Post-Thaw Storage Temperature Influenced Boar Sperm Quality and Lifespan through Apoptosis and Lipid Peroxidation
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Media
2.2. Semen Samples and Cryopreservation
2.3. Post-Thaw Measurements of Sperm Quality and Functionality
2.4. Measurements of Antioxidants and Oxidants in SPZ and SN
2.5. Statistical Analysis
3. Results
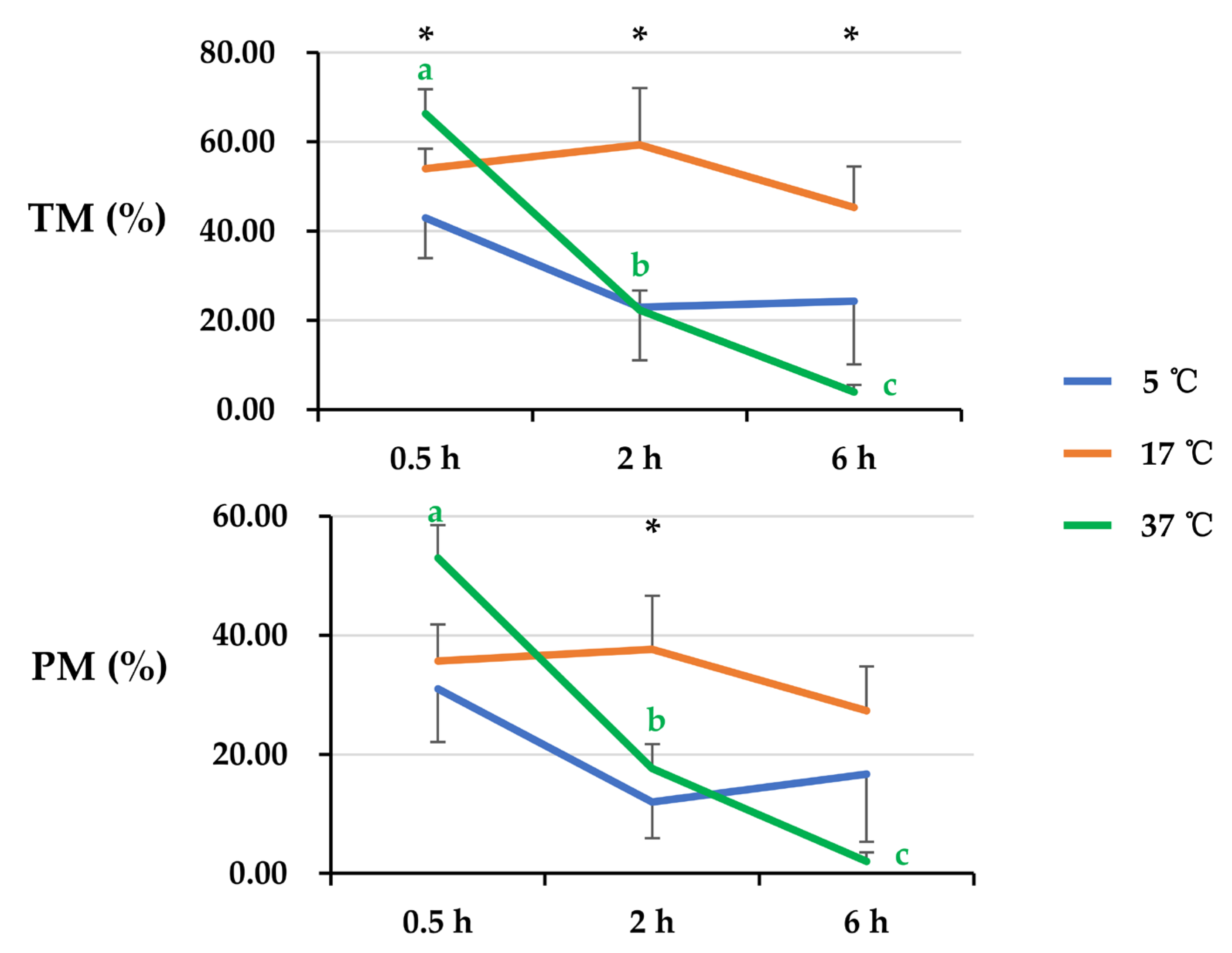
