Associations between Pleurisy and the Main Bacterial Pathogens of the Porcine Respiratory Diseases Complex (PRDC)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
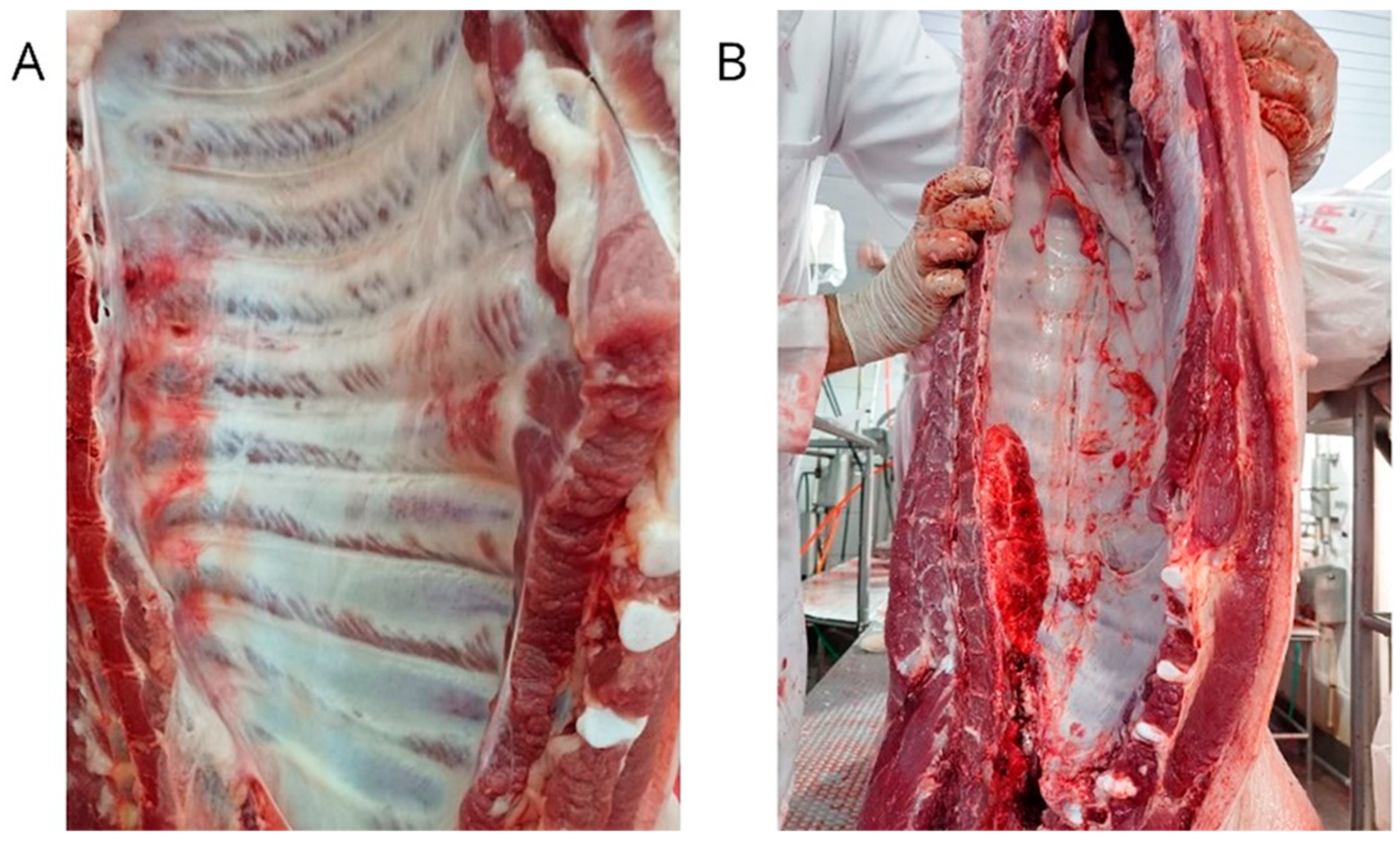
2.1. Study Design, Lung and Carcass Evaluation, and Sample Collection
2.2. DNA Extraction and PCR for the Gadph Gene
2.3. Multiplex qPCR for A. pleuropneumoniae, M. hyopneumoniae and P. multocida
2.4. qPCR Assays for G. parasuis and S. suis
2.5. Data Analysis
3. Results
3.1. Lungs and Carcass Evaluation
3.2. Multiplex qPCR Assays for A. pleuropneumoniae, M. hyopneumoniae, P. multocida, and qPCR Assays for G. parasuis and S. suis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Opriessnig, T.; Giménez-Lirola, L.G.; Halbur, P.G. Polymicrobial respiratory disease in pigs. Anim. Health Res. Rev. 2011, 12, 133–148. [Google Scholar] [CrossRef]
- Thacker, E.L. Immunology of the porcine respiratory disease complex. Vet. Clin. North Am. Food Anim. Pract. 2001, 17, 551–565. [Google Scholar] [CrossRef] [PubMed]
- Saade, G.; Deblanc, C.; Bougon, J.; Marois-Créhan, C.; Fablet, C.; Auray, G.; Belloc, C.; Leblanc-Maridor, M.; Gagnon, C.A.; Zhu, J.; et al. Coinfections and their molecular consequences in the porcine respiratory tract. Vet. Res. 2020, 51, 80. [Google Scholar] [CrossRef] [PubMed]
- Calderón Díaz, J.A.; Rodrigues da Costa, M.; Shalloo, L.; Niemi, J.K.; Leonard, F.C.; Crespo-Piazuelo, D.; Gasa, G.; Manzanilla, E.G. A bio-economic simulation study on the association between key performance indicators and pluck lesions in Irish farrow-to-finish pig farms. Porc. Health Manag. 2020, 6, 40. [Google Scholar] [CrossRef]
- Pagot, E.; Pommier, P.; Keïta, A. Relationship between groth during the fattening period and lung lesions at slaughter in swine. Revue Méd. Vet. 2007, 5, 253–259. [Google Scholar]
- Fablet, C.; Marois-Créhan, C.; Simon, G.; Grasland, B.; Jestin, A.; Kobisch, M.; Madec, F.; Rose, N. Infectious agents associated with respiratory diseases in 125 farrow-to-finish pig herds: A cross-sectional study. Vet. Microbiol. 2012, 157, 152–163. [Google Scholar] [CrossRef]
- Galdeano, J.V.B.; Baraldi, T.G.; Ferraz, M.E.S.; Almeida, H.M.d.S.; Mechler-Dreibi, M.L.; Costa, W.M.T.; Montassier, H.J.; Mathias, L.A.; Oliveira, L.G.d. Cross-sectional study of seropositivity, lung lesions and associated risk factors of the main pathogens of Porcine Respiratory Diseases Complex (PRDC) in Goiás, Brazil. Porc. Health Manag. 2019, 5, 23. [Google Scholar] [CrossRef]
