Comparative Analysis of Epididymis Cauda of Yak before and after Sexual Maturity
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Epididymal Cauda Sample Collection
2.2. Hematoxylin and Eosin Staining
2.3. RNA Library Preparation and Sequencing
2.4. Extraction and Pretreatment of Epididymal Cauda Protein
2.5. Reversed-Phase Chromatography and Liquid Chromatography-Mass Spectrometry (LC-MS)
2.6. Screening and Analysis of Differential Genes (DEGs) and Differential Proteins (DEPs)
2.7. Quantitative Real-Time PCR
2.8. 4D-parallel Reaction Monitoring
2.9. Statistical Analysis
3. Results
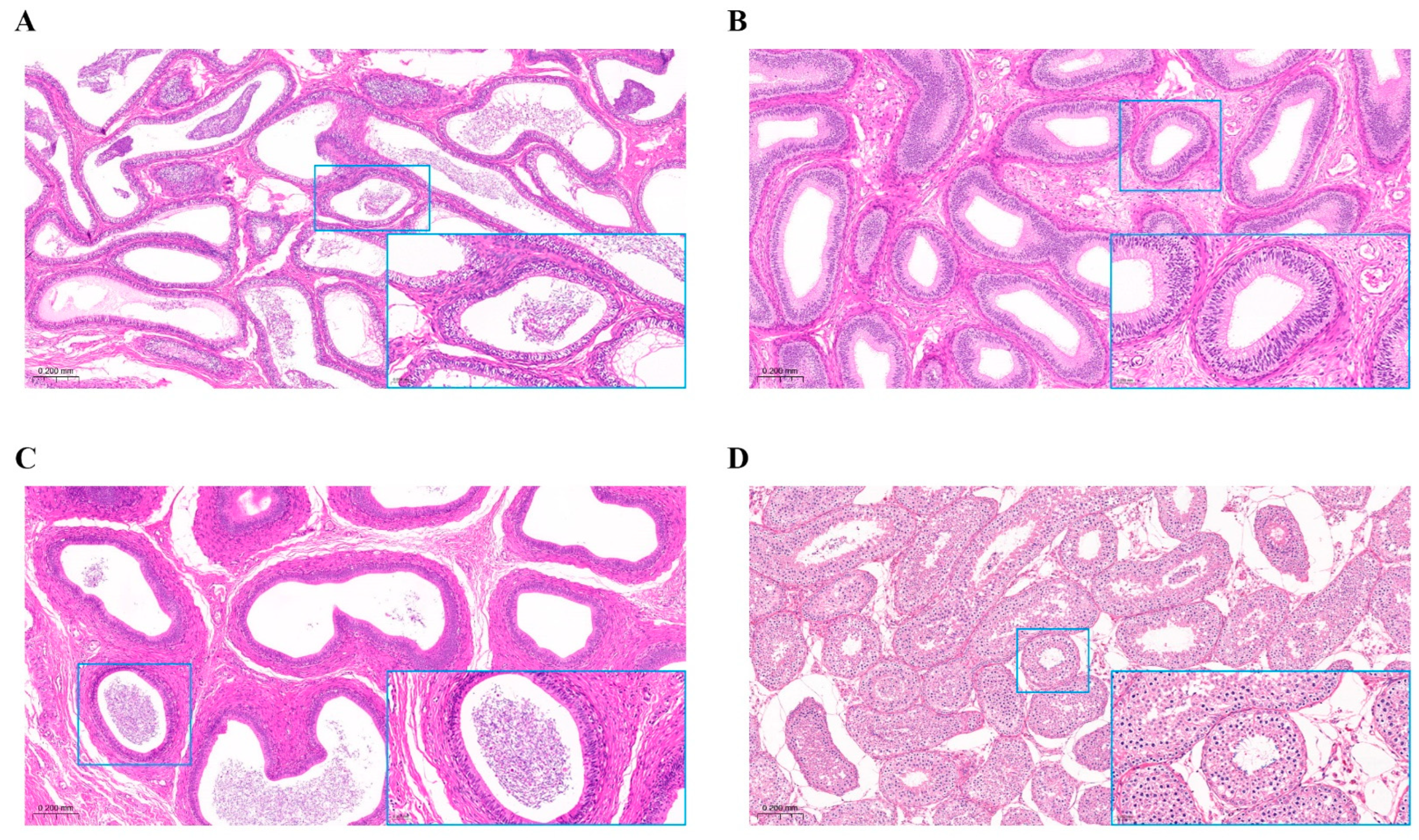
3.1. Histological Observation of Epididymis and Testis
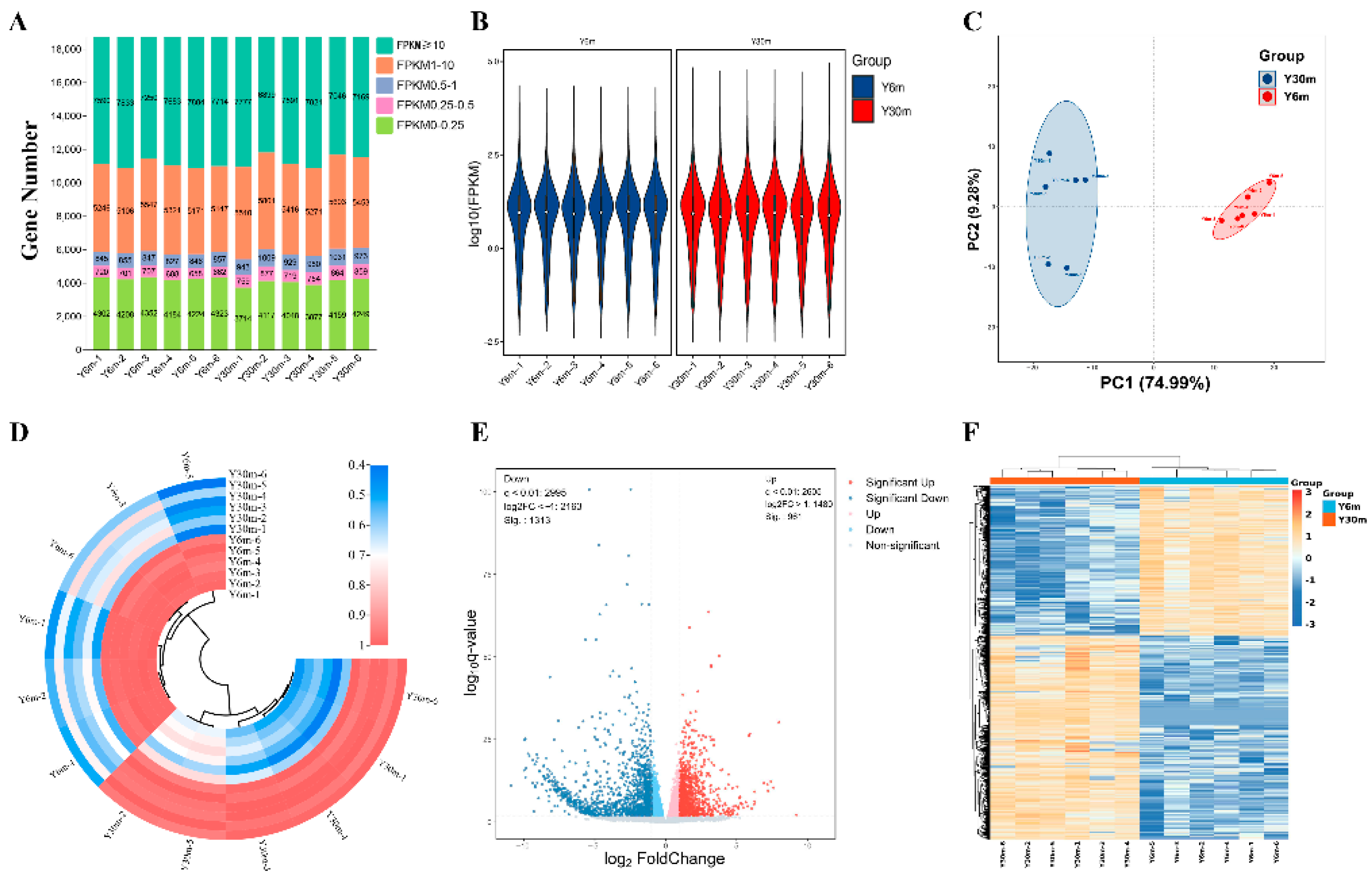
3.2. Summary of RNA-Seq Data
3.3. Identification of DEGs
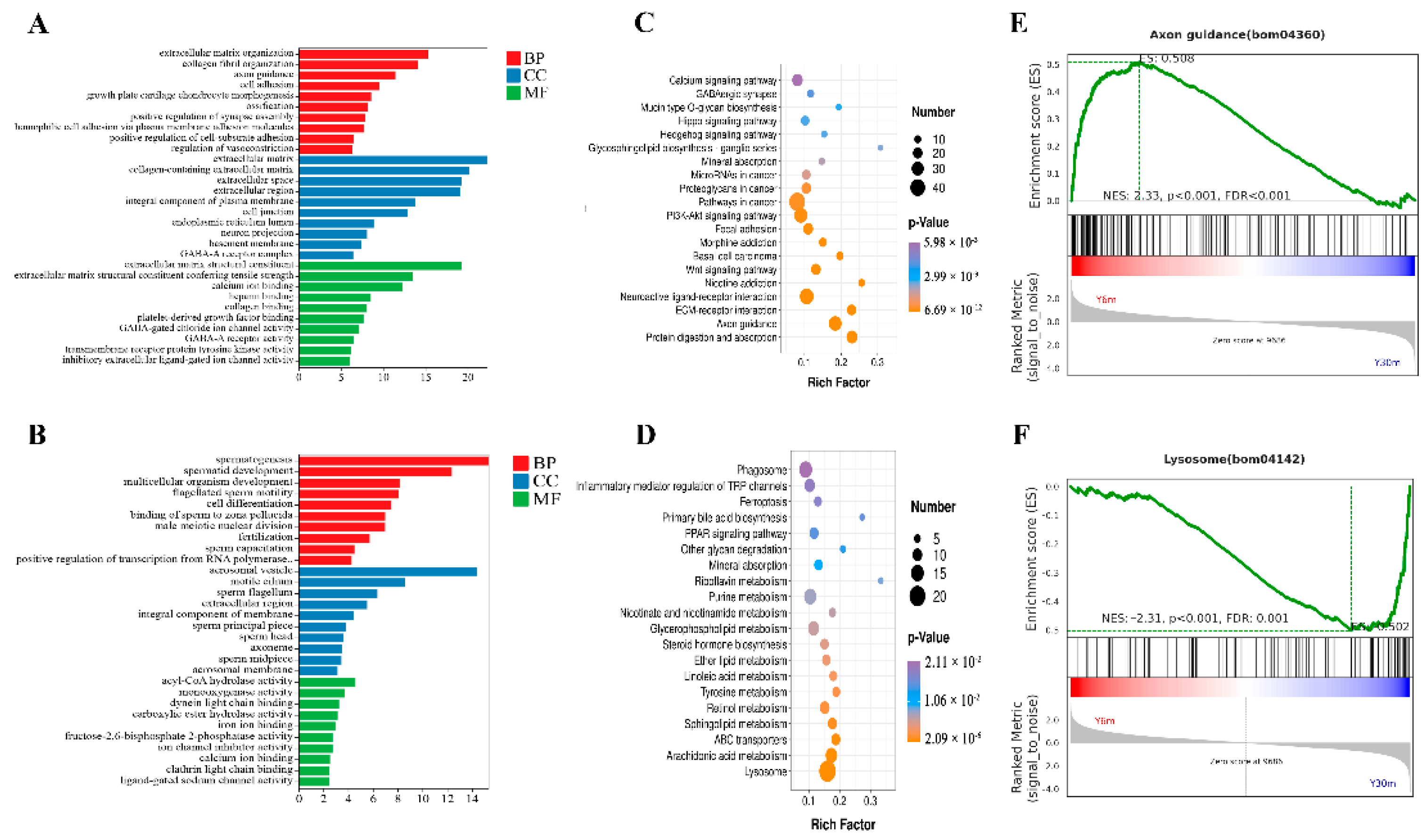
3.4. GO, KEGG Pathway and GSEA Enrichment Evaluations for DEGs
3.5. Overview of Proteome Sequencing Data and Screening and Identification of DEPs
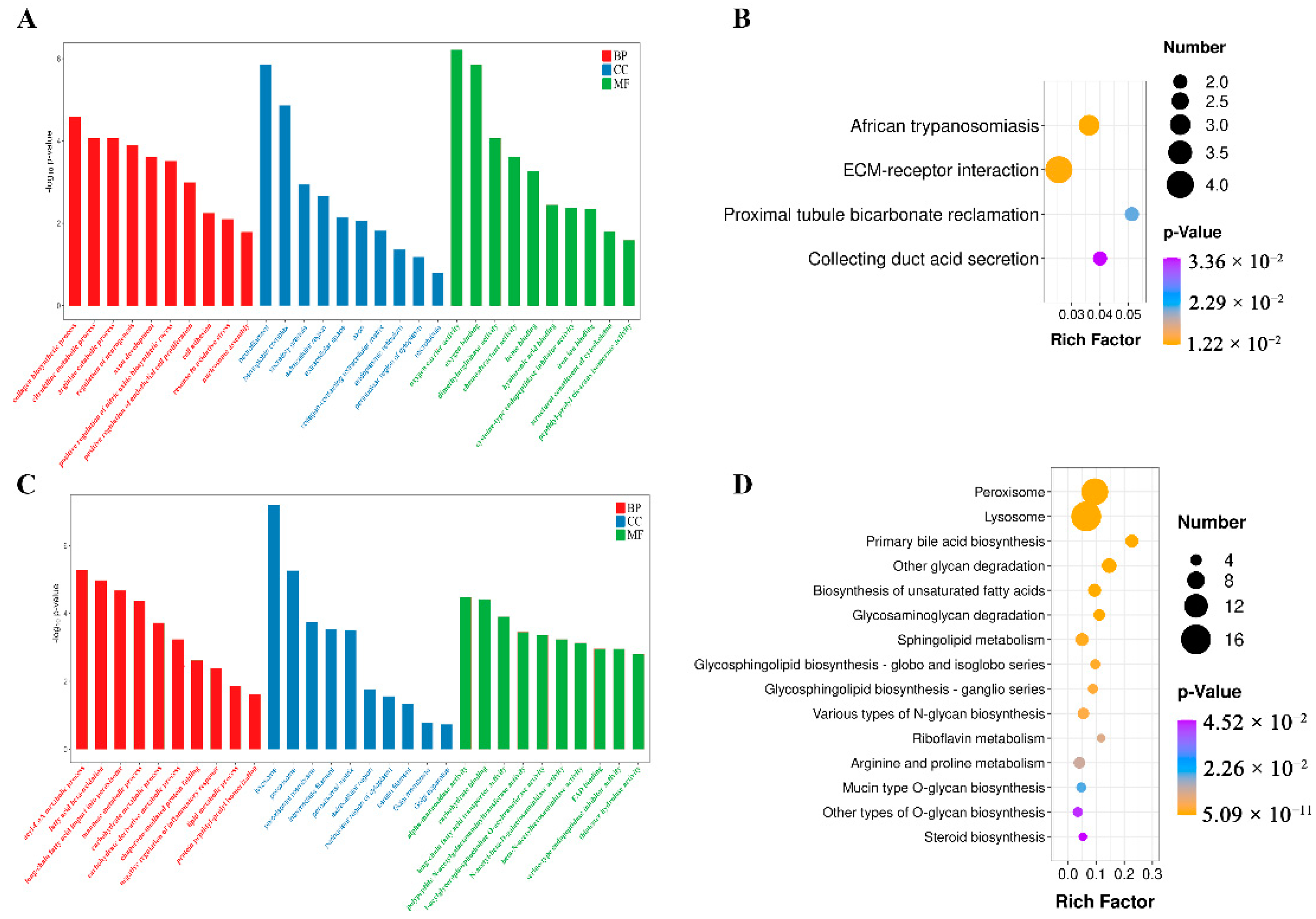
3.6. GO and KEGG Enrichment Analyses of DEPs in Epididymal Cauda
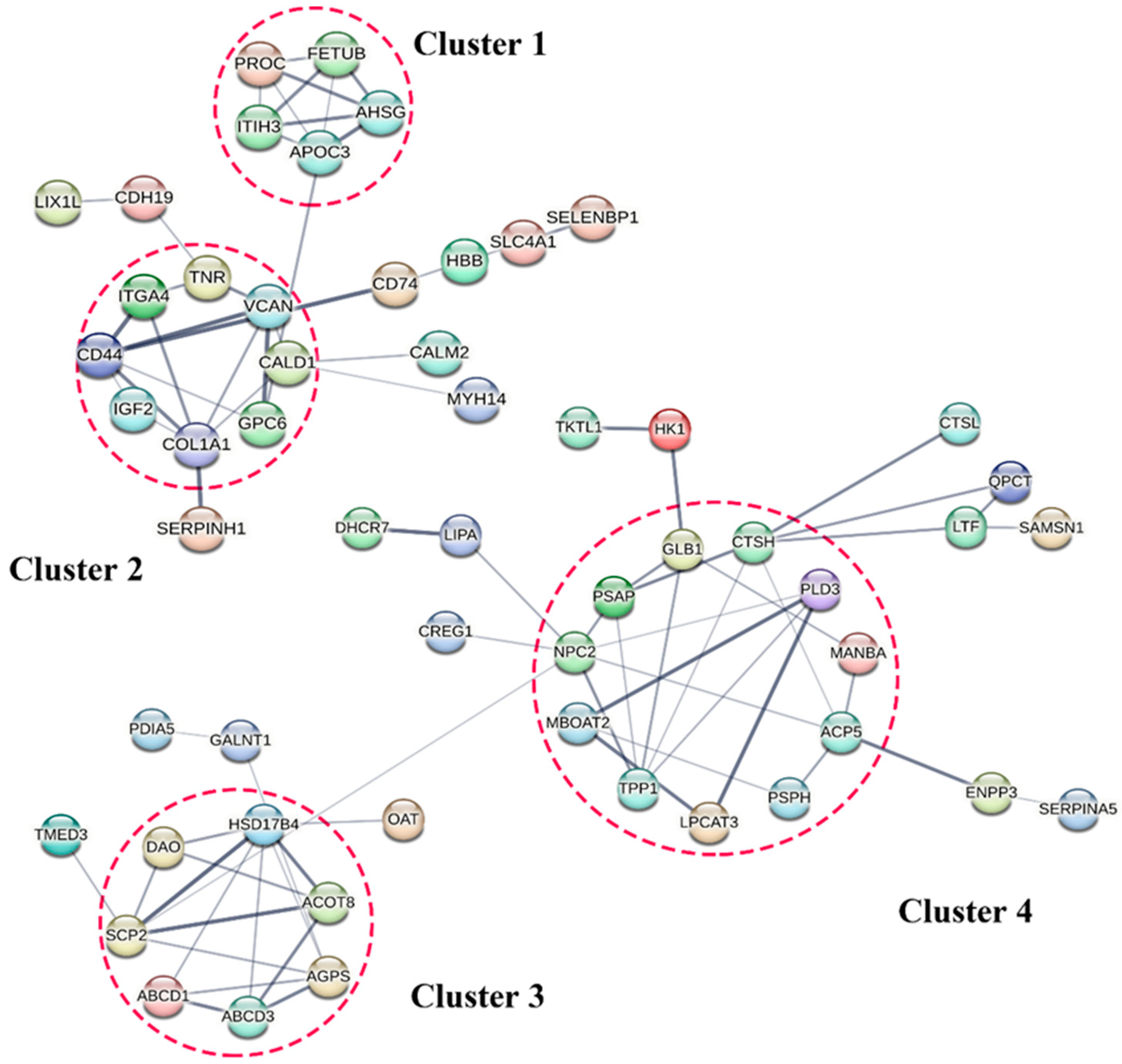
3.7. Protein–Protein Interaction (PPI) among the DEPs
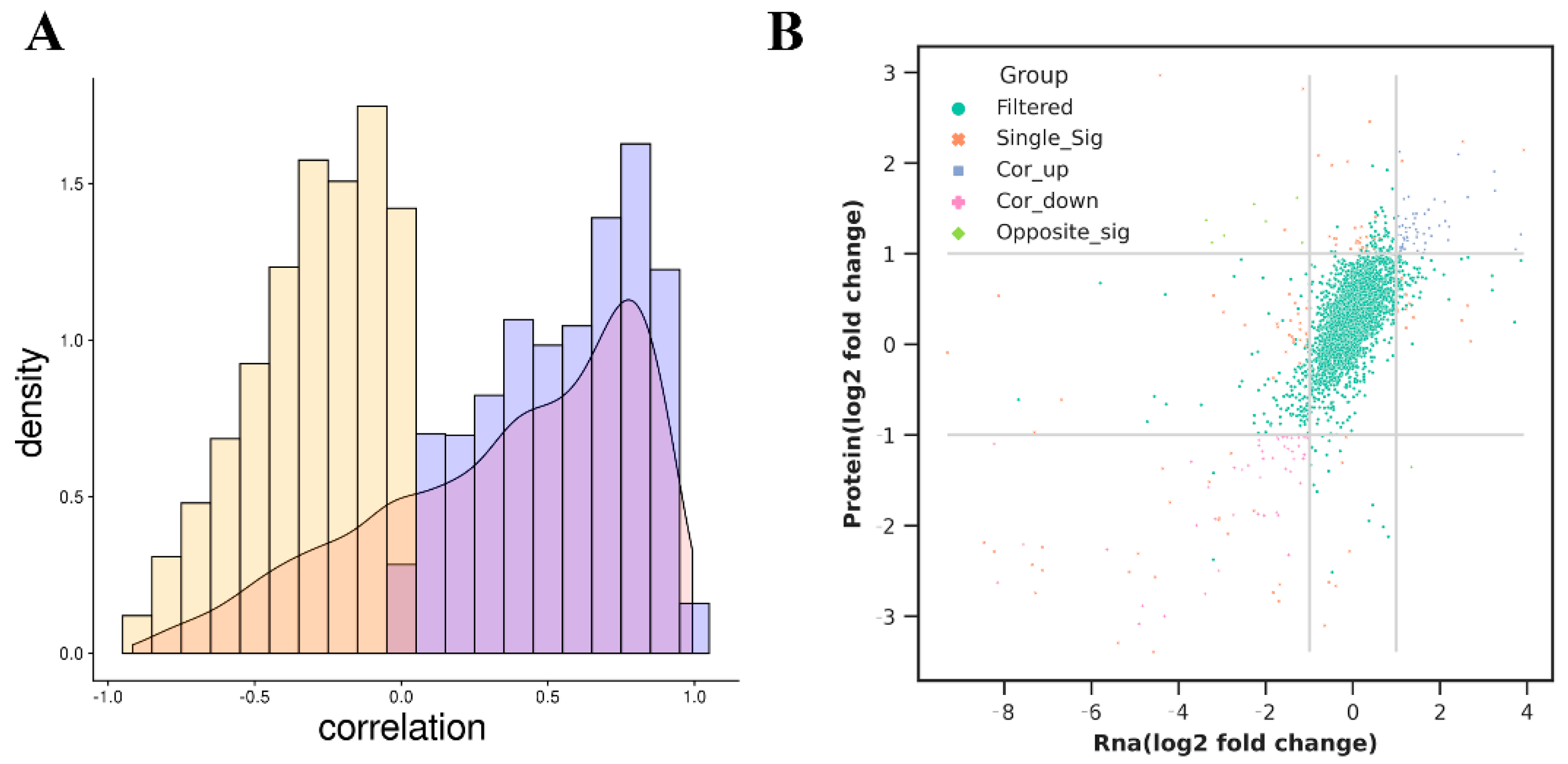
3.8. Combined Analysis of Transcriptome and Proteome
3.9. Analysis of GO and KEGG Genes with the Same Expression Trend
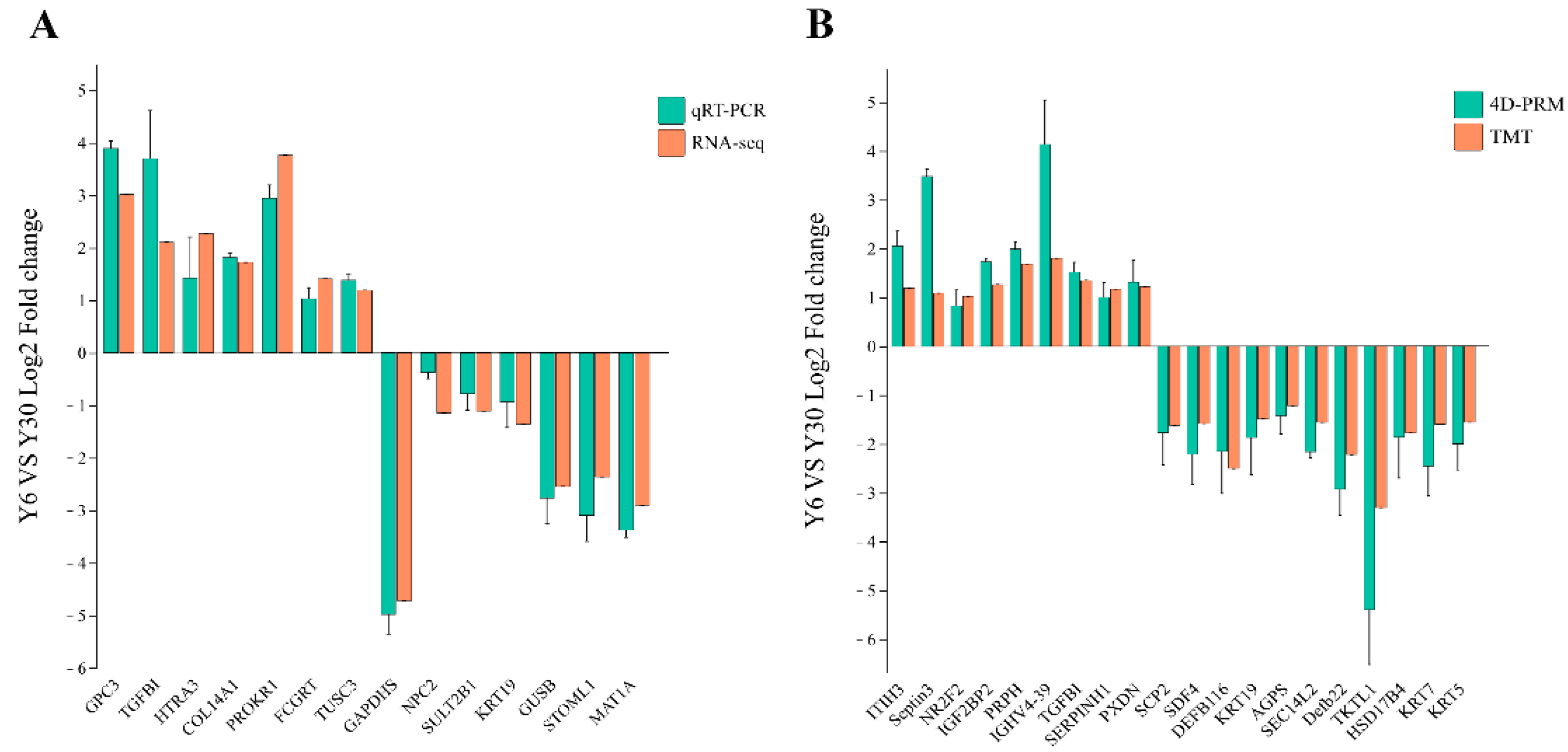
3.10. Verification of Differential Genes and Differential Proteins through qRT-PCR and 4D-RPM
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Domeniconi, R.F.; Souza, A.C.F.; Xu, B.; Washington, A.M.; Hinton, B.T. Is the Epididymis a Series of Organs Placed Side By Side? Biol. Reprod. 2016, 95, 10. [Google Scholar] [CrossRef]
- James, E.R.; Carrell, D.T.; Aston, K.I.; Jenkins, T.G.; Yeste, M.; Salas-Huetos, A. The Role of the Epididymis and the Contribution of Epididymosomes to Mammalian Reproduction. Int. J. Mol. Sci. 2020, 21, 5377. [Google Scholar] [CrossRef] [PubMed]
