Evaluation of the Analgesic Efficacy of Undiluted Intraperitoneal and Incisional Ropivacaine for Postoperative Analgesia in Dogs after Major Abdominal Surgery
Abstract
:Simple Summary
Abstract
1. Introduction
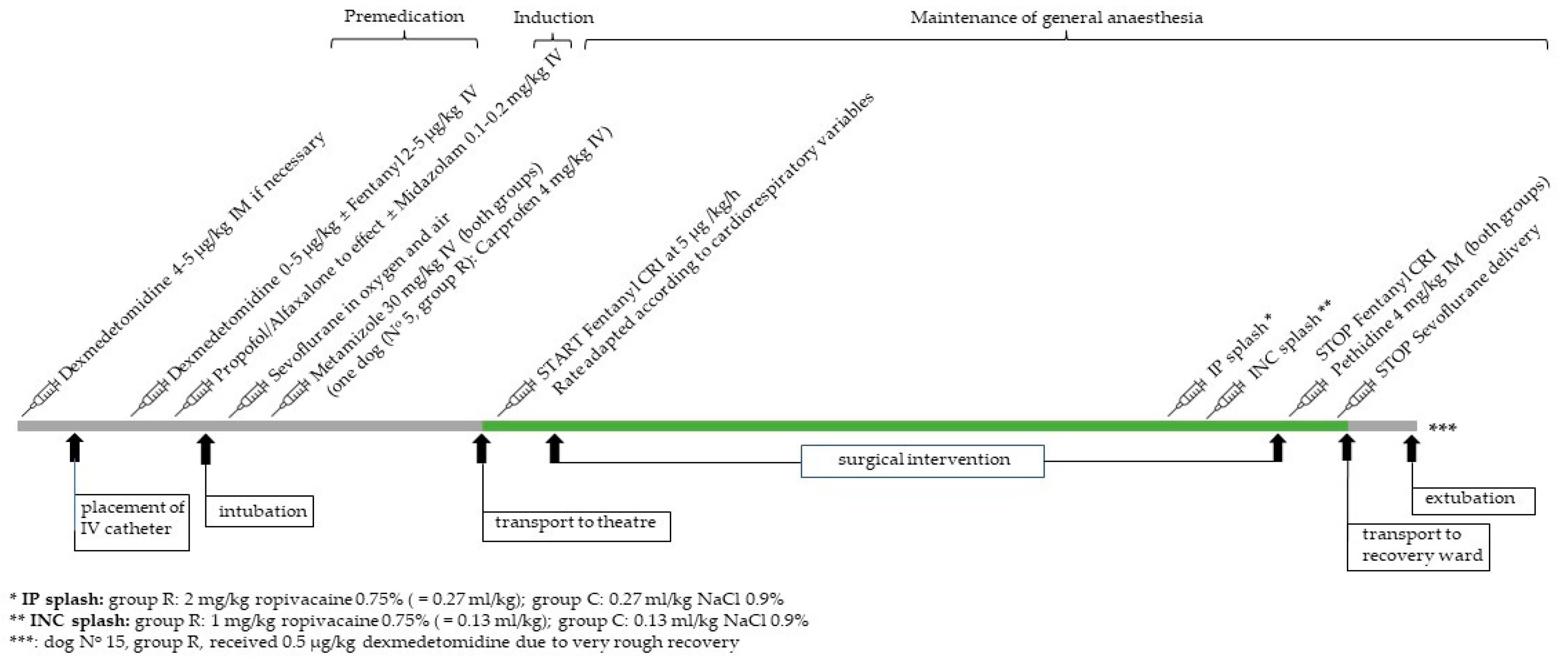
2. Materials and Methods
2.1. Animals
2.2. Anaesthesia
2.3. Postoperative Measurements
2.4. Statistical Methods
3. Results
4. Discussion
4.1. Study Design
4.2. Local Anaesthetic Protocol
4.3. Pain Scores: GCPS-SF and DIVAS
4.4. MNT in Dogs
4.5. Sedation Scores
4.6. Limitations
4.6.1. Analgesic Protocol
4.6.2. Factors Influencing Sedation and Pain Scores
4.7. Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Perret-Gentil, F.; Doherr, M.; Spadavecchia, C.; Levionnois, O. Attitudes of Swiss veterinarians towards pain and analgesia in dogs and cats. Schweiz Arch Tierheilkd. 2014, 156, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Mathews, K.; Kronen, P.W.; Lascelles, D.; Nolan, A.; Robertson, S.; Steagall, P.V.; Wright, B.; Yamashita, K. Guidelines for Recognition, Assessment and Treatment of Pain. J. Small Anim. Pract. 2014, 55, E10–E68. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, R.E.; Wilson, D.V.; Evans, A.T. Evaluation of intraperitoneal and incisional lidocaine or bupivacaine for analgesia following ovariohysterectomy in the dog. Vet. Anaesth. Analg. 2004, 31, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Campagnol, D.; Teixeira-Neto, F.J.; Monteiro, E.R.; Restitutti, F.; Minto, B.W. Effect of intraperitoneal or incisional bupivacaine on pain and the analgesic requirement after ovariohysterectomy in dogs. Vet. Anaesth. Analg. 2012, 39, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Lambertini, C.; Kluge, K.; Lanza-Perea, M.; Bruhl-Day, R.; Guerrero, K.S.K. Comparison of intraperitoneal ropivacaine and bupivacaine for postoperative analgesia in dogs undergoing ovariohysterectomy. Vet. Anaesth. Analg. 2018, 45, 865–870. [Google Scholar] [CrossRef] [PubMed]
- Khanzadeh Alishahi, M. Evaluation of Intraperitoneal Ropivacaine for Postoperative Analgesia Following Ovariohysterectomy in Dogs. Ph.D. Thesis, University of Pretoria, Pretoria, South Africa, 2018. [Google Scholar]
- Dony, P.; Dewinde, V.; Vanderick, B.; Cuignet, O.; Gautier, P.; Legrand, E.; Lavand’homme, P.; De Kock, M. The comparative toxicity of ropivacaine and bupivacaine at equipotent doses in rats. Anesth. Analg. 2000, 91, 1489–1492. [Google Scholar] [CrossRef]
