Individual Monitoring of Activity and Lameness in Conventional and Slower-Growing Breeds of Broiler Chickens Using Accelerometers
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects, Housing, and Husbandry
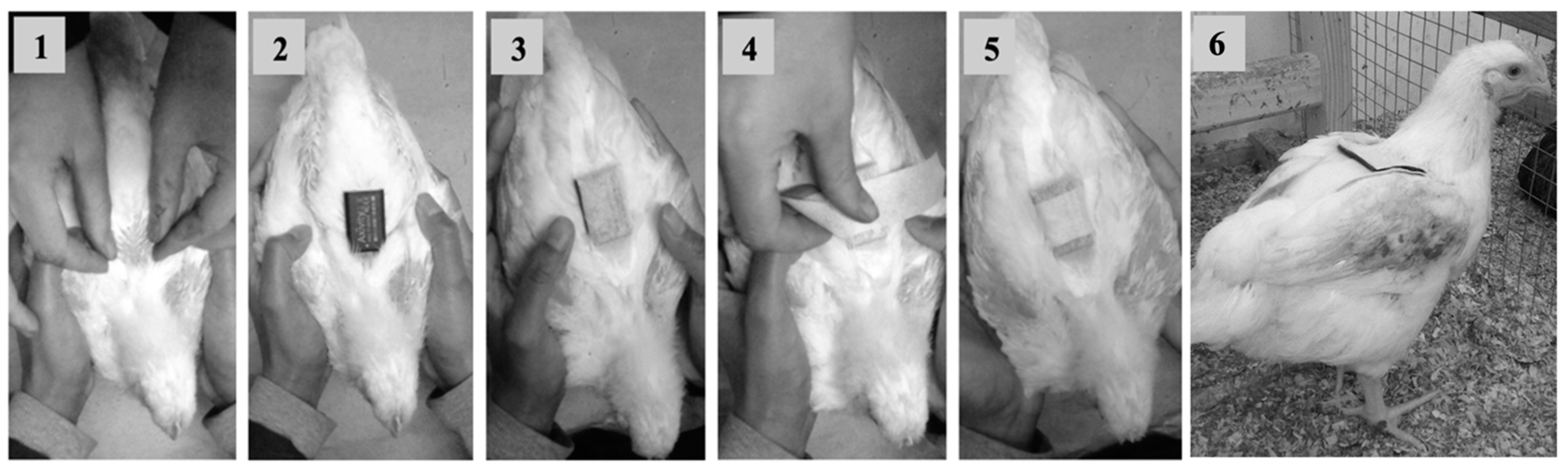
2.2. Accelerometer Attachment
2.3. Effects of Automated Monitoring Equipment on Behavioral Habituation
2.4. Welfare Assessment
2.5. Effects of Wearing an Accelerometer on Weight and Gait Score
2.6. Accelerometer Data Processing for Activity
2.7. Statistical Analysis
2.7.1. Evaluating the Effects of Wearing an Accelerometer on Behavior
2.7.2. Evaluating the Effects of Wearing an Accelerometer on Weight and Gait Score
2.7.3. Evaluating the Effect of Breed, Sex, and Weight on ActivityA
2.7.4. Evaluating the Association between ActivityA and Gait Score
3. Results
3.1. The Effect of Accelerometer Attachment on Behavior
3.2. The Effect of Accelerometer Attachment on Weight and on Gait Score
3.3. Evaluating the Effect of Breed, Sex, and Weight on ActivityA
3.4. Evaluating the Association between AcitivityA and Gait Score
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Caplen, G.; Hothersall, B.; Murrell, J.C.; Nicol, C.; Waterman-Pearson, A.E.; Weeks, C.; Colborne, G.R. Kinematic Analysis Quantifies Gait Abnormalities Associated with Lameness in Broiler Chickens and Identifies Evolutionary Gait Differences. PLoS ONE 2012, 7, e40800. [Google Scholar] [CrossRef]
- Bradshaw, R.; Kirkden, R.; Broom, D. A Review of the Aetiology and Pathology of Leg Weakness in Broilers in Relation to Welfare. Avian Poult. Biol. Rev. 2002, 13, 45–103. [Google Scholar] [CrossRef]
- Butterworth, A. Infectious components of broiler lameness: A review. Worlds Poult. Sci. J. 1999, 55, 327–352. [Google Scholar] [CrossRef]
- Sherlock, M.L.; Demmers, T.; Goodship, A.; McCarthy, I.; Wathes, C. The relationship between physical activity and leg health in the broiler chicken. Br. Poult. Sci. 2010, 51, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Shim, M.Y.; Karnuah, A.B.; Mitchell, A.D.; Anthony, N.B.; Pesti, G.M.; Aggrey, S.E. The effects of growth rate on leg morphology and tibia breaking strength, mineral density, mineral content, and bone ash in broilers. Poult. Sci. 2012, 91, 1790–1795. [Google Scholar] [CrossRef]
- Weeks, C.A.; Butterworth, A. Measuring and Auditing Broiler Welfare; CABI Publishing: Wallingford, UK, 2004. [Google Scholar]
- Granquist, E.G.; Vasdal, G.; de Jong, I.C.; Moe, R.O. Lameness and its relationship with health and production measures in broiler chickens. Animal 2019, 13, 2365–2372. [Google Scholar] [CrossRef]
