A Prospective Cohort Study Investigating the Impact of Neutering Bitches Prepubertally or Post-Pubertally on Physical Development
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Setting
2.3. Study Animals
2.4. Variables and Data Sources
2.4.1. Physical Assessments
2.4.2. Digital Images of the Vulva
2.5. Bias and Study Size
2.6. Quantitative Variables and Statistical Methods
3. Results
3.1. Participants
3.2. Height
3.2.1. Six-Month
3.2.2. 17-Month
3.2.3. Change in Height between Six- and 17-Month Physical Assessments
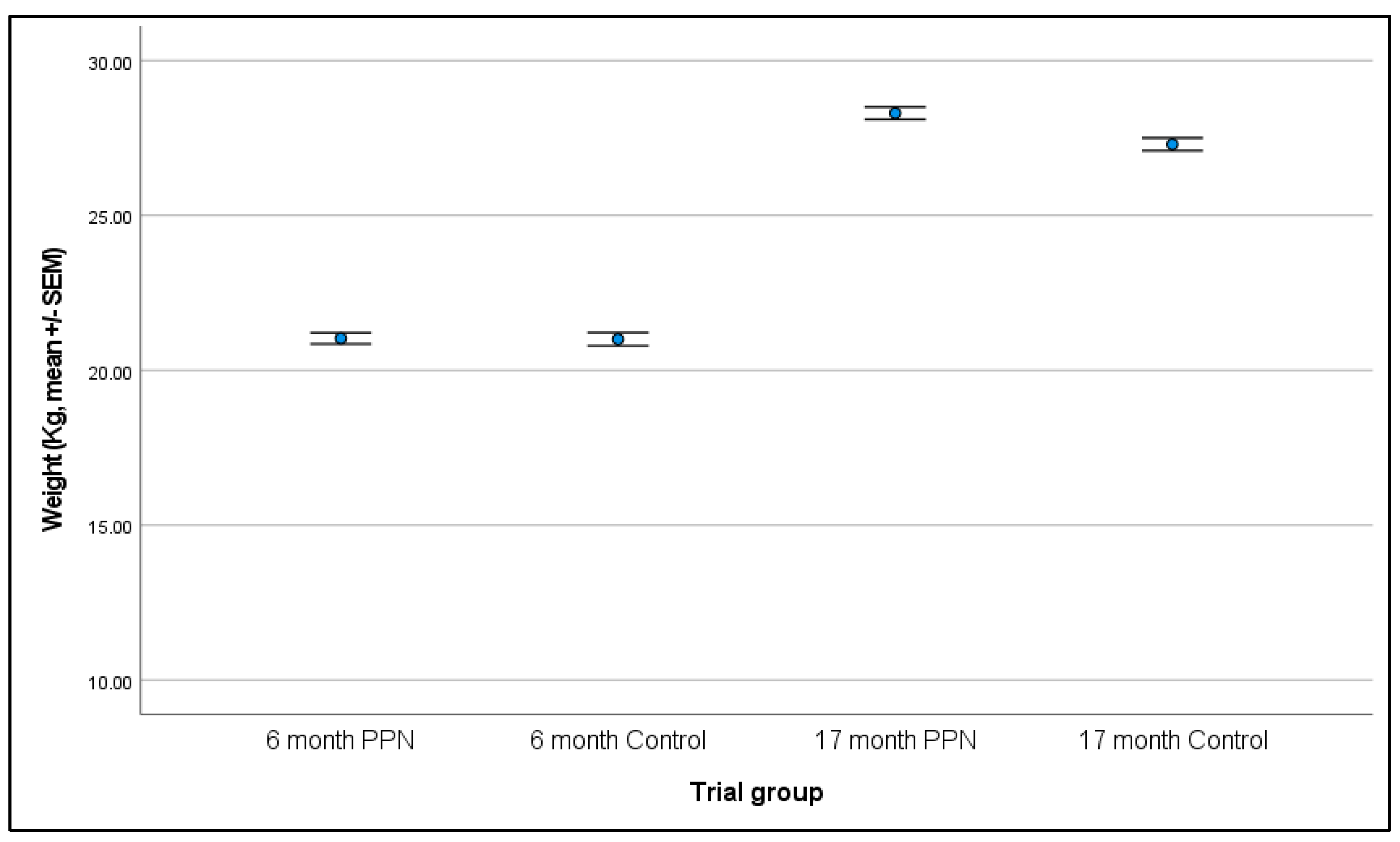
3.3. Body Weight
3.3.1. Six-Month
3.3.2. 17-Month
3.3.3. Change in Body Weight between Six- and 17-Month Physical Assessments
3.4. Body Condition Score
3.4.1. Six-Month
3.4.2. 17-Month
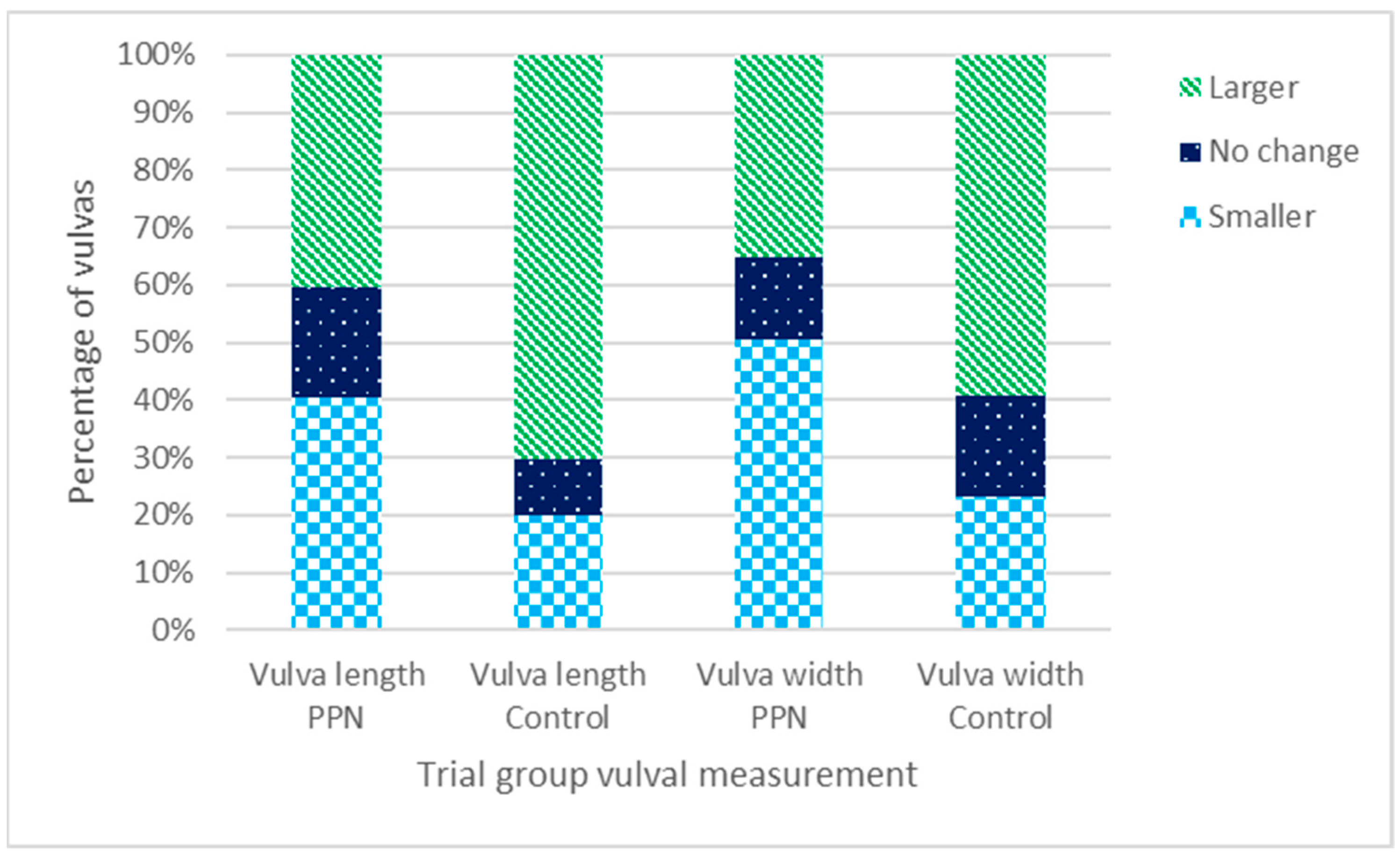
3.5. Vulval Size
3.5.1. Six-Month
3.5.2. 17-Month
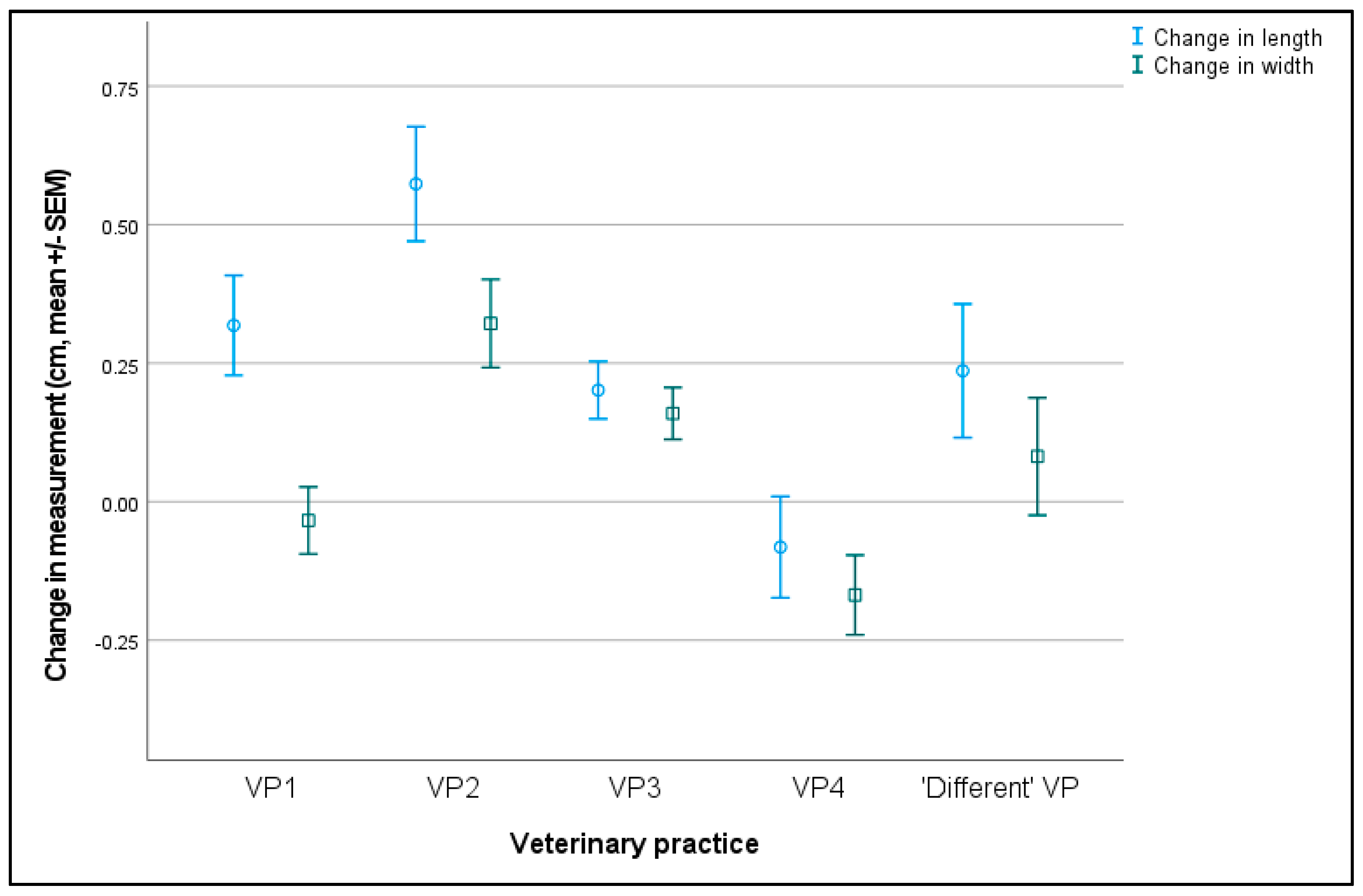
3.5.3. Change in Vulval Size between Six- and 17-Month Physical Assessments
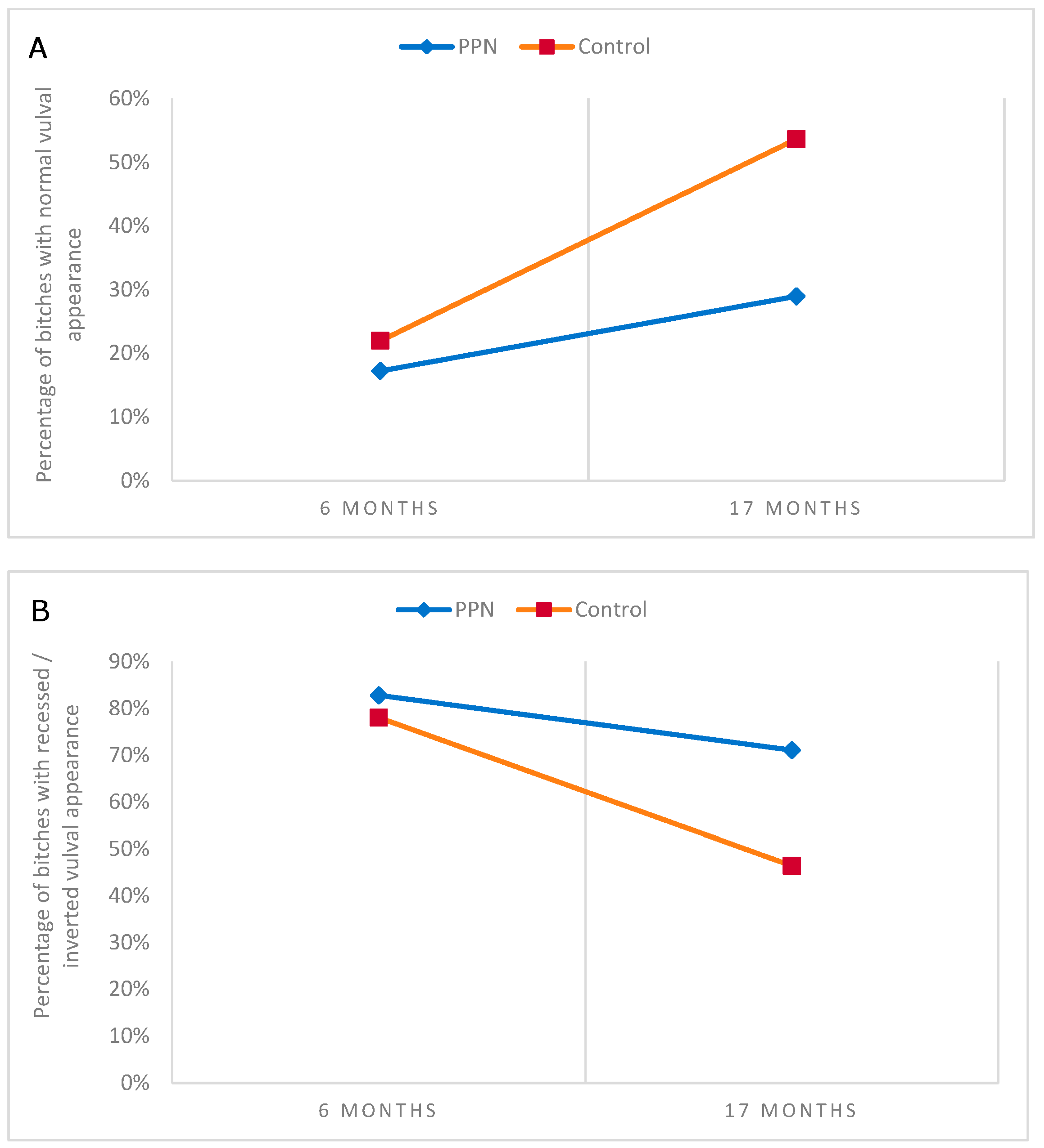
3.6. Vulval Appearance
3.6.1. Vulval Appearance at the Physical Assessments
3.6.2. Vulval Appearance from Examination of Digital Images
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stubbs, W.P.; Salmeri, K.R.; Bloomberg, M.S. Early neutering of the dog and cat. In Kirk’s Current Veterinary Therapy XII Small Animal Practice, 12th ed.; Bonagura, J.D., Ed.; WB Saunders & Co.: Philadelphia, PA, USA, 1995; pp. 1037–1040. [Google Scholar]
- Hewitt, D.; England, G. Sexual Development and Puberty in the Bitch. Vet. Nurs. J. 1999, 14, 131–135. [Google Scholar] [CrossRef]
- Kilborn, S.H.; Trudel, G.; Uhthoff, H. Review of growth plate closure compared with age at sexual maturity and lifespan in laboratory animals. J. Am. Assoc. Lab. Anim. Sci. 2002, 41, 21–26. [Google Scholar]
- Feldman, E.; Nelson, R.W. Infertility, associated breeding disorders, and disorders of sexual development. In Canine and Feline Endocrinology and Reproduction, 3rd ed.; Feldman, E., Nelson, R.W., Eds.; WB Saunders & CO.: St. Louis, MO, USA, 2004; p. 869. [Google Scholar]
- Root-Kustritz, M.V. Early spay-neuter: Clinical considerations. Clin. Tech. Small Anim. Pract. 2002, 17, 124–128. [Google Scholar] [CrossRef]
- Griffin, B.; Bushby, P.A.; McCobb, E.; White, S.C.; Rigdon-Brestle, Y.K.; Appel, L.D.; Makolinski, K.V.; Wilford, C.L.; Bohling, M.W.; Eddlestone, S.M.; et al. The Association of Shelter Veterinarians’ 2016 Veterinary Medical Care Guidelines for Spay-Neuter Programs. J. Am. Vet. Med. Assoc. 2016, 249, 165–188. [Google Scholar] [CrossRef]
- Ru, G.; Terracini, B.; Glickman, L.T. Host related risk factors for canine osteosarcoma. Vet. J. 1998, 156, 31–39. [Google Scholar] [CrossRef]