- Breton, S.; Nair, A.V.; Battistone, M.A. Epithelial Dynamics in the Epididymis: Role in the Maturation, Protection, and Storage of Spermatozoa. Andrology 2019, 7, 631–643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, W.; Quansah, E.; Yuan, M.; Gou, Q.; Mengal, K.; Li, P.; Wu, S.; Xu, C.; Yi, C.; Cai, X. Region-Specific Gene Expression in the Epididymis of Yak. Theriogenology 2019, 139, 132–146. [Google Scholar] [CrossRef]
- Belleannée, C.; Thimon, V.; Sullivan, R. Region-Specific Gene Expression in the Epididymis. Cell Tissue Res. 2012, 349, 717–731. [Google Scholar] [CrossRef]
- Mele, E.; D’Auria, R.; Scafuro, M.; Marino, M.; Fasano, S.; Viggiano, A.; Pierantoni, R.; Santoro, A.; Meccariello, R. Differential Expression of Kisspeptin System and Kisspeptin Receptor Trafficking during Spermatozoa Transit in the Epididymis. Genes 2022, 13, 295. [Google Scholar] [CrossRef]
- Ge, W.; Xiao, L.; Duan, H.; Zhao, X.; Li, J.; Hu, J. Proteomic Analysis of ITRAQ in Melatonin-treated Sheep Epididymal Epithelial Cells. Reprod. Domest. Anim. 2022, 57, 1406–1417. [Google Scholar] [CrossRef]
- Johnston, D.S.; Jelinsky, S.A.; Bang, H.J.; DiCandeloro, P.; Wilson, E.; Kopf, G.S.; Turner, T.T. The Mouse Epididymal Transcriptome: Transcriptional Profiling of Segmental Gene Expression in the Epididymis. Biol. Reprod. 2005, 73, 404–413. [Google Scholar] [CrossRef]
- Jelinsky, S.A.; Turner, T.T.; Bang, H.J.; Finger, J.N.; Solarz, M.K.; Wilson, E.; Brown, E.L.; Kopf, G.S.; Johnston, D.S. The Rat Epididymal Transcriptome: Comparison of Segmental Gene Expression in the Rat and Mouse Epididymides. Biol. Reprod. 2007, 76, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Légaré, C.; Akintayo, A.; Blondin, P.; Calvo, E.; Sullivan, R. Impact of Male Fertility Status on the Transcriptome of the Bovine Epididymis. Mol. Hum. Reprod. 2017, 23, 355–369. [Google Scholar] [CrossRef] [Green Version]
- Thimon, V.; Koukoui, O.; Calvo, E.; Sullivan, R. Region-Specific Gene Expression Profiling along the Human Epididymis. Mol. Hum. Reprod. 2007, 13, 691–704. [Google Scholar] [CrossRef]
