Effects of Eggshell Thickness, Calcium Content, and Number of Pores in Erosion Craters on Hatching Rate of Chinese Alligator Eggs
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Data Analysis
3. Results
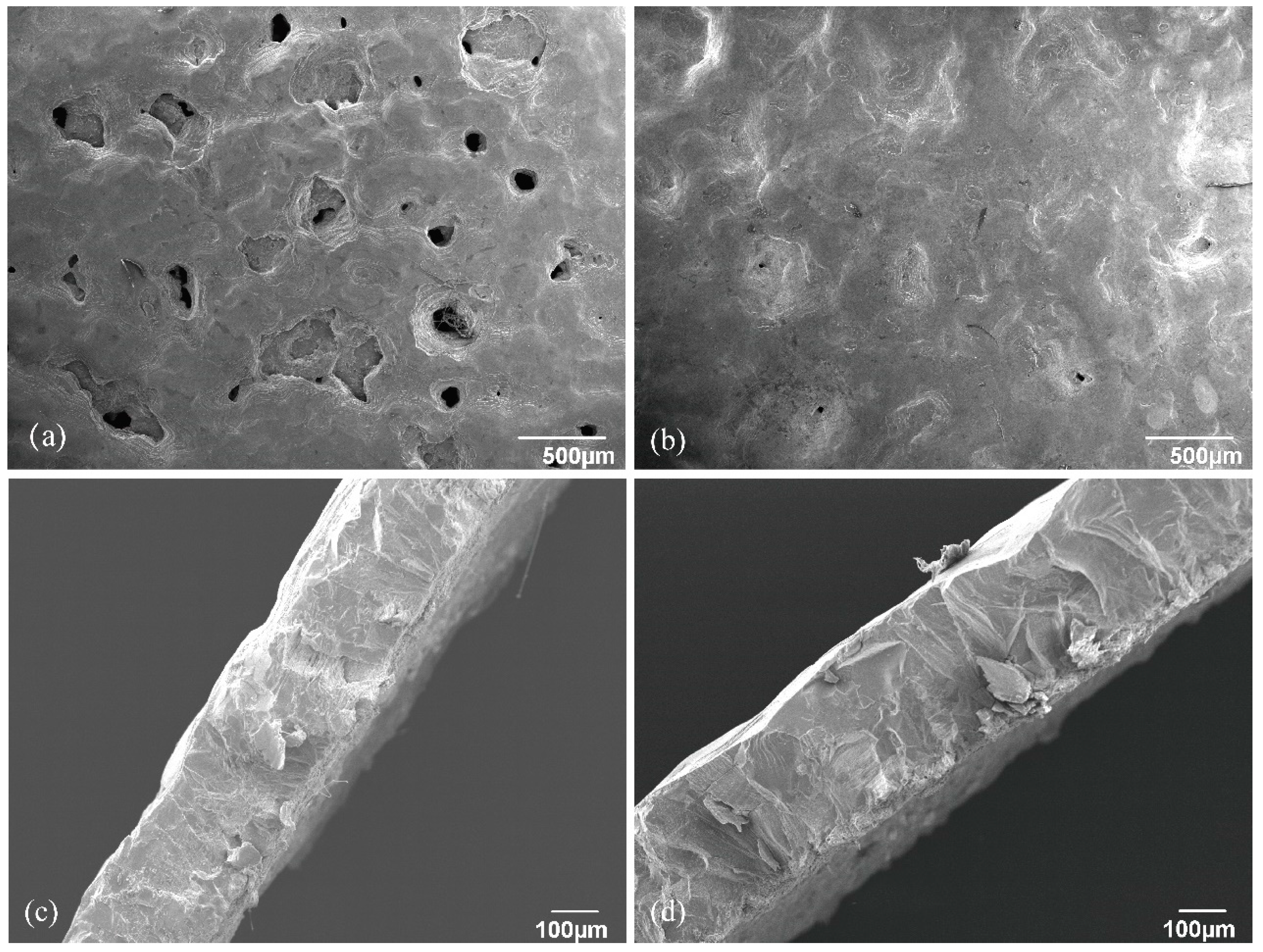
3.1. Scanning Results for the Eggshells’ Outer Surfaces
3.2. Effect of Alligator-Eggshell Thickness on the Hatching Rate
3.3. Effects of the Number of Erosion-Crater Pores on the Surfaces of Alligator Eggs on Their Hatching Rates
3.4. Eggshells’ Ca Contents Are Associated with the Hatching Rate
4. Discussion
4.1. Effect of Eggshell Thickness on the Hatching Rate
4.2. Effect of the Number of Erosion-Crater Pores on the Hatching Rate
4.3. Effect of Eggshells’ Ca Content on the Hatching Rate
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CR | critically endangered |
| CE | cracked end of the eggshell |
| ME | middle section of the eggshell |
| IE | intact end of the eggshell |
| AVG | average |
References
- Peruzzi, N.J.; Scala, N.L.; Macari, M.; Furlan, R.L.; Meyer, A.D.; Fernandez-Alarcon, M.F.; Kroetz Neto, F.L.; Souza, F.A. Fuzzy modeling to predict chicken egg hatchability in commercial hatchery. Poult. Sci. 2012, 91, 2710–2717. [Google Scholar] [CrossRef] [PubMed]
- Ulmer-Franco, A.M.; Fasenko, G.M. Hatching egg characteristics, chick quality, and broiler performance at 2 breeder flock ages and from 3 egg weights. Poult. Sci. 2010, 89, 2735–2742. [Google Scholar] [CrossRef] [PubMed]
- Narushin, V.G.; Romanov, M.N. Egg physical characteristics and hatchability. Worlds Poult. Sci. J. 2002, 58, 297–303. [Google Scholar] [CrossRef]
- Moiseyeva, I.G.; Tolokonnikova, E.V. Correlation of some quality traits of hens’ eggs and the level of hens’ productivity with chick hatchability. In Papers on Genetics and Immunogenetics of Animals, Eggs and Chicks Hatchability; Elsevier: Amsterdam, The Netherlands, 1968; Volume 2, p. 54. [Google Scholar]
- Tsarenko, P.P.; Kurova, G.M. Quality control of chicken eggs. In Effective Technologies of Poultry Production; Agropromizdat: Moscow, Russia, 1989; pp. 97–102. [Google Scholar]
- Ergun, O.F.; Yamak, U.S. The effect of eggshell thickness on hatchability of quail eggs. Vet. World. 2017, 10, 1114–1117. [Google Scholar] [CrossRef] [PubMed]
- Cedillo-Leal, C.; Simoncini, M.S.; Leiva, P.M.L.; Larriera, A.; Lang, J.W.; Pina, C.I. Eggshell structure in Caiman latirostris eggs improves embryo survival during nest inundation. Proc. Biol. Sci. 2017, 284, 20162675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koen, D.R.; Koen, G.; Marc, H.; Winy, M.; Mieke, U.; Johan, D.; Lieve, H. Influence of eggshell condensation on eggshell penetration and whole egg contamination with Salmonella enterica serovar Enteritidis. J. Food Prot. 2006, 69, 1539–1545. [Google Scholar]
- Tate, K.B.; Eme, J.; Swart, J.; Conlon, J.M.; Crossley, D.A., II. Effects of dehydration on cardiovascular development in the embryonic American alligator (Alligator mississipiensis). Comp. Biochem. Physiol. A 2012, 162, 252–258. [Google Scholar] [CrossRef]