- Weeks, C.; Danbury, T.; Davies, H.; Hunt, P.; Kestin, S. The behaviour of broiler chickens and its modification by lameness. Appl. Anim. Behav. Sci. 2000, 67, 111–125. [Google Scholar] [CrossRef] [PubMed]
- Heath, C.A.E.; Browne, W.J.; Mullan, S.; Main, D.C.J. Navigating the iceberg: Reducing the number of parameters within the Welfare Quality® assessment protocol for dairy cows. Animal 2014, 8, 1978–1986. [Google Scholar] [CrossRef]
- Mullan, S.; Browne, W.J.; Edwards, S.A.; Butterworth, A.; Whay, H.R.; Main, D.C.J. The effect of sampling strategy on the estimated prevalence of welfare outcome measures on finishing pig farms. Appl. Anim. Behav. Sci. 2009, 119, 39–48. [Google Scholar] [CrossRef]
- Abeyesinghe, S.; Chancellor, N.; Moore, D.H.; Chang, Y.-M.; Pearce, J.; Demmers, T.; Nicol, C. Associations between behaviour and health outcomes in conventional and slow-growing breeds of broiler chicken. Animal 2021, 15, 100261. [Google Scholar] [CrossRef]
- Kashiha, M.; Pluk, A.; Bahr, C.; Vranken, E.; Berckmans, D. Development of an early warning system for a broiler house using image interpretation. In Advances in Mass Data Analysis of Images and Signals in Medicine, Biotechnology, Chemistry and Food Industry, Proceedings of the 8th International Conference, MDA 2013, New York, New York, USA, 13–16 July 2013; Katholieke Universiteit Leuven: Leuven, Belgium, 2013; Volume 6, pp. 36–44. [Google Scholar]
- Dawkins, M.S.; Lee, H.-J.; Waitt, C.D.; Roberts, S.J. Optical flow patterns in broiler chicken flocks as automated measures of behaviour and gait. Appl. Anim. Behav. Sci. 2009, 119, 203–209. [Google Scholar] [CrossRef]
- Dawkins, M.S.; Roberts, S.J.; Cain, R.J.; Nickson, T.; Donnelly, C.A. Early warning of footpad dermatitis and hockburn in broiler chicken flocks using optical flow, bodyweight and water consumption. Veter. Rec. 2017, 180, 499. [Google Scholar] [CrossRef] [PubMed]
- Fraser, D. Understanding animal welfare. Acta Veter. Scand. 2008, 50, S1. [Google Scholar] [CrossRef]
- Hewson, C.J. Bien-être des animaux: Quelques définitions et courantes et leurs incidences. Can. Vet. J. 2003, 44, 496–499. [Google Scholar]
- Brown, D.D.; Kays, R.; Wikelski, M.; Wilson, R.P.; Klimley, A.P. Observing the unwatchable through acceleration logging of animal behavior. Anim. Biotelemetry 2013, 1, 20. [Google Scholar] [CrossRef]
- Nathan, R.; Spiegel, O.; Fortmann-Roe, S.; Harel, R.; Wikelski, M.; Getz, W.M. Using tri-axial acceleration data to identify behavioral modes of free-ranging animals: General concepts and tools illustrated for griffon vultures. J. Exp. Biol. 2012, 215, 986–996. [Google Scholar] [CrossRef]