- Ware, W.A.; Hopper, D.L. Cardiac tumors in dogs: 1982–1995. J. Vet. Intern. Med. 1999, 13, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Howe, L.M.; Slater, M.R.; Boothe, H.W.; Hobson, H.P.; Holcom, J.L.; Spann, A.C. Long-term outcome of gonadectomy performed at an early age or traditional age in dogs. J. Am. Vet. Med. Assoc. 2001, 218, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Cooley, D.M.; Beranek, B.C.; Schlittler, D.L.; Glickman, N.W.; Glickman, L.T.; Waters, D.J. Endogenous gonadal hormone exposure and bone sarcoma risk. Cancer Epidemiol. Biomarkers Prev. 2002, 11, 1434–1440. [Google Scholar] [PubMed]
- Slauterbeck, J.R.; Pankratz, K.; Xu, K.T.; Bozeman, S.C.; Hardy, D.M. Canine ovariohysterectomy and orchiectomy increases the prevalence of ACL injury. Clin. Orthop. Relat. Res. 2004, 429, 301–305. [Google Scholar] [CrossRef]
- Spain, C.V.; Scarlett, J.M.; Houpt, K.A. Long-term risks and benefits of early-age gonadectomy in dogs. J. Am. Vet. Med. Assoc. 2004, 224, 380–387. [Google Scholar] [CrossRef]
- Reichler, I.M.; Hung, E.; Jöchle, W.; Piché, C.A.; Roos, M.; Hubler, M.; Arnold, S. FSH and LH plasma levels in bitches with differences in risk for urinary incontinence. Therio. 2005, 63, 2164–2180. [Google Scholar] [CrossRef]
- van Hagen, M.A.; Ducro, B.J.; van den Broek, J.; Knol, B.W. Incidence, risk factors, and heritability estimates of hind limb lameness caused by hip dysplasia in a birth cohort of boxers. Am. J. Vet. Res. 2005, 66, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Witsberger, T.H.; Villamil, J.A.; Schultz, L.G.; Hahn, A.W.; Cook, J.L. Prevalence of and risk factors for hip dysplasia and cranial cruciate ligament deficiency in dogs. J. Am. Vet. Med. Assoc. 2008, 232, 1818–1824. [Google Scholar] [CrossRef] [PubMed]
- de la Riva, G.T.; Hart, B.L.; Farver, T.B.; Oberbauer, A.M.; Messam, L.L.M.; Willits, N.; Hart, L.A. Neutering dogs: Effects on joint disorders and cancers in golden retrievers. PLoS ONE 2013, 8, e55937. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.M.; Creevy, K.E.; Promislow, D.E. Reproductive capability is associated with lifespan and cause of death in companion dogs. PLoS ONE 2013, 8, e61082. [Google Scholar] [CrossRef]
- Hart, B.L.; Hart, L.A.; Thigpen, A.P.; Willits, N.H. Long-term health effects of neutering dogs: Comparison of labrador retrievers with golden retrievers. PLoS ONE 2014, 9, e102241. [Google Scholar] [CrossRef]
- Zink, M.C.; Farhoody, P.; Elser, S.E.; Ruffini, L.D.; Gibbons, T.A.; Rieger, R.H. Evaluation of the risk and age of onset of cancer and behavioral disorders in gonadectomized Vizslas. J. Am. Vet. Med. Assoc. 2014, 244, 309–319. [Google Scholar] [CrossRef]
