Population Genetic Analysis of Six Chinese Indigenous Pig Meta-Populations Based on Geographically Isolated Regions
Abstract
:Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Sample Collection
2.2. Data Quality Control
2.3. Population Structure Analysis
2.4. Genetic Diversity and Differentiation
2.5. Identification of Runs of Homozygosity and Distribution
2.6. Selective Sweep Analysis
3. Results and Discussion
3.1. Population Structure
3.2. Genetic Diversity and Differentiation
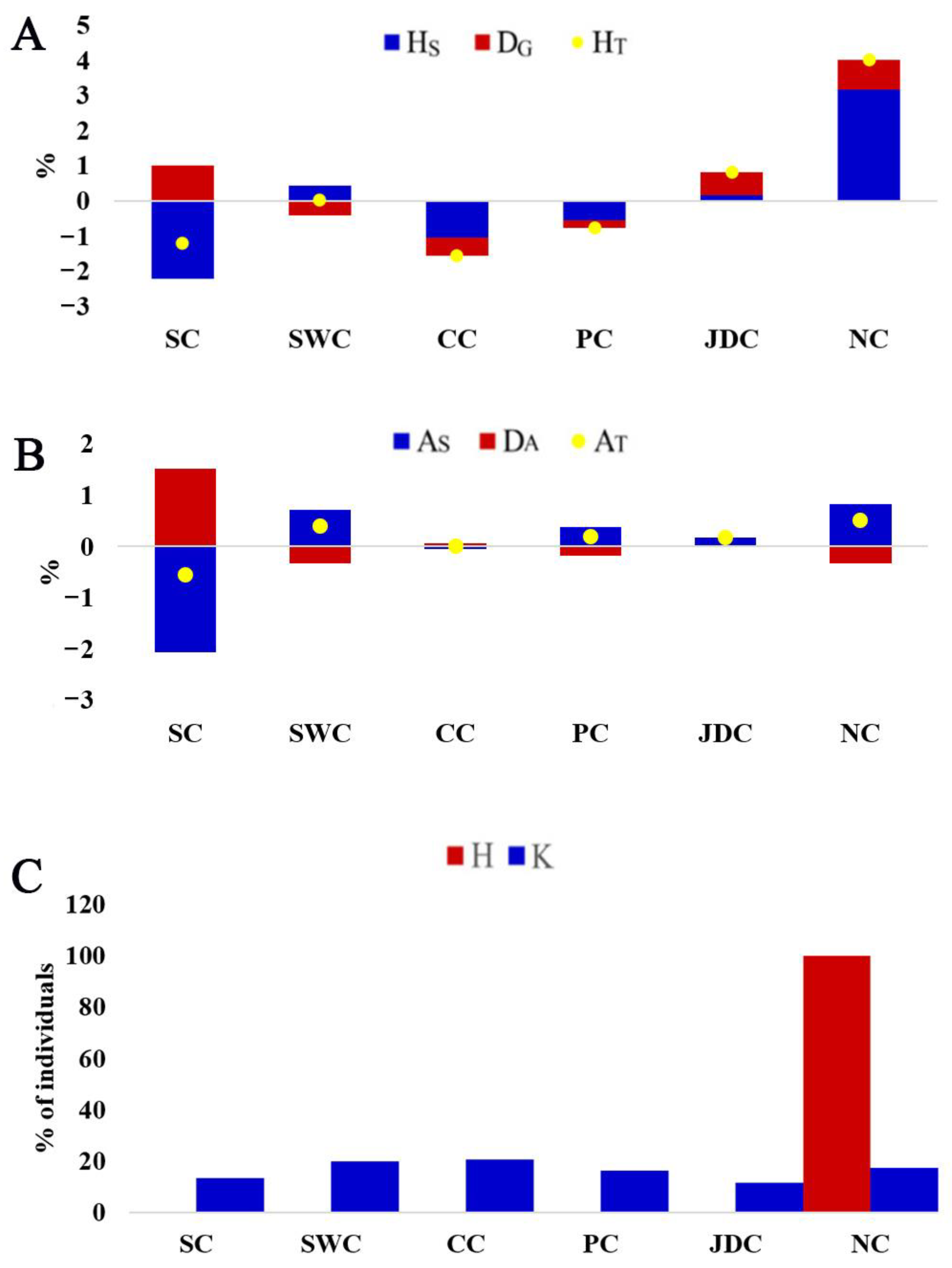
3.3. Contribution to Genetic and Allelic Diversity
3.4. Distribution of ROH and Genomic Inbreeding Coefficient
3.5. Selective Sweep Analysis for Temperature Adaptation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kijas, J.; Andersson, L. A phylogenetic study of the origin of the domestic pig estimated from the near-complete mtDNA genome. J. Mol. Evol. 2001, 52, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Larson, G.; Dobney, K.; Albarella, U.; Fang, M.; Matisoo-Smith, E.; Robins, J.; Lowden, S.; Finlayson, H.; Brand, T.; Willerslev, E.; et al. Worldwide phylogeography of wild boar reveals multiple centers of pig domestication. Science 2005, 307, 1618–1621. [Google Scholar] [CrossRef] [PubMed]
- Xiang, H.; Gao, J.; Cai, D.; Luo, Y.; Yu, B.; Liu, L.; Liu, R.; Zhou, H.; Chen, X.; Dun, W.; et al. Origin and dispersal of early domestic pigs in northern China. Sci. Rep. 2017, 7, 5602. [Google Scholar] [CrossRef]
- Zhang, Z.; Xiao, Q.; Zhang, Q.; Sun, H.; Chen, J.; Li, Z.; Xue, M.; Ma, P.; Yang, H.; Xu, N.; et al. Genomic analysis reveals genes affecting distinct phenotypes among different Chinese and western pig breeds. Sci. Rep. 2018, 8, 13352. [Google Scholar] [CrossRef] [PubMed]
- Giuffra, E.; Kijas, J.; Amarger, V.; Carlborg, O.; Jeon, J.; Andersson, L. The origin of the domestic pig: Independent domestication and subsequent introgression. Genetics 2000, 154, 1785–1791. [Google Scholar] [CrossRef] [PubMed]
- Amaral, A.; Ferretti, L.; Megens, H.; Crooijmans, R.; Nie, H.; Ramos-Onsins, S.; Perez-Enciso, M.; Schook, L.; Groenen, M. Genome-wide footprints of pig domestication and selection revealed through massive parallel sequencing of pooled DNA. PLoS ONE 2011, 6, e14782. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Quan, J.; Gao, C.; Ge, Q.; Jiao, T.; Guo, Y.; Zheng, W.; Zhao, S. Multiple Domestication Centers Revealed by the Geographical Distribution of Chinese Native Pigs. Animals 2019, 9, 709. [Google Scholar] [CrossRef] [PubMed]