- Baraldi, T.G.; Cruz, N.R.N.; Pereira, D.A.; Galdeano, J.V.B.; Gatto, I.R.H.; Silva, A.F.D.; Panzardi, A.; Linhares, D.C.L.; Mathias, L.A.; De Oliveira, L.G. Antibodies against Actinobacillus pleuropneumoniae, Mycoplasma hyopneumoniae and Influenza virus and their relationships with risk factors, clinical signs and lung lesions in pig farms with one-site production systems in Brazil. Prev. Vet. Med. 2019, 171, 104748. [Google Scholar] [CrossRef]
- Gottschalk, M.; Broes, A. Actinobacillosis. In Diseases of Swine; Zimmerman, L.A., Karriker, A.R., Schwartz, K.J., Stevenson, G.W., Jianqiang, Z., Jeffrey, J., Eds.; John Wiley & Sons Inc.: New York, NY, USA, 2019; pp. 749–766. [Google Scholar]
- Cleveland-Nielsen, A.; Nielsen, E.O.; Ersboll, A.K. Chronic pleurisy in Danish slaughter pig herds. Prev. Vet. Med. 2002, 55, 121–135. [Google Scholar] [CrossRef]
- Meyns, T.; Van Steelant, J.; Rolly, E.; Dewulf, J.; Haesebrouck, F.; Maes, D. A crosssectional study of risk factors associated with pulmonary lesions in pigs at slaughter. Vet. J. 2011, 187, 388–392. [Google Scholar] [CrossRef]
- Merialdi, G.; Dottori, M.; Bonilauri, P.; Luppi, A.; Gozio, S.; Pozzi, P.; Spaggiari, B.; Martelli, P. Survey of pleuritis and pulmonary lesions in pigs at abattoir with a focus on the extent of condition and herd risk factors. Vet. J. 2012, 193, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Sibila, M.; Aragón, V.; Fraile, L.; Segalés, J. Comparison of four lung scoring systems for the assessment of the pathological outcomes derived from Actinobacillus pleuropneumoniae experimental infections. BMC Vet. Res. 2014, 10, 165. [Google Scholar] [CrossRef] [PubMed]
- Wallgren, P.; Nörregård, E.; Molander, B.; Persson, M.; Ehlorsson, C.J. Serological patterns of Actinobacillus pleuropneumoniae, Mycoplasma hyopneumoniae, Pasteurella multocida and Streptococcus suis in pig herds affected by pleurisy. Act. Vet. Scand. 2016, 58, 71. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Morante, B.; Segalés, J.; Fraile, L.; Pérez de Rozas, A.; Maiti, H.; Coll, T.; Sibila, M. Assessment of Mycoplasma hyopneumoniae-induced Pneumonia using different lung lesion scoring systems: A comparative review. J. Comp. Pathol. 2016, 154, 125–1344. [Google Scholar] [CrossRef] [PubMed]
- Fraile, L.; Alegre, A.; López-jiménez, R.; Nofrarías, M.; Segalés, J. Risk factors associated with pleuritis and cranio-ventral pulmonary consolidation in slaughter-aged pigs. Vet. J. 2010, 184, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Christensen, G.; Sorensen, V.; Mousing, J. Diseases of the Respiratory System. In Diseases of Swine, 8th ed.; Straw, B.E., D’Allaire, S., Mengeling, W.L., Taylor, D.J., Eds.; Iowa State University Press: Ames, IA, USA, 1999; pp. 913–940. [Google Scholar]