- Moller, R.; Covino, B.G. Cardiac Electrophysiologic Properties of Bupivacaine and Lidocaine Compared with Those of Ropivacaine, A New Amide Local Anesthetic. Anesthesiology 1990, 72, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Cavaco, J.S.; Otero, P.E.; Ambrósio, A.M.; Neves, I.C.B.; Perencin, F.M.; Pereira, M.A.A.; Matera, J.M.; Fantoni, D.T. Analgesic efficacy of ultrasound-guided transversus abdominis plane block in dogs undergoing ovariectomy. Front. Vet. Sci. 2022, 9, 1031345. [Google Scholar] [CrossRef]
- Viscasillas, J.; Sanchis-Mora, S.; Burillo, P.; Esteve, V.; Del Romero, A.; Lafuente, P.; Redondo, J.I. Evaluation of Quadratus Lumborum Block as Part of an Opioid-Free Anaesthesia for Canine Ovariohysterectomy. Animals 2021, 11, 3424. [Google Scholar] [CrossRef]
- Steagall, P.; Benito, J.; Monteiro, B.; Lascelles, D.; Kronen, P.; Murrell, J.; Robertson, S.; Wright, B.; Yamashita, K. Intraperitoneal and incisional analgesia in small animals: Simple, cost-effective techniques. J. Small Anim. Pract. 2020, 61, 19–23. [Google Scholar] [CrossRef]
- Monteiro, B.P.; Lascelles, B.D.X.; Murrell, J.; Robertson, S.; Steagall, P.V.M.; Wright, B. 2022 WSAVA guidelines for the recognition, assessment and treatment of pain. J. Small Anim. Pract. 2022, 64, 177–254. [Google Scholar] [CrossRef]
- Muir, W.W.; Berry, J.; Merton-Boothe, D.; Brown, D.; Buffington, T.; Campbell, S.; Driessen, B.; Epstein, M.; Gaynor, G.; Grubb, T.; et al. Opioid-Sparing Pain Therapy in Animals: Working Task Force; FDA: Rockville, MD, USA, 2018. [Google Scholar]
- Reid, J.; Nolan, A.; Hughes, J.; Lascelles, D.; Pawson, P.; Scott, E. Development of the short-form Glasgow Composite Measure Pain Scale (CMPS-SF) and derivation of an analgesic intervention score. Anim. Welf. 2007, 16, 97. [Google Scholar] [CrossRef]
- Barletta, M.; Young, C.N.; Quandt, J.E.; Hofmeister, E.H. Agreement between veterinary students and anesthesiologists regarding postoperative pain assessment in dogs. Vet. Anaesth. Analg. 2016, 43, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Coleman, K.D.; Schmiedt, C.W.; Kirkby, K.A.; Coleman, A.E.; Robertson, S.A.; Hash, J.; Lascelles, B.D.X. Learning confounds algometric assessment of mechanical thresholds in normal dogs. Vet. Surg. 2014, 43, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Grint, N.J.; Alderson, B.; Dugdale, A.H. A comparison of acepromazine-buprenorphine and medetomidine-buprenorphine for preanesthetic medication of dogs. J. Am. Vet. Med. Assoc. 2010, 237, 1431–1437. [Google Scholar] [CrossRef]
- Wagner, M.C.; Hecker, K.G.; Pang, D.S. Sedation levels in dogs: A validation study. BMC Vet. Res. 2017, 13, 110. [Google Scholar] [CrossRef]
- Guerrero, K.S.K.; Campagna, I.; Bruhl-Day, R.; Hegamin-Younger, C.; Guerrero, T.G. Intraperitoneal bupivacaine with or without incisional bupivacaine for postoperative analgesia in dogs undergoing ovariohysterectomy. Vet. Anaesth. Analg. 2016, 43, 571–578. [Google Scholar] [CrossRef]