- Diao, S.; Huang, S.; Chen, Z.; Teng, J.; Ma, Y.; Yuan, X.; Chen, Z.; Zhang, H.; Li, J.; Zhang, Z. Genome-Wide Signatures of Selection Detection in Three South China Indigenous Pigs. Genes 2019, 10, 346. [Google Scholar] [CrossRef]
- Tian, Y.; Yue, M.; Gu, Y.; Gu, W.; Wang, Y. Single-nucleotide polymorphism analysis of GH, GHR, and IGF-1 genes in minipigs. Braz. J. Med. Biol. Res. 2014, 47, 753–758. [Google Scholar] [CrossRef]
- Cai, Y.; Fu, W.; Cai, D.; Heller, R.; Zheng, Z.; Wen, J.; Li, H.; Wang, X.; Alshawi, A.; Sun, Z.; et al. Ancient Genomes Reveal the Evolutionary History and Origin of Cashmere-Producing Goats in China. Mol. Biol. Evol. 2020, 37, 2099–2109. [Google Scholar] [CrossRef]
- Luo, H.; Zhang, H.; Li, K.; Rehman, M.; Mehmood, K.; Lan, Y.; Huang, S.; Li, J. Cysticercus tenuicollisEpidemiological Survey and Phylogenetic Characterization of Isolated from Tibetan Pigs in Tibet, China. BioMed Res. Int. 2017, 2017, 7857253. [Google Scholar] [CrossRef]
- Wang, J.; Ren, Q.; Hua, L.; Chen, J.; Zhang, J.; Bai, H.; Li, H.; Xu, B.; Shi, Z.; Cao, H.; et al. Comprehensive Analysis of Differentially Expressed mRNA, lncRNA and circRNA and Their ceRNA Networks in the Longissimus Dorsi Muscle of Two Different Pig Breeds. Int. J. Mol. Sci. 2019, 20, 1107. [Google Scholar] [CrossRef]
- Yang, Y.; Gao, C.; Yang, T.; Sha, Y.; Cai, Y.; Wang, X.; Yang, Q.; Liu, C.; Wang, B.; Zhao, S. Characteristics of Tibetan pig lung tissue in response to a hypoxic environment on the Qinghai-Tibet Plateau. Arch. Anim. Breed. 2021, 64, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yang, Q.; Xie, K.; Wang, P.; Luo, R.; Yan, Z.; Gao, X.; Zhang, B.; Huang, X.; Gun, S. HMOX1Transcriptional Regulation of Gene in Hezuo Tibetan Pigs: Roles of WT1, Sp1, and C/EBPα. Genes 2020, 11, 352. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Cui, L.; Perez-Enciso, M.; Traspov, A.; Crooijmans, R.; Zinovieva, N.; Schook, L.; Archibald, A.; Gatphayak, K.; Knorr, C.; et al. Genome-wide SNP data unveils the globalization of domesticated pigs. Genet. Sel. Evol. 2017, 49, 71. [Google Scholar] [CrossRef]
- Zheng, Z.; Li, Y.; Wang, M.; Ruan, R.; Yang, B.; Zhu, T.; Li, S.; Liu, Y.; Qiao, T.; Lin, T.; et al. Leaf spot on Alocasia macrorrhizos caused by Fusarium asiaticum in Sichuan, China. Plant Dis. 2022, 15. [Google Scholar] [CrossRef] [PubMed]
- Alexander, D.; Novembre, J.; Lange, K. Fast model-based estimation of ancestry in unrelated individuals. Genome Res. 2009, 19, 1655–1664. [Google Scholar] [CrossRef] [PubMed]