- Browne, J.A.; Yang, R.; Leir, S.-H.; Eggener, S.E.; Harris, A. Expression Profiles of Human Epididymis Epithelial Cells Reveal the Functional Diversity of Caput, Corpus and Cauda Regions. Mol. Hum. Reprod. 2016, 22, 69–82. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Yao, T.; Weng, X.; Yao, R.; Li, W.; Xie, L.; Yue, X.; Li, F. Antioxidant Properties and Transcriptome of Cauda Epididymis with Different Levels of Fertility in Hu Lambs. Theriogenology 2022, 182, 85–95. [Google Scholar] [CrossRef]
- Pihlajamaa, P.; Sahu, B.; Lyly, L.; Aittomäki, V.; Hautaniemi, S.; Jänne, O.A. Tissue-Specific Pioneer Factors Associate with Androgen Receptor Cistromes and Transcription Programs. EMBO J. 2014, 33, 312–326. [Google Scholar] [CrossRef] [PubMed]
- Dufresne, J.; Gregory, M.; Pinel, L.; Cyr, D.G. Differential Gene Expression and Hallmarks of Stemness in Epithelial Cells of the Developing Rat Epididymis. Cell Tissue Res. 2022, 389, 327–349. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Zhang, Y.; Sun, C.; Ge, S.; Tan, Y.; Shen, H.; Yang, P. A Multi-Omics Study of Human Testis and Epididymis. Molecules 2021, 26, 3345. [Google Scholar] [CrossRef] [PubMed]
- Souza, C.E.A.; Rego, J.P.A.; Lobo, C.H.; Oliveira, J.T.A.; Nogueira, F.C.S.; Domont, G.B.; Fioramonte, M.; Gozzo, F.C.; Moreno, F.B.; Monteiro-Moreira, A.C.O.; et al. Proteomic Analysis of the Reproductive Tract Fluids from Tropically-Adapted Santa Ines Rams. J. Proteom. 2012, 75, 4436–4456. [Google Scholar] [CrossRef] [PubMed]
- Dacheux, J.-L.; Belleannée, C.; Guyonnet, B.; Labas, V.; Teixeira-Gomes, A.-P.; Ecroyd, H.; Druart, X.; Gatti, J.-L.; Dacheux, F. The Contribution of Proteomics to Understanding Epididymal Maturation of Mammalian Spermatozoa. Syst. Biol. Reprod. Med. 2012, 58, 197–210. [Google Scholar] [CrossRef]
- Liang, A. iTRAQ-Based Quantitative Proteomic Analysis of Mouse Epididymal Luminal Proteins. Ph.D. Thesis, Shanghai Jiaotong University, Shanghai, China, 2016. [Google Scholar]