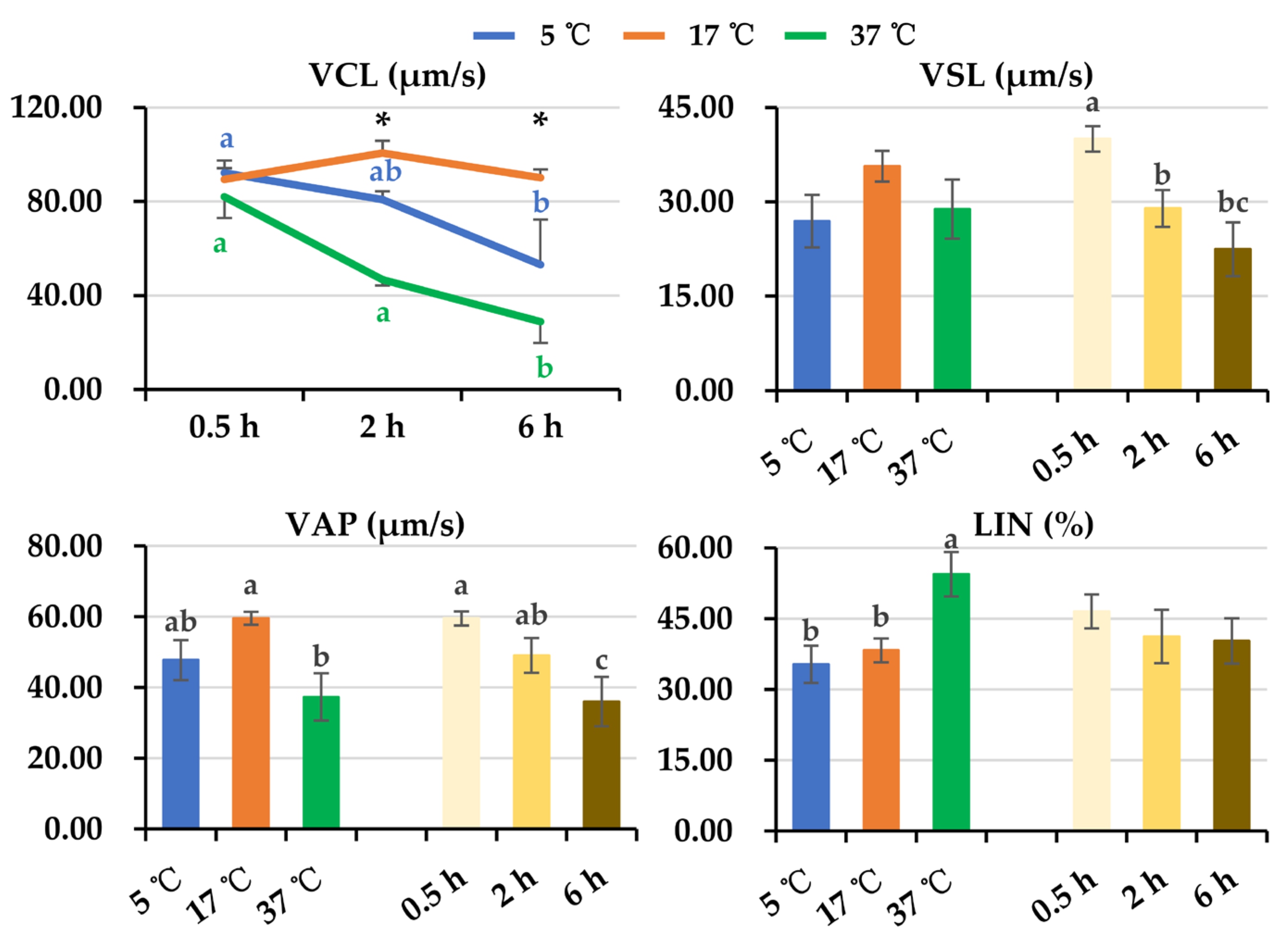
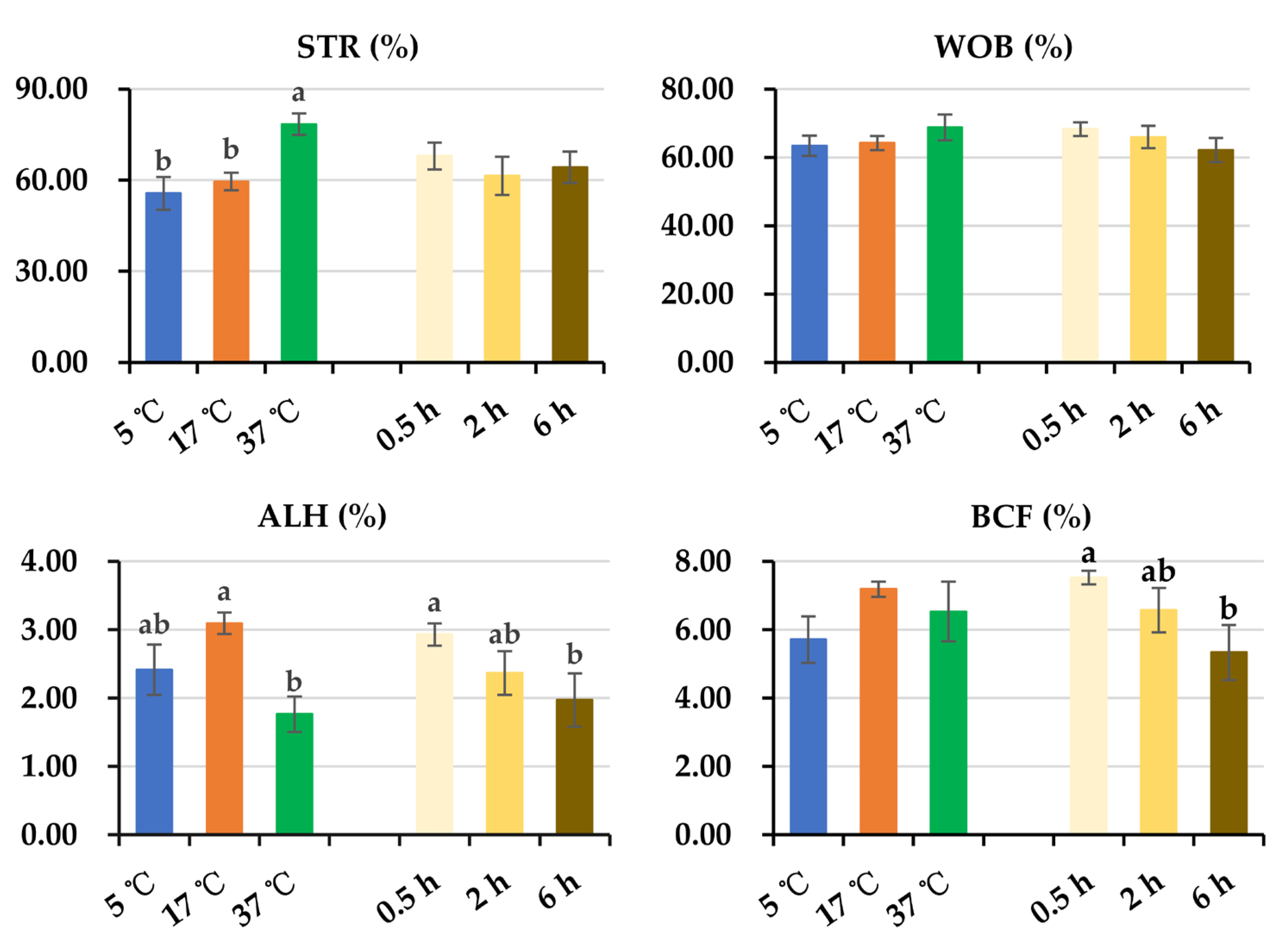
3.1. Effect of Post-Thaw Storage on Boar Sperm Motion Parameters