- Gomes, D.R.; Nicácio, I.P.; Cerazo, L.M.; Dourado, L.; Teixeira-Neto, F.J.; Cassu, R.N. Addition of magnesium sulfate to intraperitoneal ropivacaine for perioperative analgesia in canine ovariohysterectomy. J. Vet. Pharm. Ther. 2020, 43, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Gerbershagen, H.J.; Aduckathil, S.; van Wijck, A.J.M.; Peelen, L.M.; Kalkman, C.J.; Meissner, W. Pain Intensity on the First Day after Surgery: A Prospective Cohort Study Comparing 179 Surgical Procedures. Anesthesiology 2013, 118, 934–944. [Google Scholar] [CrossRef]
- Costa, G.L.; Nastasi, B.; Spadola, F.; Leonardi, F.; Interlandi, C. Effect of levobupivacaine, administered intraperitoneally, on physiological variables and on intrasurgery and postsurgery pain in dogs undergoing ovariohysterectomy. J. Vet. Behav. 2019, 30, 33–36. [Google Scholar] [CrossRef]
- Korkmaz, M.; Yilmaz, O.; Saritas, Z.K.; Demirkan, I.; Jaroszewski, J. Evaluation of intraperitoneal and incisional bupivacaine or levobupivacaine for postoperative analgesia in ovariohysterectomized dogs. Acta Sci. Vet. 2019, 47, 1666. [Google Scholar] [CrossRef]
- Labaille, T.; Mazoit, J.X.; Paqueron, X.; Franco, D.; Benhamou, D. The clinical efficacy and pharmacokinetics of intraperitoneal ropivacaine for laparoscopic cholecystectomy. Anesth Analg. 2002, 94, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Lascelles, B.D.X.; Cripps, P.J.; Jones, A.; Waterman-Pearson, A.E. Efficacy and kinetics of carprofen, administered preoperatively or postoperatively, for the prevention of pain in dogs undergoing ovariohysterectomy. Vet. Surg. 1998, 27, 568–582. [Google Scholar] [CrossRef] [PubMed]
- Duerr, F.M.; Twedt, D.C.; Monnet, E. Changes in pH of peritoneal fluid associated with carbon dioxide insufflation during laparoscopic surgery in dogs. Am. J. Vet. Res. 2008, 69, 298–301. [Google Scholar] [CrossRef] [PubMed]
- Moretz, W.H.; Erickson, W.G. Neutralization of Hydrochloric Acid in the Peritoneal Cavity. AMA Arch. Surg. 1957, 75, 834–837. [Google Scholar] [CrossRef] [PubMed]
- Briley, J.D.; Williams, M.D.; Freire, M.; Griffith, E.H.; Lascelles, B.D.X. Feasibility and repeatability of cold and mechanical quantitative sensory testing in normal dogs. Vet. J. 2014, 199, 245–250. [Google Scholar] [CrossRef]
- McKune, C.M.; Pascoe, P.J.; Lascelles, B.D.; Kass, P.H. The challenge of evaluating pain and a pre-incisional local anesthetic block. PeerJ 2014, 2, e341. [Google Scholar] [CrossRef]