- Hart, B.L.; Hart, L.A.; Thigpen, A.P.; Willits, N.H. Neutering of German Shepherd Dogs: Associated joint disorders, cancers and urinary incontinence. Vet. Med. Sci. 2016, 2, 191–199. [Google Scholar] [CrossRef]
- Sundburg, C.R.; Belanger, J.M.; Bannasch, D.L.; Famula, T.R.; Oberbauer, A.M. Gonadectomy effects on the risk of immune disorders in the dog: A retrospective study. BMC Vet. Res. 2016, 12, 278. [Google Scholar] [CrossRef]
- Pegram, C.; Brodbelt, D.C.; Church, D.B.; Hall, J.; Owen, L.; Chang, Y.M.; O’Neill, D.G. Associations between neutering and early-onset urinary incontinence in UK bitches under primary veterinary care. J. Small Anim. Pract. 2019, 60, 723–733. [Google Scholar] [CrossRef]
- Simpson, M.; Albright, S.; Wolfe, B.; Searfoss, E.; Street, K.; Diehl, K.; Page, R. Age at gonadectomy and risk of overweight/obesity and orthopedic injury in a cohort of golden retrievers. PLoS ONE 2019, 14, e0209131. [Google Scholar] [CrossRef]
- O’Neill, D.G.; James, H.; Brodbelt, D.C.; Church, D.B.; Pegram, C. Prevalence of commonly diagnosed disorders in UK dogs under primary veterinary care: Results and applications. BMC Vet. Res. 2021, 17, 69. [Google Scholar] [CrossRef]
- O’Farrell, V.; Peachey, E. Behavioural effects of ovariohysterectomy on bitches. J. Small Anim. Pract. 1990, 31, 595–598. [Google Scholar] [CrossRef]
- Salmeri, K.R.; Bloomberg, M.S.; Scruggs, S.L.; Shille, V. Gonadectomy in immature dogs: Effects on skeletal, physical, and behavioral development. J. Am. Vet. Med. Assoc. 1991, 198, 1193–1203. [Google Scholar]
- Balogh, O.; Borruat, N.; Andrea Meier, A.; Hartnack, S.; Reichler, I.M. The influence of spaying and its timing relative to the onset of puberty on urinary and general behaviour in Labrador Retrievers. Reprod. Domest. Anim. 2018, 53, 1184–1190. [Google Scholar] [CrossRef]
- Farhoody, P.; Mallawaarachchi, I.; Tarwater, P.M.; Serpell, J.A.; Duffy, D.L.; Zink, C. Aggression toward familiar people, strangers, and conspecifics in gonadectomized and intact dogs. Front. Vet. Sci. 2018, 5, 18. [Google Scholar] [CrossRef]
- Edwards, P.T.; Hazel, S.J.; Browne, M.; Serpell, J.A.; McArthur, M.L.; Smith, B.P. Investigating risk factors that predict a dog’s fear during veterinary consultations. PLoS ONE 2019, 14, e0215416. [Google Scholar] [CrossRef] [PubMed]
- Starling, M.; Fawcett, A.; Wilson, B.; Serpell, J.; McGreevy, P. Behavioural risks in female dogs with minimal lifetime exposure to gonadal hormones. PLoS ONE 2019, 14, e0223709. [Google Scholar] [CrossRef]