- López-Cortegano, E.; Pérez-Figueroa, A.; Caballero, A. metapop2: Re-implementation of software for the analysis and management of subdivided populations using gene and allelic diversity. Mol. Ecol. Resour. 2019, 19, 1095–1100. [Google Scholar] [CrossRef]
- Liu, B.; Shen, L.; Guo, Z.; Gan, M.; Chen, Y.; Yang, R.; Niu, L.; Jiang, D.; Zhong, Z.; Li, X.; et al. Single nucleotide polymorphism-based analysis of the genetic structure of Liangshan pig population. Anim. Biosci. 2021, 34, 1105–1115. [Google Scholar] [CrossRef]
- McQuillan, R.; Leutenegger, A.; Abdel-Rahman, R.; Franklin, C.; Pericic, M.; Barac-Lauc, L.; Smolej-Narancic, N.; Janicijevic, B.; Polasek, O.; Tenesa, A.; et al. Runs of homozygosity in European populations. Am. J. Hum. Genet. 2008, 83, 359–372. [Google Scholar] [CrossRef]
- Danecek, P.; Auton, A.; Abecasis, G.; Albers, C.; Banks, E.; DePristo, M.; Handsaker, R.; Lunter, G.; Marth, G.; Sherry, S.; et al. The variant call format and VCFtools. Bioinformatics 2011, 27, 2156–2158. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Patterson, N.; Reich, D. Population differentiation as a test for selective sweeps. Genome Res. 2010, 20, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Meenu, M.; Chen, P.; Xu, B. Comparative Study on Phytochemical Profiles and Antioxidant Capacities of Chestnuts Produced in Different Geographic Area in China. Antioxidants 2020, 9, 190. [Google Scholar] [CrossRef]
- Zheng, X.; Liu, L.; Sun, J.; Li, G.; Zhou, F.; Xu, J. Imaging of underground karst water channels using an improved multichannel transient Rayleigh wave detecting method. PLoS ONE 2018, 13, e0199030. [Google Scholar] [CrossRef]
- Zhai, H.; Chen, C.; Wang, N.; Chen, Y.; Nie, X.; Han, B.; Li, Q.; Xia, F.; Lu, Y. Blood lead level is associated with non-alcoholic fatty liver disease in the Yangtze River Delta region of China in the context of rapid urbanization. Environ. Health A Glob. Access Sci. Source 2017, 16, 93. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, M.; Shi, Y. Efficacy of spinal chiropractic manipulative therapy for adjusting the relationship between cervical facet joints to treat headache caused by acute mountain sickness. J. Int. Med. Res. 2020, 48, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, C.; Chen, J.; Bai, Y.; Wang, K.; Wang, Y.; Fang, M. Genome-Wide Analysis Reveals Human-Mediated Introgression from Western Pigs to Indigenous Chinese Breeds. Genes 2020, 11, 275. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, C.; Peng, Y.; Cai, X.; Hu, X.; Bosse, M.; Zhao, Y. Whole-genome analysis reveals the hybrid formation of Chinese indigenous DHB pig following human migration. Evol. Appl. 2022, 15, 501–514. [Google Scholar] [CrossRef]