- Tang, W.; Zhao, B.; Chen, Y.; Du, W. Reduced egg shell permeability affects embryonic development and hatchling traits in Lycodon rufozonatum and Pelodiscus sinensis. Integr. Zool. 2018, 13, 58–69. [Google Scholar] [CrossRef]
- Ferguson, M.W. The reproductive biology and embryology of the crocodilians. Biol. Reptilia 1981, 12, 330–491. [Google Scholar]
- Gutowska, M.S.; Mitchell, C.A. Carbonic anhydrase in the calcification of the egg shell. Poultry Sci. 1945, 24, 159–167. [Google Scholar] [CrossRef]
- Packard, M.J.; Hirsch, K.F.; Packard, G.C.; Miller, J.D.; Jones, M.E. Structure of shells from eggs of the Australian lizard Amphibolurus barbatus. Can. J. Zool. 1991, 69, 303–310. [Google Scholar] [CrossRef]
- Stahlschmidt, Z.R.; Heulin, B.; DeNardo, D.F. The role of python eggshell permeability dynamics in a respiration-hydration trade-off. Physiol. Biochem. Zool. 2010, 83, 576–586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrews, R. Patterns of embryonic development. In Reptilian Incubation: Environment, Evolution and Behaviour; Biologist: Nottingham, England, 2004; pp. 75–102. [Google Scholar]
- Vleck, C.M.; Hoyt, D.F. Metabolism and energetics of reptilian and avian embryos. In Egg Incubation: Its Effects on Embryonic Development in Birds and Reptiles; Cambridge University Press: Cambridge, UK, 1991; pp. 285–306. [Google Scholar]
- Thompson, M.B. Functional significance of the opaque white patch in eggs of Emydura macquarii. In Biology of Australasian Frogs and Reptiles; Surrey Beatty: Chipping Norton, Australia; Royal Zoological Society of New South Wales: Sydney, Australia, 1985; pp. 387–395. [Google Scholar]
- Crossley, D.A.; Altimiras, J. Cardiovascular development in embryos of the American alligator (Alligator mississippiensis): Effects of chronic and acute hypoxia. J. Exp. Biol. 2005, 208, 31–39. [Google Scholar] [CrossRef] [Green Version]
- Van Golde, J.; Mulder, T.; Blanco, C.E. Changes in mean chorioallantoic artery blood flow and heart rate produced by hypoxia in the developing chick embryo. Pediatr. Res. 1997, 42, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Kam, Y.C. Physiological effects of hypoxia on metabolism and growth of turtle embryos. Respir. Physiol. 1993, 92, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Nechaeva, M.V. Physiological responses to acute changes in temperature and oxygenation in bird and reptile embryos. Respir. Physiol. Neurobiol. 2011, 178, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Lance, V.; Joanen, T.; McNease, L. Selenium, vitamin E, and trace elements in the plasma of wild and farm-reared alligators during the reproductive cycle. Can. J. Zool. 1983, 61, 1744–1751. [Google Scholar] [CrossRef] [Green Version]
- Brown, G.J.; Forbes, P.B.; Myburgh, J.G.; Nöthling, J.O. Calcium and phosphorus in unbanded eggs of the Nile crocodile (Crocodylus niloticus). Aquac. Res. 2020, 51, 3403–3411. [Google Scholar] [CrossRef]