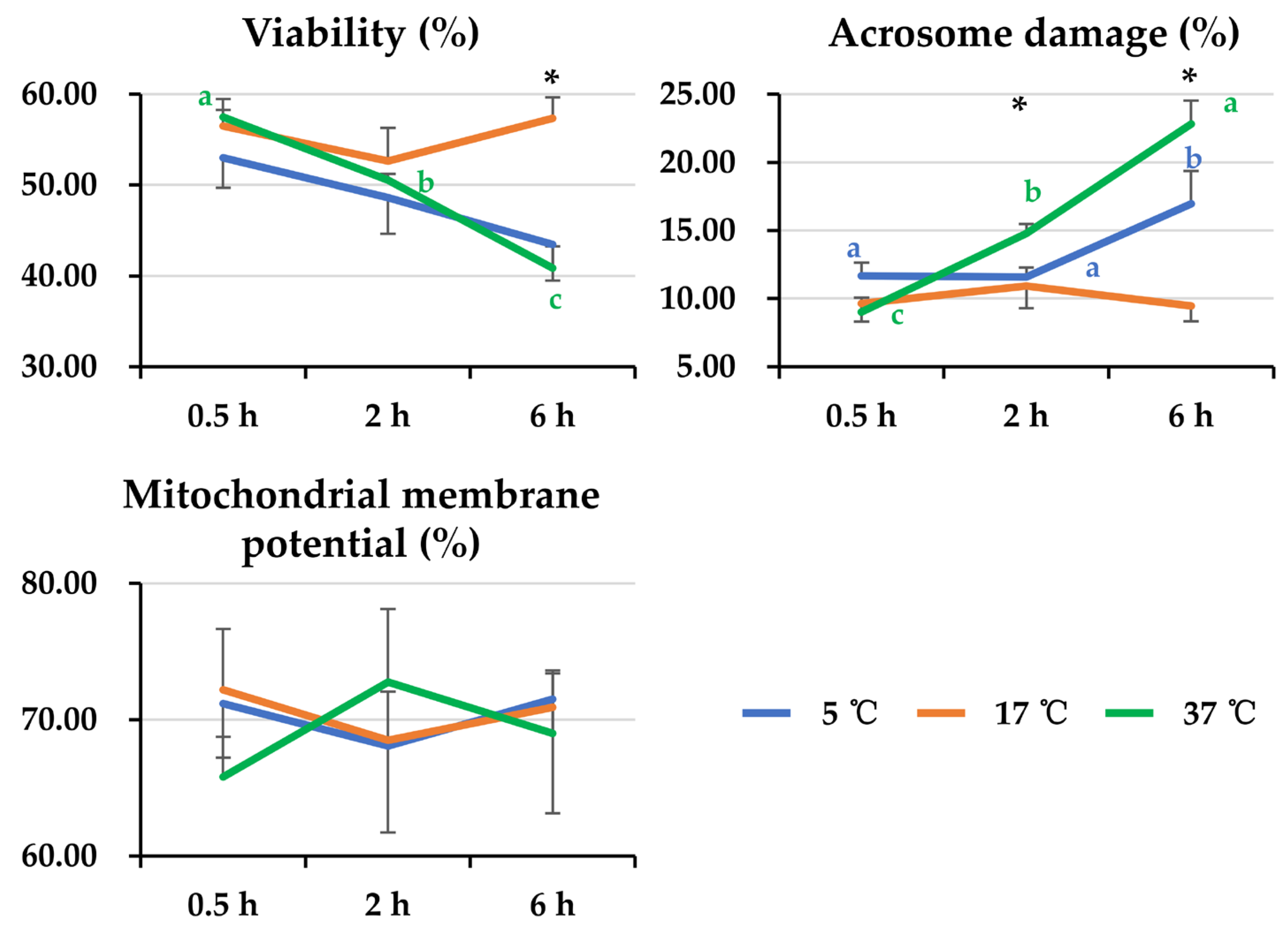
3.2. Effect of Post-Thaw Storage on Boar Sperm Morphological Status