- Sipos, W.; Cvjetković, V.; Dobrokes, B.; Sipos, S. Evaluation of the Efficacy of a Vaccination Program against Actinobacillus pleuropneumoniae Based on Lung-Scoring at Slaughter. Animals 2021, 11, 2778. [Google Scholar] [CrossRef]
- Maes, D.; Sibila, M.; Pieters, M.; Haesebrouck, F.; Segalés, J.; de Oliveira, L.G. Review on the methodology to assess respiratory tract lesions in pigs and their production impact. Vet. Res. 2023, 54, 8. [Google Scholar] [CrossRef]
- Dottori, M.; Nigrelli, A.; Bonilauri, P.; Merialdi, G.; Gozio, S.; Cominotti, F. Proposta per un Nuovo Sistema di Punteggiatura Delle Pleuriti Suine in Sede di Macellazione: La Griglia SPES (Slaughterhouse Pleurisy Evaluation System). Large Anim. Rev. 2007, 13, 161–165. Available online: https://www.vetjournal.it/riviste/item/21673-dottori-m-,-nigrelli-a-d-,-bonilauri-p-,-merialdi-g-,-gozio-s-,-cominotti-f.html (accessed on 24 March 2023).
- Di Provvido, A.; Trachtman, A.R.; Farina, E.; Vaintrub, M.O.; Fragassi, G.; Vignola, G.; Marruchella, G. Pleurisy evaluation on the parietal pleura: An alternative scoring method in slaughtered pigs. J. Swine Health Prod. 2019, 27, 312–316. [Google Scholar]
- Madec, F.; Kobisch, M. Bilan lésionnel des poumons de porcs charcutiers à l‘abattoir. Journées Rech. Porc. Fr. 1982, 14, 405–412. [Google Scholar]
- Piffer, I.A.; Brito, J.R.F. Descrição de um Modelo para Avaliação e Quantificação de Lesões Pulmonares de Suínos e Formulação de um Índice Para Classificação de Rebanhos; EMBRAPA/CNPSA: Concórdia, Brazil, 1991; p. 16. Available online: https://ainfo.cnptia.embrapa.br/digital/bitstream/item/42227/1/documento-23.pdf/ (accessed on 21 September 2022).
- Sipos, W.; Dobrokes, B.; Meppiel, L.; Sailer, J.; Friedmann, U.; Cvjetković, V. Association of lung score findings from slaughter pigs with their vaccination status against Mycoplasma hyopneumoniae and PCV2. Berl. Münch. Tierärztl. Wochenschr. 2020, 133, 1–5. [Google Scholar]
- Ruggeri, J.; Salogni, C.; Giovanni, S.; Vitale, N.; Boniotti, M.B.; Corradi, A.; Pozzi, P.; Pasquali, P.; Alborali, G.L. Association between infectious agents and lesions in post-weaned piglets and fattening heavy pigs with porcine respiratory disease complex (PRDC). Front. Vet. Sci. 2020, 7, 636. [Google Scholar] [CrossRef] [PubMed]
- Trevisan, L. Anatomopatologia e Bacteriologia de Lesões Pulmonares em Suínos ao Abate. In Dissertação (Mestrado em Ciências Agrárias). Programa de Pós-Graduação em Ciências Veterinárias; Universidade Federal do Paraná: Curitiba, Brazil, 2020; Available online: https://acervodigital.ufpr.br/handle/1884/68689 (accessed on 24 March 2023).