- Shepard, E.; Wilson, R.; Quintana, F.; Laich, A.G.; Liebsch, N.; Albareda, D.; Halsey, L.; Gleiss, A.; Morgan, D.; Myers, A.; et al. Identification of animal movement patterns using tri-axial accelerometry. Endanger. Species Res. 2008, 10, 47–60. [Google Scholar] [CrossRef]
- Dixon, L.M. Slow and steady wins the race: The behaviour and welfare of commercial faster growing broiler breeds compared to a commercial slower growing breed. PLoS ONE 2020, 15, e0231006. [Google Scholar] [CrossRef]
- Bokkers, E.A.; Koene, P. Behaviour of fast- and slow growing broilers to 12 weeks of age and the physical consequences. Appl. Anim. Behav. Sci. 2003, 81, 59–72. [Google Scholar] [CrossRef]
- Caspersen, C.J.; Powell, K.E.; Christenson, G.M. Physical activity, exercise, and physical fitness: Definitions and distinctions for health-related research. Public Health Rep. 1985, 100, 126–131. [Google Scholar]
- Miwa, M.; Oishi, K.; Nakagawa, Y.; Maeno, H.; Anzai, H.; Kumagai, H.; Okano, K.; Tobioka, H.; Hirooka, H. Application of Overall Dynamic Body Acceleration as a Proxy for Estimating the Energy Expenditure of Grazing Farm Animals: Relationship with Heart Rate. PLoS ONE 2015, 10, e0128042. [Google Scholar] [CrossRef] [PubMed]
- Fehlmann, G.; O’riain, M.J.; Hopkins, P.W.; O’sullivan, J.; Holton, M.D.; Shepard, E.L.C.; King, A.J. Identification of behaviours from accelerometer data in a wild social primate. Anim. Biotelemetry 2017, 5, 6. [Google Scholar] [CrossRef]
- Halsey, L.G.; Green, J.A.; Wilson, R.P.; Frappell, P.B. Accelerometry to Estimate Energy Expenditure during Activity: Best Practice with Data Loggers. Physiol. Biochem. Zool. 2009, 82, 396–404. [Google Scholar] [CrossRef]
- Okada, H.; Suzuki, K.; Kenji, T.; Itoh, T. Applicability of Wireless Activity Sensor Network to Avian Influenza Monitoring System in Poultry Farms. J. Sens. Technol. 2014, 4, 18–23. [Google Scholar] [CrossRef]
- Dennis, R.; Fahey, A.; Cheng, H. Different Effects of Individual Identification Systems on Chicken Well-Being. Poult. Sci. 2008, 87, 1052–1057. [Google Scholar] [CrossRef] [PubMed]
- Rault, J.-L.; Taylor, P.S. Indoor side fidelity and outdoor ranging in commercial free-range chickens in single- or double-sided sheds. Appl. Anim. Behav. Sci. 2017, 194, 48–53. [Google Scholar] [CrossRef]
- Banerjee, D.; Biswas, S.; Daigle, C.; Siegford, J.M. Remote Activity Classification of Hens Using Wireless Body Mounted Sensors. In Proceedings of the 2012 Ninth International Conference on Wearable and Implantable Body Sensor Networks, London, UK, 9–12 May 2012; pp. 107–112. [Google Scholar] [CrossRef]