- Huchzermeyer, F.W. Crocodiles: Biology, Husbandry and Diseases; CABI: Wallingford, UK, 2003. [Google Scholar]
- Lane, T.J.; Ruppert, K.C. Alligator Farming Production Review; Institute of Food and Agricultural Sciences, Universtiy of Florida, Florida Cooperative Extension Service: Beltsville, MD, USA; Washington, DC, USA, 1981. [Google Scholar]
- Packard, G.C.; Packard, M.J. Control of metabolism and growth in embryonic turtles: A test of the urea hypothesis. J. Exp. Biol. 1989, 147, 203–216. [Google Scholar] [CrossRef]
- Wu, L.; Wu, X.; Jiang, H.; Wang, C. Parameter Analysis and Population Growth Prediction of Chinese Alligator Breeding Population in Anhui Province. Acta Hydrobiol. Sin. 2006, 30, 159–165. [Google Scholar]
- Wink, C.S.; Elsey, R.M.; Bouvier, M. Porosity of eggshells from wild and captive, pen-reared alligators (Alligator mississippiensis). J. Morphol. 1990, 203, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Olesik, J.W. Elemental analysis using icp-oes and icp/ms. Anal. Chem. 1991, 63, 12A–21A. [Google Scholar] [CrossRef]
- Sharlanov, D.; Bachev, N.; Lalev, M. Investigation of correlation between some morphological parameters and hatching quality of turkey eggs. Zhivotnovudni Nauki 1988, 25, 13–17. [Google Scholar]
- Tsarenko, R.; Tsarenko, P.; Belko, A. Quality of goose eggs and their selection for incubation. Ptitsevodstvo 1978, 1, 28–30. [Google Scholar]
- Ar, A.; Paganelli, C.V.; Reeves, R.; Greene, D.; Rahn, H. The avian egg: Water vapor conductance, shell thickness, and functional pore area. The Condor 1974, 76, 153–158. [Google Scholar] [CrossRef]
- Stoker, C.; Zayas, M.A.; Ferreira, M.A.; Durando, M.; Galoppo, G.H.; Rodriguez, H.A.; Repetti, M.R.; Beldoménico, H.R.; Caldini, E.G.; Luque, E.H.; et al. The eggshell features and clutch viability of the broad-snouted caiman (Caiman latirostris) are associated with the egg burden of organochlorine compounds. Ecotoxicol. Environ. Saf. 2013, 98, 191–195. [Google Scholar] [CrossRef]
- Ketta, M.; Tůmová, E. Eggshell structure, measurements, and quality-affecting factors in laying hens: A review. Czech J. Anim. Sci. 2016, 61, 299–309. [Google Scholar] [CrossRef] [Green Version]
- Bain, M.M.; McDade, K.; Burchmore, R.; Law, A.; Wilson, P.W.; Schmutz, M.; Preisinger, R.; Dunn, I.C. Enhancing the egg’s natural defence against bacterial penetration by increasing cuticle deposition. Anim. Genet. 2013, 44, 661–668. [Google Scholar] [CrossRef] [Green Version]
- Moeller, K.T.; Butler, M.W.; DeNardo, D.F. The effect of hydration state and energy balance on innate immunity of a desert reptile. Front. Zool. 2013, 10, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Mortola, J.P. Gas exchange in avian embryos and hatchlings. Comp. Biochem. Phys. A 2009, 153, 359–377. [Google Scholar] [CrossRef]