- Valença, A.M.F.; Baptista, R.I.A.A.; Barbosa, C.N. Índice para pneumonia em granjas comerciais de suínos do estado de Pernambuco. Med. Veterinária UFRPE 2016, 10, 13–18. [Google Scholar]
- Lin, K.; Wang, C.; Murtaugh, M.P.; Ramamoorthy, S. Multiplex method for simultaneous serological detection of porcine reproductive and respiratory syndrome virus and porcine circovirus type 2. J. Clin. Microbiol. 2011, 49, 3184–3190. [Google Scholar] [CrossRef]
- Schmidt, C.; Cibulski, S.P.; Andrade, C.P.; Teixeira, T.F.; Varela AP, M.; Scheffer, C.M.; Franco, A.C.; de Almeida, L.L.; Roehe, P.M. Swine influenza virus and association with the porcine respiratory disease complex in pig farms in Southern Brazil. Zoonoses Public Health 2016, 63, 234–240. [Google Scholar] [CrossRef]
- Goecke, N.B.; Kobberø, M.; Kusk, T.K.; Hjulsager, C.K.; Pedersen, K.S.; Kristensen, C.S.; Larsen, L.E. Objective pathogen monitoring in nursery and finisher pigs by monthly laboratory diagnostic testing. Porc. Health Manag. 2020, 6, 23. [Google Scholar] [CrossRef]
- Lung, O.; Ohene-Adjei, S.; Buchanan, C.; Joseph, T.; King, R.; Erickson, A.; Detmer, S.; Ambagala, A. Multiplex PCR and Microarray for Detection of Swine Respiratory Pathogens. Transbound. Emerg. Dis. 2017, 64, 834–848. [Google Scholar] [CrossRef]
- Sunaga, F.; Tsuchiaka, S.; Kishimoto, M.; Aoki, H.; Kakinoki, M.; Kure, K.; Okumura, H.; Okumura, M.; Okumura, A.; Nagai, M.; et al. Development of a one-run real-time PCR detection system for pathogens associated with porcine respiratory diseases. J. Vet. Med. Sci. 2020, 82, 217–223. [Google Scholar] [CrossRef]
- Dalla Costa, O.A.; Dalla Costa, F.A.; Feddern, V.; dos Santos Lopes, L.; Coldebella, A.; Gregory, N.G.; Lima, G.J.M.M. Risk factors associated with pig pre-slaughtering losses. Meat Sci. 2019, 155, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Portaria nº 711/95—Normas Técnicas para Instalações e Equipamentos para Abate e Industrialização de Suínos; Ministério da Agricultura, Pecuária e Abastecimento (MAPA): Brasília, Brazil, 1995; p. 98. Available online: https://www.gov.br/agricultura/pt-br/assuntos/inspecao/produtos-animal/empresario/arquivos/Portaria_711.1995.pdf/view/ (accessed on 21 September 2022).
- Brito, J.R.; Piffer, I.A.; Sobestiansky, J.; Brito, M.A.V. Vacinação com Bordetella bronchiseptica e Pasteurella multocida, associada a alterações do manejo, no controle da rinite atrófica dos suínos. Arq. Bras. Med. Vet. Zootec. 1993, 45, 183–191. [Google Scholar]
- Davies, P.R.; Bahnson, P.B.; Grass, J.J.; Marsh, W.E.; Dial, G.D. Comparison of methods for measurement of Enzootic Pneumonia lesions in pigs. Am. J. Vet. Res. 1995, 56, 9–14. [Google Scholar]
- Kuramae-Izioka, E.E. A Rapid, Easy and High Yield Protocol for Total Genomic DNA Isolation of Colletotrichum Gloeosporioides and Fusarium oxysporum. Rev. Univ. Estadual. Mar. 1997, 19, 683–689. Available online: https://periodicos.uem.br/ojs/index.php/RevUNIMAR/article/view/4550/ (accessed on 21 September 2022).