- Xu, J.; Fu, Y.; Hu, Y.; Yin, L.; Tang, Z.; Yin, D.; Zhu, M.; Yu, M.; Li, X.; Zhou, Y.; et al. Whole genome variants across 57 pig breeds enable comprehensive identification of genetic signatures that underlie breed features. J. Anim. Sci. Biotechnol. 2020, 11, 115. [Google Scholar] [CrossRef]
- Steffen, W.; Richardson, K.; Rockström, J.; Cornell, S.; Fetzer, I.; Bennett, E.; Biggs, R.; Carpenter, S.; de Vries, W.; de Wit, C.; et al. Sustainability. Planetary boundaries: Guiding human development on a changing planet. Science 2015, 347, 1259855. [Google Scholar] [CrossRef]
- López-Cortegano, E.; Pouso, R.; Labrador, A.; Pérez-Figueroa, A.; Fernández, J.; Caballero, A. Optimal Management of Genetic Diversity in Subdivided Populations. Front. Genet. 2019, 10, 843. [Google Scholar] [CrossRef] [PubMed]
- Shang, P.; Li, W.; Tan, Z.; Zhang, J.; Dong, S.; Wang, K.; Chamba, Y. Population Genetic Analysis of Ten Geographically Isolated Tibetan Pig Populations. Animals 2020, 10, 1297. [Google Scholar] [CrossRef] [PubMed]
- Sigdel, A.; Liu, L.; Abdollahi-Arpanahi, R.; Aguilar, I.; Peñagaricano, F. Genetic dissection of reproductive performance of dairy cows under heat stress. Anim. Genet. 2020, 51, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Dikmen, S.; Wang, X.Z.; Ortega, M.S.; Cole, J.B.; Null, D.J.; Hansen, P.J. Single nucleotide polymorphisms associated with thermoregulation in lactating dairy cows exposed to heat stress. J. Anim. Breed. Genet. 2015, 132, 409–419. [Google Scholar] [CrossRef]
- Ortega, M.S.; Denicol, A.C.; Cole, J.B.; Null, D.J.; Hansen, P.J. Use of single nucleotide polymorphisms in candidate genes associated with daughter pregnancy rate for prediction of genetic merit for reproduction in Holstein cows. Anim. Genet. 2016, 47, 288–297. [Google Scholar] [CrossRef]
- Murata, H.; Kunii, H.; Kusama, K.; Sakurai, T.; Bai, H.; Kawahara, M.; Takahashi, M. Heat stress induces oxidative stress and activates the KEAP1-NFE2L2-ARE pathway in bovine endometrial epithelial cells†. Biol. Reprod. 2021, 105, 1114–1125. [Google Scholar] [CrossRef]