- Kern, M.D.; Ferguson, M.W. Gas permeability of American alligator eggs and its anatomical basis. Physiol. Zool. 1997, 70, 530–546. [Google Scholar] [CrossRef] [PubMed]
- Nechaeva, M.V.; Vladimirova, I.G.; Alekseeva, T.A. Oxygen consumption as related to the development of the extraembryonic membranes and cardiovascular system in the European pond turtle (Emys orbicularis) embryogenesis. Comp. Biochem. Phys. A 2007, 148, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Packard, M.J.; Clark, N.B. Aspects of calcium regulation in embryonic lepidosaurians and chelonians and a review of calcium regulation in embryonic archosaurians. Physiol. Zool. 1996, 69, 435–466. [Google Scholar] [CrossRef]
- Packard, M.J.; Packard, G.C. Mobilization of calcium, phosphorus, and magnesium by embryonic alligators (Alligator mississippiensis). Am. J. Physiol. -Regul. Integr. Comp. Physiol. 1989, 257, R1541–R1547. [Google Scholar] [CrossRef]



| Levene Test | T Test of Mean Equation | ||||||
|---|---|---|---|---|---|---|---|
| F | Sig. | T | df | Sig. (Both Sides) | Mean Value Difference | Standard Deviation | |
| CE | 0.488 | 0.487 | 5.765 | 78 | 0.000 | 0.47 | 0.081 |
| ME | 4.825 | 0.031 | 68.369 | 78 | 0.000 | 0.571 | 0.084 |
| IE | 0.019 | 0.89 | 3.682 | 78 | 0.000 | 0.347 | 0.094 |
| AVG | 1.643 | 0.204 | 6.722 | 78 | 0.000 | 0.463 | 0.069 |
| CE | ME | IE | AVG | |
|---|---|---|---|---|
| Total N | 80 | 80 | 80 | 80 |
| Mann–Whitney U | 1205.500 | 1141.500 | 1154.000 | 1222.500 |
| Wilcoxon | 2025.500 | 1961.500 | 1974.500 | 2042.500 |
| Test statistic | 1205.500 | 1141.500 | 1154.500 | 1222.500 |
| Standard error | 103.835 | 103.883 | 103.836 | 103.914 |
| Standardized testing | 3.905 | 3.287 | 3.414 | 4.066 |
| Progressive significance (Z-sided test) | 0.000 | 0.001 | 0.001 | 0.000 |
| Levene Test | T Test of Mean Equation | |||||
|---|---|---|---|---|---|---|
| F | Sig. | T | df | Sig. (Both Sides) | Mean Value Difference | Standard Deviation |
| 0.02 | 0.889 | 2.569 | 12 | 0.025 | 47,312.49917 | 18,416.94162 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, N.; Zhang, H.; Fan, G.; Sun, K.; Jiang, Q.; Lv, Z.; Han, B.; Nie, Z.; Shao, Y.; Zhou, Y.; et al. Effects of Eggshell Thickness, Calcium Content, and Number of Pores in Erosion Craters on Hatching Rate of Chinese Alligator Eggs. Animals 2023, 13, 1405. https://doi.org/10.3390/ani13081405
Zhang N, Zhang H, Fan G, Sun K, Jiang Q, Lv Z, Han B, Nie Z, Shao Y, Zhou Y, et al. Effects of Eggshell Thickness, Calcium Content, and Number of Pores in Erosion Craters on Hatching Rate of Chinese Alligator Eggs. Animals. 2023; 13(8):1405. https://doi.org/10.3390/ani13081405
Chicago/Turabian StyleZhang, Naijing, Huabin Zhang, Guangwei Fan, Ke Sun, Qingqing Jiang, Zhuowen Lv, Boyang Han, Zhenyuan Nie, Yujie Shao, Yongkang Zhou, and et al. 2023. "Effects of Eggshell Thickness, Calcium Content, and Number of Pores in Erosion Craters on Hatching Rate of Chinese Alligator Eggs" Animals 13, no. 8: 1405. https://doi.org/10.3390/ani13081405