- Birkenheuer, A.J.; Levy, M.G.; Breitschwerdt, E.B. Development and Evaluation of a Seminested PCR for Detection and Differentiation of Babesia Gibsoni (Asian Genotype) and B. Canis DNA in Canine Blood Samples. J. Clin. Microbiol. 2003, 41, 4172–4177. [Google Scholar] [CrossRef]
- Toledo, M.A.; Leite, A.I.; Gonçalves, L.R.; Sousa, K.C.M.; Amaral, R.B.d.; da Silva, G.C.P.; Machado, R.Z.; André, M.R. High occurrence of Mycoplasma suis infection in swine herds from non-technified farms in Mossoró, state of Rio Grande do Norte, Northeastern Brazil. Rev. Bras. Parasitol. Vet. 2016, 25, 414–417. [Google Scholar] [CrossRef] [PubMed]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef]
- Goecke, N.B.; Hjulsager, C.K.; Krog, J.S.; Skovgaard, K.; Larsen, L.E. Development of a high-throughput real-time PCR system for detection of enzootic pathogens in pigs. J. Vet. Diagn. Investig. 2020, 1, 51–64. [Google Scholar] [CrossRef]
- Fourour, S.; Fablet, C.; Tocqueville, V.; Dorenlor, V.; Eono, F.; Eveno, E.; Kempf, I.; Marois-Créhan, C. A new multiplex real-time TaqMan® PCR for quantification of Mycoplasma hyopneumoniae, M. hyorhinis and M. flocculare: Exploratory epidemiological investigations to research mycoplasmal association in enzootic pneumonia-like lesions in slaughtered pigs. J. Appl. Microbiol. 2018, 125, 345–355. [Google Scholar] [CrossRef]
- Han, Q.; Wang, J.; Li, R.; Han, Q.; Yuan, W.; Wang, J. Development of a recombinase polymerase amplification assay for rapid detection of Haemophilus parasuis in tissue samples. Vet. Med. Sci. 2020, 6, 894–900. [Google Scholar] [CrossRef]
- Jirawattanapong, P.; Stockhofe-Zurwieden, N.; van Leengoed, L.; Wisselink, H.; Raymakers, R.; Cruijsen, T.; van der Peet-Schwering, C.; Nielen, M.; van Nes, A. Pleuritis in slaughter pigs: Relations between lung lesions and bacteriology in 10 herds with high pleuritis. Res. Vet. Sci. 2010, 88, 11–15. [Google Scholar] [CrossRef]
- Enøe, C.; Mousing, J.; Schirmer, A.L.; Willeberg, P. Infectious and rearing-system related risk factors for chronic pleuritis in slaughter pigs. Prev. Vet. Med. 2002, 54, 337–349. [Google Scholar] [CrossRef]
- De Conti, E.R.; Takeuti, K.L.; Schwertz, C.I.; Bianchi, R.M.; Driemeier, D.; de Barcellos, D.E. Agents of pneumonia in slaughtered pigs in southern Brazil. Pesqui. Veterinária Bras. 2021, 41, e06669. [Google Scholar] [CrossRef]
- Ferraz, M.; Almeida, H.; Storino, G.Y.; Sonálio, K.; Souza, M.R.; Moura, C.; Costa, W.; Lunardi, L.; Linhares, D.; de Oliveira, L.G. Lung consolidation caused by Mycoplasma hyopneumoniae has a negative effect on productive performance and economic revenue in finishing pigs. Prev. Vet. Med. 2020, 182, 105091. [Google Scholar] [CrossRef]
- Takeuti, K.L.; Watanabe, T.T.N.; de Castro, L.A.; Driemeier, D.; de Barcellos, D.E.S.N. Caracterização histopatológica e imuno-histoquímica da pneumonia causada pela co-infecção por Pasteurella multocida e Mycoplasma hyopneumoniae em suínos. Acta Sci. Vet. 2016, 41, 1117. [Google Scholar]
- Park, C.; Jeong, J.; Kang, I.; Choi, K.; Park, S.J.; Chae, C. Increased fucosyl glycoconjugate by Mycoplasma hyopneumoniae enhances adherences of Pasteurella multocida type A in the ciliated epithelial cells of the respiratory tract. BMC Vet. Res. 2016, 12, 25. [Google Scholar] [CrossRef]