- Palestrini, C.; Mazzola, S.M.; Caione, B.; Groppetti, D.; Pecile, A.M.; Minero, M.; Cannas, S. Influence of gonadectomy on canine behavior. Animals 2021, 11, 553. [Google Scholar] [CrossRef] [PubMed]
- Stellato, A.C.; Flint, H.E.; Dewey, C.E.; Widowski, T.M.; Niel, L. Risk-factors associated with veterinary-related fear and aggression in owned domestic dogs. Appl. Anim. Behav. Sci. 2021, 241, 105374. [Google Scholar] [CrossRef]
- Nap, R.C.; Hazewinkel, H.A.W. Growth and skeletal development in the dog in relation to nutrition; a review. Vet. Q. 1994, 16, 50–59. [Google Scholar] [CrossRef]
- Van der Eerden, B.C.J.; Karperien, M.; Wit, J. Systemic and local regulation of the growth plate. Endocr. Rev. 2003, 24, 782–801. [Google Scholar] [CrossRef]
- Von Pfeil, D.J.F.; DeCamp, C.E.; Abood, S.K. The epiphyseal plate: Nutritional and hormonal influences; hereditary and other disorders. Compend. Contin. Educ. Vet. 2009, 31, E1–E13. [Google Scholar]
- Makielski, K.M.; Mills, L.J.; Sarver, A.L.; Henson, M.S.; Spector, L.G.; Naik, S.; Modiano, J.F. Risk factors for development of canine and human osteosarcoma: A comparative review. Vet. Sci. 2019, 6, 48. [Google Scholar] [CrossRef] [PubMed]
- May, C.; Bennett, D.; Downham, D.Y. Delayed physeal closure associated with castration in cats. J. Small Anim. Pract. 1991, 32, 326–328. [Google Scholar] [CrossRef]
- Stubbs, W.P.; Bloomberg, M.S.; Scruggs, S.L.; Shille, V.M.; Lane, T.J. Effects of prepubertal gonadectomy on physical and behavioral development in cats. J. Am. Vet. Med. Assoc. 1996, 209, 1864–1871. [Google Scholar] [PubMed]
- Root, M.V.; Johnston, S.D.; Olson, P.N. The effect of prepuberal and postpuberal gonadectomy on radial physeal closure in male and female domestic cats. Vet. Radiol. Ultrasound. 1997, 38, 42–47. [Google Scholar] [CrossRef]
- Oberbauer, A.M.; Belanger, J.M.; Famula, T.R. A review of the impact of neuter status on expression of inherited conditions in dogs. Front. Vet. Sci. 2019, 6, 397. [Google Scholar] [CrossRef] [PubMed]
- Sontas, B.; Ekici, H. Short-term effects of prepubertal ovariohysterectomy on skeletal, physical and behavioural development of dogs up to 24 weeks of age. Acta Vet. Hung. 2007, 55, 379–387. [Google Scholar] [CrossRef]
- Verstegen-Onclin, K.; Verstegen, J. Non-reproductive Effects of Spaying and Neutering: Effects on the Urogenital System. In Proceedings of the Third International Symposium on Non-Surgical Contraceptive Methods for Pet Population Control, Alexandria, VA, USA, 9–12 November 2006. [Google Scholar]
- Morgan, R.V. Handbook of Small Animal Practice, 5th ed.; Saunders Elsevier: St. Louis, Mo, USA, 2008; pp. 527, 543–549, 584–586. [Google Scholar]