- KuKanich, B.; Lascelles, B.D.; Papich, M.G. Assessment of a von Frey device for evaluation of the antinociceptive effects of morphine and its application in pharmacodynamic modeling of morphine in dogs. Am. J. Vet. Res. 2005, 66, 1616–1622. [Google Scholar] [CrossRef]
- Slingsby, L.S.; Taylor, P.M.; Murrell, J.C. A study to evaluate buprenorphine at 40 μg kg−1 compared to 20 μg kg−1 as a post-operative analgesic in the dog. Vet. Anaesth. Analg. 2011, 38, 584–593. [Google Scholar] [CrossRef]
- Harris, L.K.; Murrell, J.C.; van Klink, E.G.M.; Whay, H.R. Influence of experimental protocol on response rate and repeatability of mechanical threshold testing in dogs. Vet. J. 2015, 204, 82–87. [Google Scholar] [CrossRef]
- Dent, B.T.; Aarnes, T.K.; Wavreille, V.A.; Lakritz, J.; Lerche, P.; KuKanich, B.; Pereira, C.H.R.; Bednarski, R.M. Pharmacokinetics and pharmacodynamic effects of oral transmucosal and intravenous administration of dexmedetomidine in dogs. Am. J. Vet. Res. 2019, 80, 969–975. [Google Scholar] [CrossRef]
- Aarnes, T.K.; Dent, B.T.; Lakritz, J.; KuKanich, B.; Wavreille, V.A.; Lerche, P.; Pereira, C.H.R.; Bednarski, R.M. Pharmacokinetics and pharmacodynamics of intramuscular dexmedetomidine in dogs. Am. J. Vet. Res. 2023, 84. [Google Scholar] [CrossRef] [PubMed]
- Kalchofner Guerrero, K.S.; Schwarz, A.; Wuhrmann, R.; Feldmann, S.; Hartnack, S.; Bettschart-Wolfensberger, R. Comparison of a new metamizole formulation and carprofen for extended post-operative analgesia in dogs undergoing ovariohysterectomy. Vet. J. 2015, 204, 99–104. [Google Scholar] [CrossRef]
- Vettorato, E.; Bacco, S. A comparison of the sedative and analgesic properties of pethidine (meperidine) and butorphanol in dogs. J. Small Anim. Pract. 2011, 52, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Waterman, A.E.; Kalthum, W. Pharmacokinetics of pethidine administered intramuscularly and intravenously to dogs over 10 years old. Res. Vet. Sci. 1990, 48, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Ellwood, B.; Murison, P.J. Investigating the effect of anxiety on pain scores in dogs. Vet. Anaesth. Analg. 2022, 49, 135–142. [Google Scholar] [CrossRef]
- Grouper, H.; Eisenberg, E.; Pud, D. More Insight on the Role of Personality Traits and Sensitivity to Experimental Pain. J. Pain Res. 2021, 14, 1837. [Google Scholar] [CrossRef] [PubMed]
- Gruen, M.E.; White, P.; Hare, B. Do dog breeds differ in pain sensitivity? Veterinarians and the public believe they do. PLoS ONE 2020, 15, e0230315. [Google Scholar] [CrossRef] [PubMed]

| Group Ropivacaine | Group Control | p-Value | |
|---|---|---|---|
| Age (months) | 75 (9–135) | 59 (6–122) | 0.77 |
| Bodyweight (kg) | 13 (6.8–30) | 9 (2.5–33) | 0.94 |
| Duration of anaesthesia (minutes) | 134 (106–170) | 124 (111–208) | 0.32 |