- Buijs, S.; Booth, F.; Richards, G.; McGaughey, L.; Nicol, C.J.; Edgar, J.; Tarlton, J.F. Behavioural and physiological responses of laying hens to automated monitoring equipment. Appl. Anim. Behav. Sci. 2017, 199, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, D.; Daigle, C.L.; Dong, B.; Wurtz, K.; Newberry, R.; Siegford, J.; Biswas, S. Detection of jumping and landing force in laying hens using wireless wearable sensors. Poult. Sci. 2014, 93, 2724–2733. [Google Scholar] [CrossRef]
- Stadig, L.M.; Rodenburg, T.B.; Ampe, B.; Reubens, B.; Tuyttens, F.A. An automated positioning system for monitoring chickens’ location: Effects of wearing a backpack on behaviour, leg health and production. Appl. Anim. Behav. Sci. 2018, 198, 83–88. [Google Scholar] [CrossRef]
- Dawson, L.C.; Widowski, T.; Liu, Z.; Edwards, A.; Torrey, S. In pursuit of a better broiler: A comparison of the inactivity, behavior, and enrichment use of fast- and slower-growing broiler chickens. Poult. Sci. 2021, 100, 101451. [Google Scholar] [CrossRef]
- Hall, J.; Abeyesinghe, S.; Daley, M. Quantitative analysis of locomotion as an indicator of bird personality. In Proceedings of the Measuring Animal Emotion Workshop, the 50th Congress of the International Society for Applied Ethology, Edinburgh, UK, 12 July 2016. [Google Scholar]
- Venäläinen, E.; Valaja, J.; Jalava, T. Effects of dietary metabolisable energy, calcium and phosphorus on bone mineralisation, leg weakness and performance of broiler chickens. Br. Poult. Sci. 2006, 47, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Kestin, S.C.; Gordon, S.; Su, G. Relationships in broiler chickens between lameness, liveweight, growth rate and age. Vet. Rec. 2001, 148, 195–198. [Google Scholar] [CrossRef] [PubMed]
- Fernández, A.P.; Norton, T.; Tullo, E.; van Hertem, T.; Youssef, A.; Exadaktylos, V.; Vranken, E.; Guarino, M.; Berckmans, D. Real-time monitoring of broiler flock’s welfare status using camera-based technology. Biosyst. Eng. 2018, 173, 103–114. [Google Scholar] [CrossRef]
- Wathes, C.; Kristensen, H.; Aerts, J.-M.; Berckmans, D. Is precision livestock farming an engineer’s daydream or nightmare, an animal’s friend or foe, and a farmer’s panacea or pitfall? Comput. Electron. Agric. 2008, 64, 2–10. [Google Scholar] [CrossRef]
- RSPCA. Broiler Breed Welfare Assessment Protocol. 2017. Available online: https://science.rspca.org.uk/sciencegroup/farmanimals/standards/chickens (accessed on 26 March 2021).