- Schäfer-Somi, S.; Kaya, D.; Gültiken, N.; Aslan, S. Suppression of Fertility in Pre-pubertal Dogs and Cats. Reprod. Domest. Anim. 2014, 49, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Nelson, R.W.; Couto, C.G. Small Animal Internal Medicine, 4th ed.; Nelson, R.W., Couto, C.G., Eds.; Mosby Elsevier: St. Louis, MO, USA, 2009; p. 914. [Google Scholar]
- Johnson, C.A. Diagnosis and treatment of chronic vaginitis in the bitch. Vet. Clin. N. Am. Small. Anim. Pract. 1991, 21, 523–531. [Google Scholar] [CrossRef]
- Lightner, B.A.; McLoughlin, M.A.; Chew, D.J.; Beardsley, S.M.; Matthews, H.K. Episioplasty for the treatment of perivulvar dermatitis or recurrent urinary tract infections in dogs with excessive perivulvar skin folds: 31 cases (1983–2000). J. Am. Vet. Med. Assoc. 2001, 219, 1577–1581. [Google Scholar] [CrossRef]
- Mathews, K.G. Surgery of the canine vagina and vulva. Vet. Clin. N. Am. Small. Anim. Pract. 2001, 31, 271–290. [Google Scholar] [CrossRef]
- Miller, W.H.; Griffin, C.E.; Campbell, K.L.; Muller, G.H.; Scott, D.W. Chapter 16. Environmental Skin Diseases. In Muller & Kirk’s Small Animal Dermatology, 7th ed.; Elsevier Inc.: St. Louis, MO, USA, 2012; p. 680. [Google Scholar]
- Root-Kustritz, M.V. Determining the optimal age for gonadectomy of dogs and cats. J. Am. Vet. Med. Assoc. 2007, 231, 1665–1675. [Google Scholar] [CrossRef] [PubMed]
- Moxon, R.; Freeman, S.L.; Payne, R.; Corr, S.; England, G.C. A prospective cohort study investigating the peri-and postoperative outcomes following ovariohysterectomy in bitches neutered prepubertally or post-pubertally. Theriogenology 2023, 197, 283–294. [Google Scholar] [CrossRef]
- Godfrey-Hunt, J.; Pagett, C.; Moxon, R.; England, G. Vulval conformation in the entire bitch and the effects of pre and post-pubertal neutering on vulva development and urogenital disease. In BSAVA Congress Proceedings; British Small Animal Veterinary Association: Gloucester, UK, 2020; p. 384. [Google Scholar] [CrossRef]
- Kaya, D.; Schäfer-Somi, S.; Kurt, B.; Kuru, M.; Kaya, S.; Kaçar, C.; Aksoy, Ö.; Aslan, S. Clinical use of deslorelin implants for the long-term contraception in prepubertal bitches: Effects on epiphyseal closure, body development, and time to puberty. Theriogenology 2015, 83, 1147–1153. [Google Scholar] [CrossRef]
- Reichler, I.M. Gonadectomy in cats and dogs: A review of risks and benefits. Reprod. Domest. Anim. 2009, 44, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Balsa, I.; Robinson, D. Juvenile Orthopedic Disease in Dogs & Cats. Part 1: Musculoskeletal Development & Pediatric Bone Diseases. Today’s Vet. Pract. 2016, 6, 38–45. [Google Scholar]
