | 57.5 ± 1.8 c | 66.8 ± 2.0 b | 58.5 ± 2.3 c | 68.4 ± 2.2 ab | 72.6 ± 2.2 ab | <0.01 |
| Cotyledon density | 0.1 ± 0.006 | 0.12 ± 0.005 | 0.12 ± 0.006 | 0.13 ± 0.006 | 0.12 ± 0.006 | 0.11 ± 0.005 | NS |
| Item | AL | RF | PG | MS | RPC | PMC |
|---|---|---|---|---|---|---|
| Left hind-leg girth | 8.7 ± 0.1 b | 8.6 ± 0.1 b | 8.7 ± 0.1 b | 8.8 ± 0.1 b | 9.4 ± 0.1 a | 9.0 ± 0.1 ab |
| Left fore-leg girth | 8.5 ± 0.1 b | 7.9 ± 0.1 b | 8.4 ± 0.1 b | 8.4 ± 0.1 b | 8.8 ± 0.1 a | 8.6 ± 0.1 b |
| Rump height | 39.0 ± 0.2 ab | 38.0 ± 0.2 b | 40.0 ± 0.19 a | 39.0 ± 0.2 ab | 39.8 ± 0.2 a | 39.5 ± 0.2 a |
| Withers height | 35.4 ± 0.2 b | 34.2 ± 0.2 b | 36.9 ± 0.2 a | 35.5 ± 0.2 b | 37.1 ± 0.2 a | 37.0 ± 0.2 a |
| Abdomen girth | 41.3 ± 0.2 c | 39.0 ± 0.3 d | 42.7 ± 0.3 bc | 41.4 ± 0.3 c | 43.8 ± 0.3 ab | 44.4 ± 0.2 a |
| Heart girth | 39.3 ± 0.3 b | 36.6 ± 0.3 d | 36.8 ± 0.3 c | 40.2 ± 0.3 ab | 41.2 ± 0.3 ab | 41.7 ± 0.3 a |
| Head girth | 20.1 ± 0.1 ab | 19.0 ± 0.1 c | 19.4 ± 0.1 bc | 19.1 ± 0.1 c | 20.3 ± 0.1 a | 20.6 ± 0.1 a |
| CRL | 50.3 ± 0.3 c | 47.5 ± 0.3 d | 53.1 ± 0.3 ab | 51.6 ± 0.3 bc | 52.2 ± 0.3 abc | 53.8 ± 0.3 a |
| CCRL | 31.6 ± 0.2 b | 28.9 ± 0.2 c | 34.0 ± 0.2 a | 32.7 ± 0.2 ab | 33.3 ± 0.2 a | 34.0 ± 0.2 a |
| Pin bone width | 10.8 ± 0.1 ab | 9.7 ± 0.1 b | 10.2 ± 0.1 ab | 9.6 ± 0.1 b | 10.4 ± 0.1 a | 10.6 ± 0.1 a |
| Hook bone width | 12.2 ± 0.1 abc | 11.7 ± 0.1 c | 12.4 ± 0.1 ab | 11.8 ± 0.1 bc | 12.5 ± 0.1 a | 12.9 ± 0.1 a |
| Number of lambs | 11 | 12 | 12 | 11 | 12 | 13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmadzadeh-Gavahan, L.; Hosseinkhani, A.; Palangi, V.; Lackner, M. Supplementary Feed Additives Can Improve Lamb Performance in Terms of Birth Weight, Body Size, and Survival Rate. Animals 2023, 13, 993. https://doi.org/10.3390/ani13060993
Ahmadzadeh-Gavahan L, Hosseinkhani A, Palangi V, Lackner M. Supplementary Feed Additives Can Improve Lamb Performance in Terms of Birth Weight, Body Size, and Survival Rate. Animals. 2023; 13(6):993. https://doi.org/10.3390/ani13060993
Chicago/Turabian StyleAhmadzadeh-Gavahan, Leila, Ali Hosseinkhani, Valiollah Palangi, and Maximilian Lackner. 2023. "Supplementary Feed Additives Can Improve Lamb Performance in Terms of Birth Weight, Body Size, and Survival Rate" Animals 13, no. 6: 993. https://doi.org/10.3390/ani13060993
APA StyleAhmadzadeh-Gavahan, L., Hosseinkhani, A., Palangi, V., & Lackner, M. (2023). Supplementary Feed Additives Can Improve Lamb Performance in Terms of Birth Weight, Body Size, and Survival Rate. Animals, 13(6), 993. https://doi.org/10.3390/ani13060993