- Wu, C.; Wang, C.; Zhai, B.; Zhao, Y.; Zhao, Z.; Yuan, Z.; Zhang, M.; Tian, K.; Fu, X. Study of MicroRNA Expression Profile in Different Regions of Ram Epididymis. Reprod. Domest. Anim. 2021, 56, 1209–1219. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. Fastp: An Ultra-Fast All-in-One FASTQ Preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A Fast Spliced Aligner with Low Memory Requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [Green Version]
- Roberts, A.; Trapnell, C.; Donaghey, J.; Rinn, J.L.; Pachter, L. Improving RNA-Seq Expression Estimates by Correcting for Fragment Bias. Genome Biol. 2011, 12, R22. [Google Scholar] [CrossRef] [Green Version]
- Anders, S.; Pyl, P.T.; Huber, W. HTSeq—A Python Framework to Work with High-Throughput Sequencing Data. Bioinformatics 2015, 31, 166–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, A.; Yadav, B.; Swain, D.K.; Anand, M.; Madan, A.K.; Yadav, R.K.S.; Kushawaha, B.; Yadav, S. Dynamics of HSPA1A and Redox Status in the Spermatozoa and Fluid from Different Segments of Goat Epididymis. Cell Stress Chaperones 2020, 25, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Wang, X.; Guo, S.; Kang, Y.; Pei, J.; Guo, X. F1 Male Sterility in Cattle-Yak Examined through Changes in Testis Tissue and Transcriptome Profiles. Animals 2022, 12, 2711. [Google Scholar] [CrossRef]
- Udomsinprasert, W.; Angkathunyakul, N.; Klaikeaw, N.; Vejchapipat, P.; Poovorawan, Y.; Honsawek, S. Hepatic Glypican-3 and Alpha-Smooth Muscle Actin Overexpressions Reflect Severity of Liver Fibrosis and Predict Outcome after Successful Portoenterostomy in Biliary Atresia. Surgery 2020, 167, 560–568. [Google Scholar] [CrossRef]
- Li, X. Study on the expression of GPC3 in clear cell carcinoma and its clinical-pathological characteristics. Master’s Thesis, Nanjing Medical University, Nanjing, China, 2015. [Google Scholar]
- Shimizu, Y.; Suzuki, T.; Yoshikawa, T.; Endo, I.; Nakatsura, T. Next-Generation Cancer Immunotherapy Targeting Glypican-3. Front. Oncol. 2019, 9, 248. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Danshina, P.V.; Mohr, K.; Qu, W.; Goodson, S.G.; O’Connell, T.M.; O’Brien, D.A. Sperm Function, Protein Phosphorylation, and Metabolism Differ in Mice Lacking Successive Sperm-Specific Glycolytic Enzymes. Biol. Reprod. 2017, 97, 586–597. [Google Scholar] [CrossRef] [Green Version]