- Gottschalk, M.; Broes, A. Actinobacillosis. In Diseases of Swine, 11th ed.; Zimmerman, J.J., Karriker, L.A., Ramirez, A., Schwartz, K.J., Stevenson, G.W., Zhang, J., Eds.; Wiley: Chichester, UK, 2019. [Google Scholar]
- Van de Kerkhof, A.; Haesebrouck, F.; Chiers, K.; Ducatelle, R.; Kamp, E.M.; Smits, M.A. Influence of Actinobacillus pleuropneumoniae and its metabolites on porcine alveolar epithelial cells. Infect. Immun. 1996, 64, 3905–3907. [Google Scholar] [CrossRef] [PubMed]
- Jäger, H.C.; McKinley, T.J.; Wood, J.L.; Pearce, G.P.; Williamson, S.; Strugnell, B.; Done, S.; Habernoll, H.; Palzer, A.; Tucker, A.W. Factors associated with pleurisy in pigs: A case-control analysis of slaughter pig data for England and Wales. PLoS ONE 2012, 7, 2. [Google Scholar] [CrossRef]
- Liggett, A.D.; Harrison, L.R.; Farrell, R.L. Sequential study of lesion development in experimental Haemophilus pleuropneumonia. Res Vet Sci. 1987, 42, 204–212. [Google Scholar] [CrossRef]
- Amass, S.F.; Clark, L.K.; van Alstine, W.G.; Bowersock, T.L.; Murphy, D.A.; Knox, K.E.; Albregts, S.R. Interaction of Mycoplasma hyopneumoniae and Pasteurella multocida infections in swine. J. Am. Vet. Med. Assoc. 1994, 204, 102–107. [Google Scholar]
- Pors, S.E.; Hansen, M.S.; Bisgaard, M.; Jensen, H.E. Occurrence and associated lesions of Pasteurella multocida in porcine bronchopneumonia. Vet. Microbiol. 2011, 150, 160–166. [Google Scholar] [CrossRef]
- Filho, J.X.D.O.; Morés, M.A.; Rebelatto, R.; Agnol, A.M.; Plieski, C.L.; Klein, C.S.; Barcellos, D.E.; Morés, N. Pasteurella multocida type A as the primary agent of pneumonia and septicaemia in pigs. Pesqui. Veterinária Bras. 2015, 35, 716–724. [Google Scholar] [CrossRef]
- Paladino, E.S.; Gabardo, M.d.P.; Lunardi, P.N.; Morés, N.; Guedes, R.M.C. Anatomopathological pneumonic aspects associated with highly pathogenic Pasteurella multocida in finishing pigs. Pesq. Vet. Bras. 2017, 37, 1091–1100. [Google Scholar] [CrossRef]
- Piva, M.M.; Schwertz, C.I.; Bianchi, R.M.; Henker, L.C.; Morés MA, Z.; Rebelatto, R.; Kemper, R.T.; Goslar, M.S.; Nagae, R.Y.; Pavarini, S.P. Pasteurella multocida polyserositis in growing-finishing pigs. J. Comp. Pathol. 2023, 202, 16–22. [Google Scholar] [CrossRef]
- Turni, C.; Meers, J.; Parke, K.; Singh, R.; Yee, S.; Templeton, J.; Mone, N.; Blackall, P.; Barnes, T. Pathogens associated with pleuritic pig lungs at an abattoir in Queensland Australia. Aust. Vet. J. 2021, 99, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Zachary, J.F. Chapter 4—Mechanisms of Microbial Infections. In Pathologic Basis of Veterinary Disease; Zachary, J.F., Ed.; Elsevier: Urbana, IL, USA, 2017; pp. 132–241. [Google Scholar]
- O’Sullivan, T.; Friendship, R.; Blackwell, T.; Pearl, D.; McEwen, B.; Carman, S.; Dewey, C. Microbiological identification and analysis of swine tonsils collected from carcasses at slaughter. Can. J. Vet. Res. 2011, 75, 106–111. [Google Scholar]