| Duration of surgery (minutes) | 70 (50–85) | 66 (43–123) | 0.52 |
| Ratio LOI: XTP (%) | 56 (36–71) | 55 (32–85) | 0.85 |
| NRS of anticipated pain intensity (surgeon) | 3.5 (3–5) | 3 (3–4) | 0.23 |
| Dog N° and Group | Breed | Premedication | Induction | Procedure | |||
|---|---|---|---|---|---|---|---|
| FEN (µg/kg) | DEX (µg/kg) | PRO (mg/kg) | ALF (mg/kg) | MIDA (mg/kg) | |||
| 1C | Irish Soft Coated Wheaten Terrier | 4.0 | 5.0 IV | 2.0 | - | 0.2 | Partial pancreatectomy |
| 2C | Chihuahua | 5.0 | 2.0 IV | 1.0 | - | 0.2 | Nephrectomy |
| 3R | Mixed breed | 5.0 | - | 2.5 | - | 0.2 | Adrenalectomy |
| 4R | German Pinscher | 3.0 | 5.0 IM | - | 0.25 | 0.2 | Splenectomy, Liver biopsies |
| 5R | Dachshund | 5.0 | 2.0 IV | 1.0 | - | 0.2 | Cystotomy |
| 6C | Podenco Ibicenco | 5.0 | 1.0 IV | 1.0 | - | 0.2 | Splenectomy, Liver biopsies |
| 7C | Mixed breed | 5.0 | 2.0 IV | 0.75 | - | 0.2 | Ureteroneostomy |
| 8R | Miniature Schnauzer | 5.0 | - | 3.0 | - | - | Closure of portosystemic shunt, Ovariectomy |
| 9C | Havanese | 5.0 | - | - | 2.0 | - | Closure of portosystemic shunt |
| 10R | Mixed breed | 5.0 | 4.0 IM | 2.0 | - | - | Closure of portosystemic shunt |
| 11C | Mixed breed | 5.0 | 5.0 IM | 2.0 | - | - | Closure of portosystemic shunt |
| 12R | Basset | - | 5.0 IM | 0.75 | - | 0.1 | Cholecystectomy |
| 13C | Mixed breed | 2.0 | 0.5 IV | 2.0 | - | 0.2 | Splenectomy |
| 14R | Boxer | 5.0 | 0.5 IV | - | 1.25 | 0.2 | Nephrectomy |
| 15R | West Highland White Terrier | 5.0 | 2.0 IV | - | 0.5 | 0.2 | Ovariohysterectomy (metropathy) |
| 16C | Weimaraner | 5.0 | 2.0 IV | - | 0.75 | 0.2 | Enterotomy |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Henze, I.S.; Navarro Altuna, V.; Steiger, J.I.; Torgerson, P.R.; Kutter, A.P.N. Evaluation of the Analgesic Efficacy of Undiluted Intraperitoneal and Incisional Ropivacaine for Postoperative Analgesia in Dogs after Major Abdominal Surgery. Animals 2023, 13, 1489. https://doi.org/10.3390/ani13091489
Henze IS, Navarro Altuna V, Steiger JI, Torgerson PR, Kutter APN. Evaluation of the Analgesic Efficacy of Undiluted Intraperitoneal and Incisional Ropivacaine for Postoperative Analgesia in Dogs after Major Abdominal Surgery. Animals. 2023; 13(9):1489. https://doi.org/10.3390/ani13091489
Chicago/Turabian StyleHenze, Inken S., Victoria Navarro Altuna, Joëlle I. Steiger, Paul R. Torgerson, and Annette P. N. Kutter. 2023. "Evaluation of the Analgesic Efficacy of Undiluted Intraperitoneal and Incisional Ropivacaine for Postoperative Analgesia in Dogs after Major Abdominal Surgery" Animals 13, no. 9: 1489. https://doi.org/10.3390/ani13091489