- Hall, J. Unpublished Thesis: Quantitative Analysis of Locomotion as an Indicator of Bird Personality; Royal Veterinary College: London, UK, 2018. [Google Scholar]
- Chielo, L.I.; Pike, T.; Cooper, J. Ranging behavior of commercial free-range laying hens. Animals 2016, 6, 28. [Google Scholar] [CrossRef]
- Villagrá, A.; Olivas, I.; Althaus, R.; Gómez, E.; Láinez, M.; Torres, A. Behavior of broiler chickens in four different substrates: A choice test. Braz. J. Poult. Sci. 2014, 16, 67–75. [Google Scholar] [CrossRef]
- Kestin, S.C.; Knowles, T.; Tinch, A.E.; Gregory, N.G. Prevalence of leg weakness in broiler chickens and its relationship with genotype. Veter. Rec. 1992, 131, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Menke, W.; Menke, J. 9—Detecting correlations among data. In Environmental Data Analysis with MatLab; Academic Press: Cambridge, MA, USA, 2012; pp. 167–201. [Google Scholar]
- Green, J.A.; Halsey, L.; Wilson, R.P.; Frappell, P.B. Estimating energy expenditure of animals using the accelerometry technique: Activity, inactivity and comparison with the heart-rate technique. J. Exp. Biol. 2009, 212, 471–482. [Google Scholar] [CrossRef]
- Gervasi, V.; Brunberg, S.; Swenson, J.E. An Individual-Based Method to Measure Animal Activity Levels: A Test on Brown Bears. Wildl. Soc. Bull. 2006, 34, 1314–1319. [Google Scholar] [CrossRef]
- Leppink, J.; van Merriënboer, J.J.G. The beast of aggregating cognitive load measures in technology-based learning. Educ. Technol. Soc. 2015, 18, 230–245. [Google Scholar]
- Baxter, M.; Richmond, A.; Lavery, U.; O’Connell, N.E. A comparison of fast growing broiler chickens with a slower-growing breed type reared on Higher Welfare commercial farms. PLoS ONE 2021, 16, e0259333. [Google Scholar] [CrossRef]
- Aydin, A.; Cangar, O.; Ozcan, S.E.; Bahr, C.; Berckmans, D. Application of a fully automatic analysis tool to assess the activity of broiler chickens with different gait scores. Comput. Electron. Agric. 2010, 73, 194–199. [Google Scholar] [CrossRef]
- Van Hertem, T.; Norton, T.; Berckmans, D.; Vranken, E. Predicting broiler gait scores from activity monitoring and flock data. Biosyst. Eng. 2018, 173, 93–102. [Google Scholar] [CrossRef]
- Silvera, A.M.; Knowles, T.; Butterworth, A.; Berckmans, D.; Vranken, E.; Blokhuis, H. Lameness assessment with automatic monitoring of activity in commercial broiler flocks. Poult. Sci. 2017, 96, 2013–2017. [Google Scholar] [CrossRef] [PubMed]
- Paxton, H.; Daley, M.A.; Corr, S.A.; Hutchinson, J.R. The gait dynamics of the modern broiler chicken: A cautionary tale of selective breeding. J. Exp. Biol. 2013, 216, 3237–3248. [Google Scholar] [CrossRef] [PubMed]
- Paxton, H.; Anthony, N.B.; Corr, S.A.; Hutchinson, J.R. The effects of selective breeding on the architectural properties of the pelvic limb in broiler chickens: A comparative study across modern and ancestral populations. J. Anat. 2010, 217, 153–166. [Google Scholar] [CrossRef]
- Abdoli, A.; Alaee, S.; Imani, S.; Murillo, A.; Gerry, A.; Hickle, L.; Keogh, E. Fitbit for Chickens? Time Series Data Mining Can Increase the Productivity of Poultry Farms. In Proceedings of the Proceedings of the 26th ACM SIGKDD International Conference on Knowledge Discovery & Data Mining, Virtual Event, 23–27 August 2020. [Google Scholar] [CrossRef]
- Barwick, J.; Lamb, D.; Dobos, R.; Schneider, D.; Welch, M.; Trotter, M. Predicting Lameness in Sheep Activity Using Tri-Axial Acceleration Signals. Animals 2018, 8, 12. [Google Scholar] [CrossRef]


| Age (Days) | 26 | 27 | 28 | 29 | 30 | 31 | 32 | 33 | 34 | 35 | 36 | 37 | 38 | 39 | 40 | 41 | 42 | 43 | 44 | 45 | 46 | 47 | 48 | 49 | 50 | 51 | 52 | 53 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CNV (N = 15) | A | D | A | D | WA | |||||||||||||||||||||||
| SGH (N = 25) | A | D | A | D | A | D | WA | A | D 1 | |||||||||||||||||||
| SGN (N = 15) | A | D | A | D | A | D | A | D | WA |
| Behaviors | Definition (Modified from An Ethogram Supplied by University of Guelph as Part of An Alignment of Methods in the Wider Study [11]) |
|---|---|
| State Behaviors (Mutually Exclusive) Measured as Frequencies | |