- Miki, K.; Qu, W.; Goulding, E.H.; Willis, W.D.; Bunch, D.O.; Strader, L.F.; Perreault, S.D.; Eddy, E.M.; O’Brien, D.A. Glyceraldehyde 3-Phosphate Dehydrogenase-S, a Sperm-Specific Glycolytic Enzyme, Is Required for Sperm Motility and Male Fertility. Proc. Natl. Acad. Sci. USA 2004, 101, 16501–16506. [Google Scholar] [CrossRef] [Green Version]
- Elkina, Y.L.; Kuravsky, M.L.; Bragina, E.E.; Kurilo, L.F.; Khayat, S.S.; Sukhomlinova, M.Y.; Schmalhausen, E.V. Detection of a Mutation in the Intron of Sperm-Specific Glyceraldehyde-3-Phosphate Dehydrogenase Gene in Patients with Fibrous Sheath Dysplasia of the Sperm Flagellum. Andrologia 2017, 49, e12606. [Google Scholar] [CrossRef]
- Tang, W.; Zhou, M.; Dorsey, T.H.; Prieto, D.A.; Wang, X.W.; Ruppin, E.; Veenstra, T.D.; Ambs, S. Integrated Proteotranscriptomics of Breast Cancer Reveals Globally Increased Protein-MRNA Concordance Associated with Subtypes and Survival. Genome Med. 2018, 10, 94. [Google Scholar] [CrossRef] [Green Version]
- Corona, A.; Blobe, G.C. The Role of the Extracellular Matrix Protein TGFBI in Cancer. Cell Signal. 2021, 84, 110028. [Google Scholar] [CrossRef] [PubMed]
- Ween, M.P.; Oehler, M.K.; Ricciardelli, C. Transforming Growth Factor-Beta-Induced Protein (TGFBI)/(Βig-H3): A Matrix Protein with Dual Functions in Ovarian Cancer. Int. J. Mol. Sci. 2012, 13, 10461–10477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahlfeld, S.K.; Wang, J.; Gao, Y.; Snider, P.; Conway, S.J. Initial Suppression of Transforming Growth Factor-β Signaling and Loss of TGFBI Causes Early Alveolar Structural Defects Resulting in Bronchopulmonary Dysplasia. Am. J. Pathol. 2016, 186, 777–793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiavarina, B.; Costanza, B.; Ronca, R.; Blomme, A.; Rezzola, S.; Chiodelli, P.; Giguelay, A.; Belthier, G.; Doumont, G.; Van Simaeys, G.; et al. Metastatic Colorectal Cancer Cells Maintain the TGFβ Program and Use TGFBI to Fuel Angiogenesis. Theranostics 2021, 11, 1626–1640. [Google Scholar] [CrossRef] [PubMed]