- Asadollahi, H.; Vaez Torshizi, R.; Ehsani, A.; Masoudi, A.A. An association of CEP78, MEF2C, VPS13A and ARRDC3 genes with survivability to heat stress in an F2 chicken population. J. Anim. Breed. Genet. 2022, 139, 574–582. [Google Scholar] [CrossRef]
- Urs, S.; Venkatesh, D.; Tang, Y.; Henderson, T.; Yang, X.; Friesel, R.; Rosen, C.; Liaw, L. Sprouty1 is a critical regulatory switch of mesenchymal stem cell lineage allocation. FASEB J. 2010, 24, 3264–3273. [Google Scholar] [CrossRef]
- Urs, S.; Henderson, T.; Le, P.; Rosen, C.; Liaw, L. Tissue-specific expression of Sprouty1 in mice protects against high-fat diet-induced fat accumulation, bone loss and metabolic dysfunction. Br. J. Nutr. 2012, 108, 1025–1033. [Google Scholar] [CrossRef]
- Xiao, C.; Sun, T.; Yang, Z.; Xu, W.; Wang, J.; Zeng, L.; Deng, J.; Yang, X. Transcriptome landscapes of differentially expressed genes related to fat deposits in Nandan-Yao chicken. Funct. Integr. Genom. 2021, 21, 113–124. [Google Scholar] [CrossRef]
- Harris, R.; Alcott, C.; Sullivan, E.; Takahashi, D.; McCurdy, C.; Comstock, S.; Baquero, K.; Blundell, P.; Frias, A.; Kahr, M.; et al. Genomic Variants Associated with Resistance to High Fat Diet Induced Obesity in a Primate Model. Sci. Rep. 2016, 6, 36123. [Google Scholar] [CrossRef] [PubMed]
- Rovadoscki, G.; Pertile, S.; Alvarenga, A.; Cesar, A.; Pértille, F.; Petrini, J.; Franzo, V.; Soares, W.; Morota, G.; Spangler, M.; et al. Estimates of genomic heritability and genome-wide association study for fatty acids profile in Santa Inês sheep. BMC Genom. 2018, 19, 375. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cheng, H.; Wang, S.; Luo, X.; Ma, X.; Sun, L.; Chen, N.; Zhang, J.; Qu, K.; Wang, M.; et al. Genomic Diversity and Selection Signatures for Weining Cattle on the Border of Yunnan-Guizhou. Front. Genet. 2022, 13, 848951. [Google Scholar] [CrossRef] [PubMed]
- Song, N.; Chang, S.; Li, D.; Villanueva, C.; Park, K. Induction of thermogenic adipocytes: Molecular targets and thermogenic small molecules. Exp. Mol. Med. 2017, 49, e353. [Google Scholar] [CrossRef] [PubMed]





| Meta-Population | Origin | Breed Number | Number | HO | HE | F | fii |
|---|---|---|---|---|---|---|---|
| PC | Plateau of China | 6 | 110 | 0.2488 | 0.2733 | 0.3536 | 0.7428 |
| NC | North China | 5 | 84 | 0.2790 | 0.3224 | 0.3533 | 0.6817 |
| SC | Southern China | 6 | 101 | 0.2525 | 0.2953 | 0.4407 | 0.7676 |
| CC | Central China | 8 | 130 | 0.2246 | 0.2752 | 0.4278 | 0.7470 |
| JDC | Jianghai District of China | 4 | 66 | 0.2289 | 0.2882 | 0.4748 | 0.7318 |