- Salt, C.; Morris, P.J.; German, A.J.; Wilson, D.; Lund, E.M.; Cole, T.J.; Butterwick, R.F. Growth standard charts for monitoring bodyweight in dogs of different sizes. PLoS ONE 2017, 12, e0182064. [Google Scholar] [CrossRef]
- Von Pfeil, D.J.F.; DeCamp, C.E. The epiphyseal plate: Physiology, anatomy and trauma. Compend. Contin. Educ. Vet. 2009, 31, E1–E11. [Google Scholar] [PubMed]
- Hammond, G.; McConnell, F. Radiology of the appendicular skeleton. In BSAVA Manual of Canine and Feline A Foundation Manual Radiography and Radiology, 1st ed.; Holloway, A., McConnell, F., Eds.; British Small Animal Veterinary Association: Gloucester, UK, 2013; p. 243. [Google Scholar]
- Marino, G.; Rizzo, S.; Quartuccio, M.; Macrì, F.; Pagano, G.; Taormina, A.; Cristarella, S.; Zanghì, A. Deslorelin Implants in Pre-pubertal Female Dogs: Short-and Long-Term Effects on the Genital Tract. Reprod. Domest. Anim. 2014, 49, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Rubion, S.; Desmoulins, P.O.; Rivière-Godet, E.; Kinziger, M.; Salavert, F.; Rutten, F.; Flochlay-Sigognault, A.; Driancourt, M.A. Treatment with a subcutaneous GnRH agonist containing controlled release device reversibly prevents puberty in bitches. Theriogenology 2006, 66, 1651–1654. [Google Scholar] [CrossRef] [PubMed]
- Fontbonne, A. Clinical approach to conditions of the non-pregnant and neutered bitch. In BSAVA Manual of Canine and Feline Reproduction and Neonatology, 2nd ed.; England, G.C.W., von Heimendahl, A., Eds.; British Small Animal Veterinary Association: Gloucester, UK, 2010; p. 166. [Google Scholar]






| Variable | BCS 3 | BCS 4 | BCS 5 | BCS 6 |
|---|---|---|---|---|
| Breed | ||||
| Golden Retriever cross Labrador (n = 200) | 2 | 149 | 46 | 3 |
| Backcrosses (n = 58) | 0 | 34 | 17 | 7 |
| Labrador cross Golden Retriever (n = 43) | 0 | 31 | 12 | 0 |
| Veterinary practice | ||||
| 1 (n = 65) | 1 | 48 | 16 | 0 |
| 2 (n = 52) | 0 | 6 | 39 | 7 |
| 3 (n = 118) | 0 | 103 | 12 | 3 |
| 4 (n = 66) | 1 | 57 | 8 | 0 |
| Trial group | ||||
| PPN (n = 152) | 1 | 106 | 44 | 1 |
| Control (n = 149) | 1 | 108 | 31 | 9 |
| Variable | BCS 4 | BCS 5 | BCS 6 |
|---|---|---|---|
| Breed Golden Retriever cross Labrador (n = 171) | 6 | 149 | 16 |
| Backcrosses (n = 49) | 0 | 39 | 10 |
| Labrador cross Golden Retriever (n = 32) | 2 | 25 | 5 |
| Veterinary practice | |||
| 1 (n = 52) | 4 | 43 | 5 |
| 2 (n = 44) | 0 | 29 | 15 |
| 3 (n = 87) | 1 | 78 | 8 |
| 4 (n = 50) | 0 | 49 | 1 |
| Other (n=) | 3 | 14 | 2 |
| Trial group PPN (n = 125) | 4 | 105 | 16 |
| Control (n = 127) | 4 | 108 | 15 |
| Statistic | Change in Vulval Length (cm) | Change in Vulval Width (cm) | ||
|---|---|---|---|---|
| PPN N = 119 | Control N = 125 | PPN N = 119 | Control N = 125 | |