- Steitz, A.M.; Steffes, A.; Finkernagel, F.; Unger, A.; Sommerfeld, L.; Jansen, J.M.; Wagner, U.; Graumann, J.; Müller, R.; Reinartz, S. Tumor-Associated Macrophages Promote Ovarian Cancer Cell Migration by Secreting Transforming Growth Factor Beta Induced (TGFBI) and Tenascin C. Cell Death Dis. 2020, 11, 249. [Google Scholar] [CrossRef] [Green Version]
- He, Z.; Feng, L.; Zhang, X.; Geng, Y.; Parodi, D.A.; Suarez-Quian, C.; Dym, M. Expression of Col1a1, Col1a2 and Procollagen I in Germ Cells of Immature and Adult Mouse Testis. Reproduction 2005, 130, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Kuivaniemi, H.; Tromp, G. Type III Collagen (COL3A1): Gene and Protein Structure, Tissue Distribution, and Associated Diseases. Gene 2019, 707, 151–171. [Google Scholar] [CrossRef]
- Yue, F.; Cheng, Y.; Breschi, A.; Vierstra, J.; Wu, W.; Ryba, T.; Sandstrom, R.; Ma, Z.; Davis, C.; Pope, B.D.; et al. A Comparative Encyclopedia of DNA Elements in the Mouse Genome. Nature 2014, 515, 355–364. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; He, B.; Yin, C.; Chang, Z.; Zhao, R. Transcriptomic Responses of Porcine Cumulus Cells to Heat Exposure during Oocytes in Vitro Maturation. Mol. Reprod. Dev. 2021, 88, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Aberkane, A.; Essahib, W.; Spits, C.; De Paepe, C.; Sermon, K.; Adriaenssens, T.; Mackens, S.; Tournaye, H.; Brosens, J.J.; Van de Velde, H. Expression of Adhesion and Extracellular Matrix Genes in Human Blastocysts upon Attachment in a 2D Co-Culture System. Mol. Hum. Reprod. 2018, 24, 375–387. [Google Scholar] [CrossRef]
- Keber, R.; Rozman, D.; Horvat, S. Sterols in Spermatogenesis and Sperm Maturation. J. Lipid Res. 2013, 54, 20–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schuler, G.; Sánchez-Guijo, A.; Hartmann, M.F.; Wudy, S.A. Simultaneous Profiles of Sulfonated Androgens, Sulfonated Estrogens and Sulfonated Progestogens in