| SWC | Southwestern China | 8 | 122 | 0.2415 | 0.2773 | 0.3823 | 0.7308 |
| Class | 0–6 Mb | 6–12 Mb | 12–24 Mb | 24–48 Mb | >48 Mb | Total |
|---|---|---|---|---|---|---|
| PC | 35,759 (95.07%) | 1265 (3.36%) | 372 (0.99%) | 142 (0.38%) | 74 (0.20%) | 37,612 |
| NC | 21,126 (91.63%) | 1204 (5.22%) | 438 (1.90%) | 197 (0.85%) | 92 (0.40%) | 23,057 |
| SC | 36,375 (93.72%) | 1713 (4.41%) | 467 (1.20%) | 152 (0.39%) | 107 (0.28%) | 38,814 |
| CC | 41,530 (92.92%) | 1945 (4.35%) | 703 (1.57%) | 326 (0.73%) | 189 (0.42%) | 44,693 |
| JDC | 20,526 (90.18%) | 1376 (6.05%) | 541 (2.38%) | 237 (1.04%) | 81 (0.36%) | 22,761 |
| SWC | 37,518 (93.32%) | 1731 (4.31%) | 622 (1.55%) | 231 (0.57%) | 102 (0.25%) | 40,204 |
| Group | CHR | Start (bp) | End (bp) | Gene | FST | XP-CLR | Function |
|---|---|---|---|---|---|---|---|
| SNC | 3 | 94,167,759 | 94,253,662 | EPAS1 | 0.4493 | 13.6013 | Heat tolerance in dairy cows [33,34,35] |
| SNC | 15 | 82,967,485 | 83,146,185 | NFE2L2 | 0.3681 | 7.8295 | Protecting the body of dairy cows under heat stress [36] |
| SNC | 1 | 230,069,339 | 230,331,343 | VPS13A | 0.4295 | 8.3043 | Reducing physiological damage in animals under heat stress [37] |
| SNC | 8 | 100,449,476 | 100,863,780 | SPRY1 | 0.4900 | 6.9364 | Fat deposition [38,39] |
| NSC | 9 | 127,853,581 | 128,164,825 | PLA2G4A | 0.1960 | 5.0068 | The key regulators for fat deposition [40,41] |
| NSC | 1 | 83,140,479 | 83,295,865 | UBE3D | 0.2612 | 7.2031 | Related to fat composition in sheep [42,43,44] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Zhang, S.; Zhan, F.; Song, M.; Shang, P.; Zhu, F.; Li, J.; Yang, F.; Li, X.; Qiao, R.; et al. Population Genetic Analysis of Six Chinese Indigenous Pig Meta-Populations Based on Geographically Isolated Regions. Animals 2023, 13, 1396. https://doi.org/10.3390/ani13081396
Zhang L, Zhang S, Zhan F, Song M, Shang P, Zhu F, Li J, Yang F, Li X, Qiao R, et al. Population Genetic Analysis of Six Chinese Indigenous Pig Meta-Populations Based on Geographically Isolated Regions. Animals. 2023; 13(8):1396. https://doi.org/10.3390/ani13081396
Chicago/Turabian StyleZhang, Lige, Songyuan Zhang, Fengting Zhan, Mingkun Song, Peng Shang, Fangxian Zhu, Jiang Li, Feng Yang, Xiuling Li, Ruimin Qiao, and et al. 2023. "Population Genetic Analysis of Six Chinese Indigenous Pig Meta-Populations Based on Geographically Isolated Regions" Animals 13, no. 8: 1396. https://doi.org/10.3390/ani13081396