| Mean ± SEM | 0.08 ± 0.05 | 0.39 ± 0.05 | −0.05 ± 0.04 | 0.19 ± 0.04 |
| Range | −1.4 to 1.7 | −1.0 to 1.9 | −1.6 to 1.5 | −1.0 to 1.3 |
| Vulval Measurement | Comparison | Mean Difference | SEM | 91% CI | p-Value |
|---|---|---|---|---|---|
| Vulval length | PPN vs. Control * | −0.377 | 0.079 | −0.511 to −0.243 | <0.001 |
| Vulval width | PPN vs. Control * | −0.221 | 0.063 | −0.328 to −0.113 | <0.001 |
| Vulval length | VP3 vs. VP2 * | −0.373 | 0.111 | −0.666 to −0.079 | 0.005 |
| Vulval length | VP4 vs. VP1 * | −0.397 | 0.112 | −0.693 to −0.102 | 0.005 |
| Vulval length | VP4 vs. VP2 * | −0.676 | 0.122 | −0.997 to −0.354 | <0.001 |
| Vulval length | VP4 vs. VP3 * | −0.303 | 0.108 | −0.588 to −0.019 | 0.054 |
| Vulval width | VP1 vs. VP2 * | −0.353 | 0.095 | −0.603 to −0.103 | 0.003 |
| Vulval width | VP4 vs. VP2 * | −0.468 | 0.098 | −0.726 to −0.211 | <0.001 |
| Vulval width | VP4 vs. VP3 * | −0.304 | 0.086 | −0.531 to −0.076 | 0.005 |
| Reported Anomaly in Vulval Appearance | Six-Month Assessment Observed % (N) | Chi-Square (p Value) | 17-Month Assessment Observed % (N) | Chi-Square (p Value) |
|---|---|---|---|---|
| Swollen vulva | ||||
| PPN group | 0.7 (1) | 1.551 * (0.213) | 0.0 (0) | Analysis not possible |
| Control group | 3.3 (5) | 0.0 (0) | ||
| Vaginal discharge | ||||
| PPN group | 9.2 (14) | 4.867 (0.027) | 4.8 (6) | 0.130 * (0.718) |
| Control group | 17.9 (27) | 3.1 (4) | ||
| Juvenile | ||||
| PPN group | 15.1 (23) | 1.389 (0.238) | 16.0 (20) | 14.834 * (<0.001) |
| Control group | 10.6 (16) | 1.6 (2) | ||
| Recessed/inverted | ||||
| PPN group | 14.5 (22) | 0.428 (0.513) | 13.6 (17) | 7.792 * (0.005) |
| Control group | 17.2 (26) | 3.1 (4) | ||
| Prominent perivulval skin folds | ||||
| PPN group | 3.3 (5) | 1.788 (0.181) | 4.8 (6) | 1.237 (0.266) |
| Control group | 6.6 (10) | 1.6 (2) | ||
| Perivulval dermatitis | ||||
| PPN group | 0.7 (1) | 6.092 * (0.014) | 0.0 (0) | Analysis not possible |
| Control group | 6.6 (10) | 1.6 (2) | ||
| Reported Anomaly in Vulval Appearance | Six-Month PPN % (N) | Six-Month Control % (N) | Chi-Square (p Value) | 17-Month PPN % (N) | 17-Month Control % (N) | Chi-Square (p Value) |
|---|---|---|---|---|---|---|
| Vulval discharge | ||||||
| Present on image | 49.3 (36) | 55.0 (44) | 0.494 (0.482) | 28.8 (23) | 22.6 (21) | 0.863 (0.353) |
| Not present | 50.7 (37) | 45.0 (36) | 71.3 (57) | 77.4 (72) | ||
| Vulval discharge categorisation | ||||||
| Mucoid | 13.7 (10) | 7.5 (6) | 2.541 (0.281) * | 8.8 (7) | 6.5 (6) | 4.398 (0.222) * |
| Mucoid purulent | 12.3 (9) | 18.8 (15) | 6.3 (5) | 9.8 (9) | ||
| Purulent | 23.3 (17) | 27.5 (22) | 13.8 (11) | 5.4 (5) | ||