Postpubertal Boars (Sus Scrofa Domestica) Measured by LC-MS/MS. J. Steroid Biochem. Mol. Biol. 2018, 179, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, B.; Tenbusch, L.; Klymiuk, M.C.; Dezhkam, Y.; Schuler, G. SULFATION PATHWAYS: Expression of SULT2A1, SULT2B1 and HSD3B1 in the Porcine Testis and Epididymis. J. Mol. Endocrinol. 2018, 61, M41–M55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selvaraju, S.; Ramya, L.; Parthipan, S.; Swathi, D.; Binsila, B.K.; Kolte, A.P. Deciphering the Complexity of Sperm Transcriptome Reveals Genes Governing Functional Membrane and Acrosome Integrities Potentially Influence Fertility. Cell Tissue Res. 2021, 385, 207–222. [Google Scholar] [CrossRef] [PubMed]
- Barrachina, F.; Battistone, M.A.; Castillo, J.; Mallofré, C.; Jodar, M.; Breton, S.; Oliva, R. Sperm Acquire Epididymis-Derived Proteins through Epididymosomes. Hum. Reprod. 2022, 37, 651–668. [Google Scholar] [CrossRef]
- Trépos-Pouplard, M.; Lardenois, A.; Staub, C.; Guitton, N.; Dorval-Coiffec, I.; Pineau, C.; Primig, M.; Jégou, B. Proteome Analysis and Genome-Wide Regulatory Motif Prediction Identify Novel Potentially Sex-Hormone Regulated Proteins in Rat Efferent Ducts. Int. J. Androl. 2010, 33, 661–674. [Google Scholar] [CrossRef]
- Meng, Y.; Heybrock, S.; Neculai, D.; Saftig, P. Cholesterol Handling in Lysosomes and Beyond. Trends Cell Biol. 2020, 30, 452–466. [Google Scholar] [CrossRef]
- Zmudzinska, A.; Wisniewski, J.; Mlynarz, P.; Olejnik, B.; Mogielnicka-Brzozowska, M. Age-Dependent Variations in Functional Quality and Proteomic Characteristics of Canine (Canis Lupus Familiaris) Epididymal Spermatozoa. Int. J. Mol. Sci. 2022, 23, 9143. [Google Scholar] [CrossRef]
- Belleannée, C.; Labas, V.; Teixeira-Gomes, A.-P.; Gatti, J.L.; Dacheux, J.-L.; Dacheux, F. Identification of Luminal and Secreted Proteins in Bull Epididymis. J. Proteom. 2011, 74, 59–78. [Google Scholar] [CrossRef]