- Maes, D.; Sibila, M.; Kuhnert, P.; Segales, J.; Haesebrouck, F.; Pieters, M. Update on Mycoplasma hyopneumoniae infections in pigs: Knowledge gaps for improved disease control. Transbound Emerg. Dis. 2018, 65 (Suppl. 1), 110–124. [Google Scholar] [CrossRef] [PubMed]
| Score | Features of Pleurisy, Considering the Extension and Localization of Lesions. |
|---|---|
| 0 | Absence of macroscopic lesions. |
| 1 | Pleurisy affecting the cranial-ventral portion of the lung; interlobar adhesion. |
| 2 | Discrete, unilateral pleurisy of the diaphragmatic lobe. |
| 3 | Discrete, bilateral pleurisy of both the diaphragmatic lobes; large, unilateral adhesion affecting the diaphragmatic lobe. |
| 4 | Large, bilateral adhesions between both the diaphragmatic lobes on one side and the chest cavity on the other. |
| Gene | N° of Access on GenBank | Primers and Probes | The Nucleotide Sequence (5′ → 3′) | Amplicon Size (bp) | Reference |
|---|---|---|---|---|---|
| omIA | [gb|NC_009053|] | F_App_ omIA | AGTGCTTACCGCATGTAGTGGC | 153 | [41] |
| R_App_ omIA | TTGGTGCGGACATATCAACCTTA | ||||
| P_App_ omIA | 5′-Cy5-CGATGAACCCGATGAGCCGCC-3′-IB®RQ | ||||
| p102 | [gb|AE017332.1|] | F_Mhp_p102 | 5′-TAAGGGTCAAAGTCAAAGTC-3′ | 150 | [42] |
| R_Mhp_p102 | 5′-AAATTAAAAGCTGTTCAAATGC-3′ | ||||
| P_Mhp_p102 | 5′-FAM-AACCAGTTTCCACTTCATCGCC-3′-BHQ1 | ||||
| kmt1 | [gb|NC_017027.1|] | F_Pmt_kmt1 | 5′-GGGCTTGTCGGTAGTCTTT-3′ | 148 | [43] |
| R_Pmt_kmt1 | 5′-CGGCAAATAACAATAAGCTGAGTA-3′ | ||||
| P_Pmt_kmt1 | 5′-TexRd- CGGCGCAACTGATTGGACGTTATT3′-IB®RQ |
| Lot 1 | Lot 2 | Lot 3 | Lot 4 | Lot 5 | Lot 6 | Mean | Median Standard Error | p-Value | |
|---|---|---|---|---|---|---|---|---|---|
| Evaluated carcasses | 128 | 151 | 107 | 111 | 113 | 116 | 121 | 14.91 | - |
| Pulmonary consolidation (%) | 9.05 a | 5.95 a | 10.00 a | 16.32 b | 14.57 b | 8.32 a | 10.70 | 3.61 | p = 0.03 |
| Pneumonia index (PI) | 1.25 a | 1.03 b | 1.40 a | 1.89 a | 1.81 a | 1.35 a | 1.46 | 0.30 | p = 0.04 |
| Occurrence of pleuritis (%) | 10.16 a | 5.95 a | 5.61 a | 18.92 b | 15.93 b | 17.24 b | 12.30 | 5.34 | p = 0.03 |
| App index | 0.10 a | 0.00 b | 0.13 a | 0.21 a | 0.19 a | 0.17 a | 0.13 | 0.07 | p = 0.04 |
| Groups | A. pleuropneumoniae | M. hyopneumoniae | P. multocida | G. parasuis | S. suis | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Lungs | Pleura | Lungs | Pleura | Lungs | Pleura | Lungs | Pleura | Lungs | Pleura | |
| G1 (n = 10) | 4 | 2 | 10 | 1 | 3 | 0 | 9 | 10 | 9 | 9 |
| G2 (n = 11) | 2 | 6 | 10 | 1 | 5 | 0 | 11 | 11 | 11 | 9 |
| G3 (n = 10) | 8 | 5 | 9 | 5 | 4 | 2 | 9 | 10 | 8 | 6 |
| G4 (n = 11) | 5 | 4 | 11 | 4 | 8 | 4 | 9 | 11 | 11 | 8 |
| G5 (n = 11) | 5 | 4 | 10 | 2 | 2 | 0 | 10 | 10 | 11 | 10 |
| Lung Samples | Pleura Samples | |||||
|---|---|---|---|---|---|---|
| Score of Pleurisy | Median * | Median Standard Error | Score of Pleurisy | Median * | Median Standard Error | |
| M. hyopneumoniae | 0 | 3.30 × 10−1 b | 8.77 × 10−1 | 0 | 4.73 × 10−1 a | 1.25 × 100 |
| 1 | 9.71 × 102 ab | 2.46 × 101 | 1 | 6.40 × 10−2 a | 1.62 × 10−1 | |
| 2 | 6.75 × 103 b | 1.72 × 102 | 2 | 3.06 × 102 a | 8.47 × 10−2 | |
| 3 | 2.11 × 103 ab | 5.34 × 102 | 3 | 4.36 × 101 a | 1.10 × 100 | |
| 4 | 1.13 × 104 a | 9.35 × 102 | 4 | 6.48 × 101 a | 1.72 × 100 | |
| P. multocida | 0 | 4.64 × 103 b | 1.23 × 10−2 | 0 | 0.00 × 100 b | 0.00 × 100 |