| Haemorrhagic | 0.0 (0) | 1.3 (1) | 0.0 (0) | 0.0 (0) | ||
| Dorsal skin folds | ||||||
| Present on image | 98.9 (88) | 100.0 (98) | Analysis not possible | 97.8 (89) | 99.0 (95) | 0.002 (0.963) † |
| Not present | 1.1 (1) | 0.0 (0) | 2.2 (2) | 1.0 (1) | ||
| Estimated dorsal skin fold coverage | ||||||
| 0% | 1.2 (1) | 0.0 (0) | 2.500 (0.475) ** | 2.6 (2) | 0.0 (0) | 4.089 (0.252) ** |
| 10% | 1.2 (1) | 3.3 (3) | 7.8 (6) | 10.2 (9) | ||
| 20% | 26.7 (23) | 21.7 (20) | 32.5 (25) | 35.2 (31) | ||
| 30% | 24.4 (21) | 34.8 (32) | 28.6 (22) | 37.5 (33) | ||
| 40% | 29.1 (25) | 27.2 (25) | 19.5 (15) | 13.6 (12) | ||
| 50% | 16.3 (14) | 10.9 (10) | 9.1 (7) | 3.4 (3) | ||
| 60% | 1.2 (1) | 1.1 (1) | 0.0 (0) | 0.0 (0) | ||
| 70% | 0.0 (0) | 1.1 (1) | 0.0 (0) | 0.0 (0) | ||
| Recessed/inverted appearance | ||||||
| Present on image | 82.8 (72) | 78.0 (71) | 0.632 (0.427) | 71.1 (54) | 46.3 (38) | 9.902 (0.002) |
| Not present | 17.2 (15) | 22.0 (20) | 28.9 (22) | 53.7 (44) | ||
| Perivulval skin changes | ||||||
| Present on image | 98.2 (112) | 99.1 (109) | 0.001 (0.975) † | 96.4 (107) | 99.2 (118) | 0.967 (0.325) † |
| Not present | 1.8 (2) | 0.9 (1) | 3.6 (4) | 0.8 (1) | ||
| Nature of perivulval changes | ||||||
| Skin discolouration | 28.8 (32) | 31.8 (34) | 0.187 (0.666) ‡ | 23.4 (26) | 38.7 (46) | 5.563 (0.018) ‡ |
| Hair discolouration | 2.7 (3) | 2.8 (3) | 0.9 (1) | 0.8 (1) | ||
| Skin and hair discolouration | 69.4 (77) | 67.3 (72) | 72.1 (80) | 59.7 (71) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moxon, R.; Freeman, S.L.; Payne, R.; Godfrey-Hunt, J.; Corr, S.; England, G.C.W. A Prospective Cohort Study Investigating the Impact of Neutering Bitches Prepubertally or Post-Pubertally on Physical Development. Animals 2023, 13, 1431. https://doi.org/10.3390/ani13091431
Moxon R, Freeman SL, Payne R, Godfrey-Hunt J, Corr S, England GCW. A Prospective Cohort Study Investigating the Impact of Neutering Bitches Prepubertally or Post-Pubertally on Physical Development. Animals. 2023; 13(9):1431. https://doi.org/10.3390/ani13091431
Chicago/Turabian StyleMoxon, Rachel, Sarah L. Freeman, Richard Payne, Jasmine Godfrey-Hunt, Sandra Corr, and Gary C. W. England. 2023. "A Prospective Cohort Study Investigating the Impact of Neutering Bitches Prepubertally or Post-Pubertally on Physical Development" Animals 13, no. 9: 1431. https://doi.org/10.3390/ani13091431
APA StyleMoxon, R., Freeman, S. L., Payne, R., Godfrey-Hunt, J., Corr, S., & England, G. C. W. (2023). A Prospective Cohort Study Investigating the Impact of Neutering Bitches Prepubertally or Post-Pubertally on Physical Development. Animals, 13(9), 1431. https://doi.org/10.3390/ani13091431