- Girouard, J.; Frenette, G.; Sullivan, R. Seminal Plasma Proteins Regulate the Association of Lipids and Proteins within Detergent-Resistant Membrane Domains of Bovine Spermatozoa. Biol. Reprod. 2008, 78, 921–931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sullivan, R.; Legare, C.; Thabet, M.; Thimon, V. Gene Expression in the Epididymis of Normal and Vasectomized Men: What Can We Learn about Human Sperm Maturation? J. Androl. 2011, 32, 686–697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moura, M.T.; Latorraca, L.B.; Paula-Lopes, F.F. Contextualizing Autophagy during Gametogenesis and Preimplantation Embryonic Development. Int. J. Mol. Sci. 2021, 22, 6313. [Google Scholar] [CrossRef]
- Sun, X.; Kovacs, T.; Hu, Y.-J.; Yang, W.-X. The Role of Actin and Myosin during Spermatogenesis. Mol. Biol. Rep. 2011, 38, 3993–4001. [Google Scholar] [CrossRef]
- Yang, C.; Wang, X. Lysosome Biogenesis: Regulation and Functions. J. Cell Biol. 2021, 220, e202102001. [Google Scholar] [CrossRef] [PubMed]
- Moreno, R.D.; Alvarado, C.P. The Mammalian Acrosome as a Secretory Lysosome: New and Old Evidence. Mol. Reprod. Dev. 2006, 73, 1430–1434. [Google Scholar] [CrossRef]
- Wang, H.; Wan, H.; Li, X.; Liu, W.; Chen, Q.; Wang, Y.; Yang, L.; Tang, H.; Zhang, X.; Duan, E.; et al. Atg7 Is Required for Acrosome Biogenesis during Spermatogenesis in Mice. Cell Res. 2014, 24, 852–869. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, Z.; Xiong, L.; Wang, X.; Guo, S.; Cao, M.; Kang, Y.; La, Y.; Bao, P.; Pei, J.; Guo, X. Comparative Analysis of Epididymis Cauda of Yak before and after Sexual Maturity. Animals 2023, 13, 1355. https://doi.org/10.3390/ani13081355
Ding Z, Xiong L, Wang X, Guo S, Cao M, Kang Y, La Y, Bao P, Pei J, Guo X. Comparative Analysis of Epididymis Cauda of Yak before and after Sexual Maturity. Animals. 2023; 13(8):1355. https://doi.org/10.3390/ani13081355
Chicago/Turabian StyleDing, Ziqiang, Lin Xiong, Xingdong Wang, Shaoke Guo, Mengli Cao, Yandong Kang, Yongfu La, Pengjia Bao, Jie Pei, and Xian Guo. 2023. "Comparative Analysis of Epididymis Cauda of Yak before and after Sexual Maturity" Animals 13, no. 8: 1355. https://doi.org/10.3390/ani13081355