| Walking | Slow movement forward where one foot is always placed on the ground and breast is above ground. Start from movement, a slight shift in body weight just before foot is raised off ground. Ends when both feet are placed onto the ground and when neither foot has moved for 2 s or, when another behavior commences. |
| Sit Inactive | Sat down, immobile with entire breast touching the ground and legs tucked underneath bird. Start from cessation of movement for 2 s. Ends when another state behavior commences (event behaviors can occur simultaneously). |
| Standing | Immobile on both legs with body not touching ground. Start from cessation of movement for 2 s. Ends when another state behavior commences (event behaviors can occur simultaneously) (modified from [41]). |
| Feeding | Downward pecking in feeder while sitting or standing. Start from the first peck at feed. Ends when bird has not pecked at feed for 3 s or when another behavior commences (modified from [42]). |
| Drinking | Downward pecking in drinker while sitting or standing. Start from the first peck in drinker defined as direct beak contact with water. Ends when bird has not lowered head to drink for 3 s or when another behavior commences. |
| Preen Sitting | Moving the beak through feathers while sitting. Start at the first movement of beak moving through feathers. Ends when beak loses contact with feathers for 3 s or when another behavior commences. |
| Preen Standing | Moving the beak through the feather while standing. Start at the first movement of beak moving through feathers. Ends when beak loses contact with feathers for 3 s or when another behavior commences. |
| Gait Score | Definition |
|---|---|
| 0 | The bird displays smooth, fluid locomotion. Typically, the foot is picked up and put down smoothly and each foot is brought under the bird’s center of gravity as it walks (rather than the bird swaying). Often, the toes are partially curled while the foot is in the air. |
| 1 | The bird has a slight defect in its gait that is difficult to define precisely. The bird may take unduly large strides, be unsteady, or wobble when it walks, which produces an uneven gait, but the problem leg is unclear/cannot be easily identified. |
| 2 | The bird has a definite and identifiable gait abnormality, but this does not affect its ability to move. The bird may make short, quick, unsteady steps with one leg, but is not sufficiently lame to seriously compromise its ability to move, i.e., maneuver, accelerate, and run. |
| 3 | The bird has an obvious gait defect that affects its ability to move. The bird may have a limp, jerky, or unsteady strut, or splay one leg as it moves. The bird often prefers to squat when not coerced to move and will not run. |
| 4 | The bird has a severe gait defect. The bird is capable of walking, but only with difficulty and when driven or strongly motivated. Otherwise, it squats down at the first available opportunity. |
| 5 | The bird is incapable of sustained walking on its feet. Although it may be able to stand, the bird cannot walk except with the assistance of the wings or by crawling on the shanks. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pearce, J.; Chang, Y.-M.; Abeyesinghe, S. Individual Monitoring of Activity and Lameness in Conventional and Slower-Growing Breeds of Broiler Chickens Using Accelerometers. Animals 2023, 13, 1432. https://doi.org/10.3390/ani13091432
Pearce J, Chang Y-M, Abeyesinghe S. Individual Monitoring of Activity and Lameness in Conventional and Slower-Growing Breeds of Broiler Chickens Using Accelerometers. Animals. 2023; 13(9):1432. https://doi.org/10.3390/ani13091432
Chicago/Turabian StylePearce, Justine, Yu-Mei Chang, and Siobhan Abeyesinghe. 2023. "Individual Monitoring of Activity and Lameness in Conventional and Slower-Growing Breeds of Broiler Chickens Using Accelerometers" Animals 13, no. 9: 1432. https://doi.org/10.3390/ani13091432
APA StylePearce, J., Chang, Y.-M., & Abeyesinghe, S. (2023). Individual Monitoring of Activity and Lameness in Conventional and Slower-Growing Breeds of Broiler Chickens Using Accelerometers. Animals, 13(9), 1432. https://doi.org/10.3390/ani13091432