| 1 | 4.91 × 103 b | 1.31 × 10−2 | 1 | 0.00 × 100 b | 0.00 × 100 | |
| 2 | 7.4 × 100 ab | 1.84 × 101 | 2 | 0.00 × 100 b | 0.00 × 100 | |
| 3 | 1.3 × 102 ab | 9.30 × 10−2 | 3 | 5.17 × 103 ab | 1.31 × 10−2 | |
| 4 | 8.37 × 101 a | 2.22 × 100 | 4 | 7.68 × 101 a | 2.04 × 100 | |
| A. pleuropneumoniae | 0 | 6.84 × 102 bc | 1.81 × 10−1 | 0 | 6.83 × 100 a | 6.88 × 10−2 |
| 1 | 7.42 × 103 bc | 1.88 × 10−2 | 1 | 6.24 × 100 a | 1.16 × 10−2 | |
| 2 | 1.20 × 104 a | 4.68 × 100 | 2 | 6.68 × 100 a | 7.47 × 10−1 | |
| 3 | 2.06 × 102 ab | 5.22 × 102 | 3 | 7.43 × 100 a | 4.25 × 10−1 | |
| 4 | 6.43 × 103 c | 1.71 × 10−2 | 4 | 6.60 × 100 a | 1.93 × 10−1 | |
| G. parasuis | 0 | 1.15 × 101 a | 3.06 × 10−1 | 0 | 1.76 × 101 a | 4.68 × 10−1 |
| 1 | 2.70 × 101 a | 6.84 × 10−1 | 1 | 2.23 × 101 a | 5.63 × 10−1 | |
| 2 | 4.66 × 102 a | 1.18 × 10−1 | 2 | 2.57 × 101 a | 7.11 × 10−1 | |
| 3 | 4.65 × 101 a | 1.18 × 100 | 3 | 1.53 × 100 a | 3.87 × 100 | |
| 4 | 4.20 × 101 a | 1.11 × 101 | 4 | 1.66 × 101 a | 4.40 × 10−1 | |
| S. suis | 0 | 9.44 × 10−2 a | 2.50 × 10−1 | 0 | 1.65 × 10−1 a | 4.39 × 10−1 |
| 1 | 5.80 × 10−2 a | 1.47 × 10−1 | 1 | 1.03 × 10−1 a | 2.61 × 10−1 | |
| 2 | 4.21 × 10−2 a | 1.09 × 10−1 | 2 | 2.20 × 10−1 a | 6.10 × 10−1 | |
| 3 | 1.86 × 100 a | 4.71 × 100 | 3 | 9.94 × 10−2 a | 2.51 × 10−1 | |
| 4 | 8.03 × 10−2 a | 2.13 × 10−1 | 4 | 1.40 × 10−1 a | 3.70 × 10−1 | |
| Pleurisy Lesion Severity | Interaction | Spearman’s Correlation | p-Value |
|---|---|---|---|
| Score 2 | A. pleuropneumoniae and M. hyopneumoniae | R = 0.42 | p = 0.04 |
| Score 3 | G. parasuis and S. suis | R = 0.45 | p = 0.04 |
| Score 4 | M. hyopneumoniae and P. multocida | R = 0.58 | p = 0.03 |
| M. hyopneumoniae and S. suis | R = 0.63 | p = 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petri, F.A.M.; Ferreira, G.C.; Arruda, L.P.; Malcher, C.S.; Storino, G.Y.; Almeida, H.M.d.S.; Sonalio, K.; Silva, D.G.d.; Oliveira, L.G.d. Associations between Pleurisy and the Main Bacterial Pathogens of the Porcine Respiratory Diseases Complex (PRDC). Animals 2023, 13, 1493. https://doi.org/10.3390/ani13091493
Petri FAM, Ferreira GC, Arruda LP, Malcher CS, Storino GY, Almeida HMdS, Sonalio K, Silva DGd, Oliveira LGd. Associations between Pleurisy and the Main Bacterial Pathogens of the Porcine Respiratory Diseases Complex (PRDC). Animals. 2023; 13(9):1493. https://doi.org/10.3390/ani13091493
Chicago/Turabian StylePetri, Fernando Antônio Moreira, Geovana Coelho Ferreira, Laíza Pinto Arruda, Clarisse Sena Malcher, Gabriel Yuri Storino, Henrique Meiroz de Souza Almeida, Karina Sonalio, Daniela Gomes da Silva, and Luís Guilherme de Oliveira. 2023. "Associations between Pleurisy and the Main Bacterial Pathogens of the Porcine Respiratory Diseases Complex (PRDC)" Animals 13, no. 9: 1493. https://doi.org/10.3390/ani13091493
APA StylePetri, F. A. M., Ferreira, G. C., Arruda, L. P., Malcher, C. S., Storino, G. Y., Almeida, H. M. d. S., Sonalio, K., Silva, D. G. d., & Oliveira, L. G. d. (2023). Associations between Pleurisy and the Main Bacterial Pathogens of the Porcine Respiratory Diseases Complex (PRDC). Animals, 13(9), 1493. https://doi.org/10.3390/ani13091493