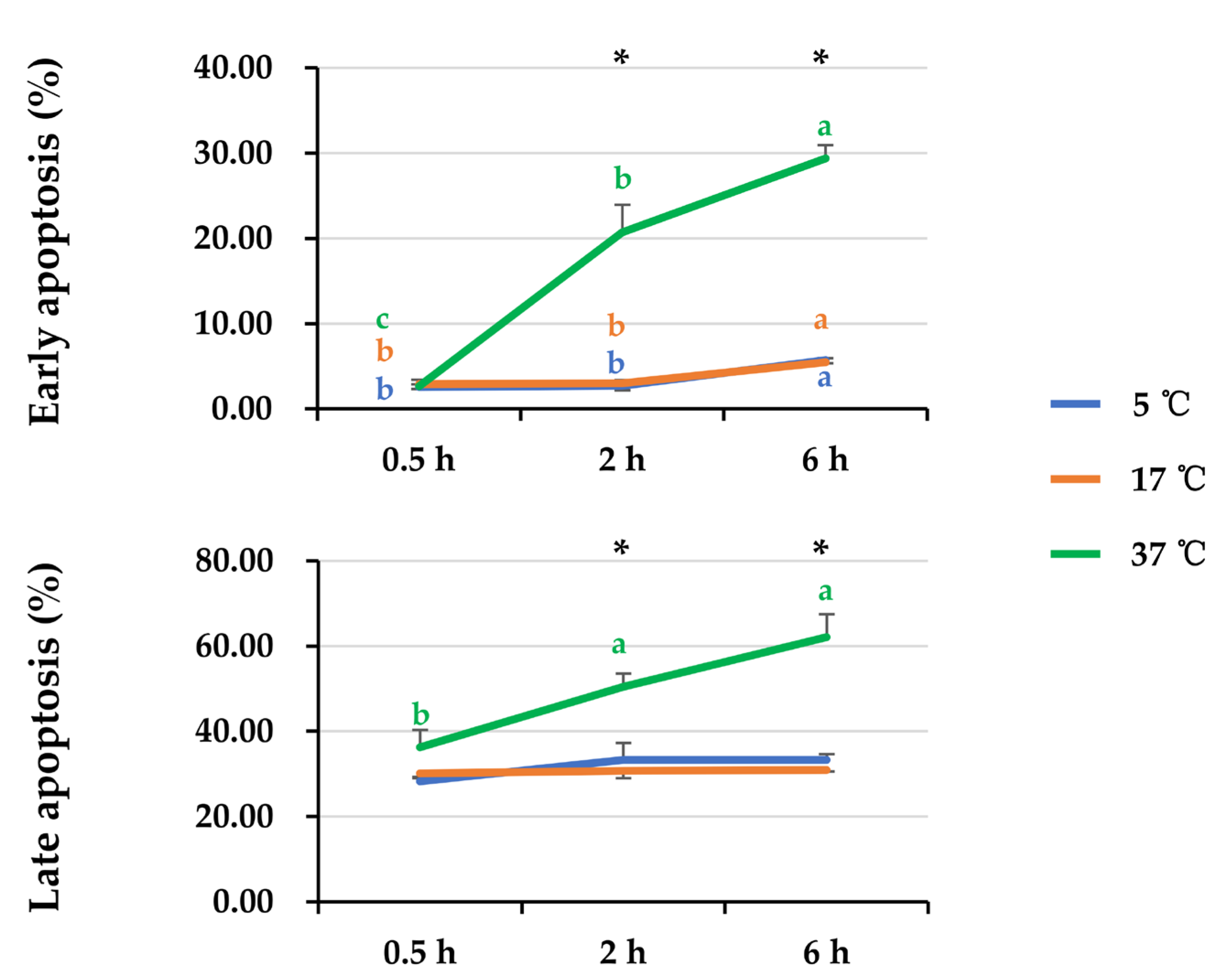
3.3. Effect of Post-Thaw Storage on Boar Sperm Apoptotic Changes
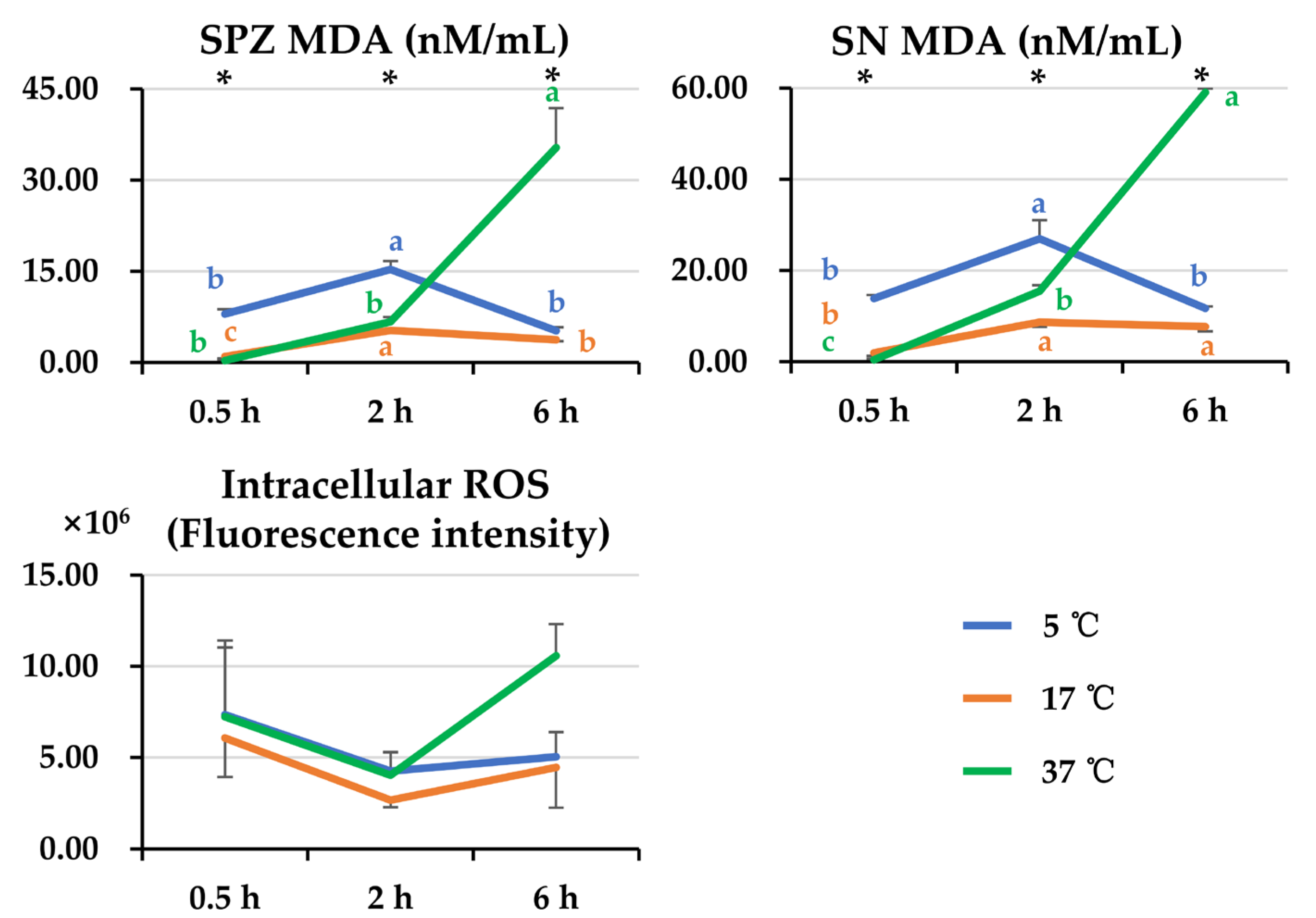
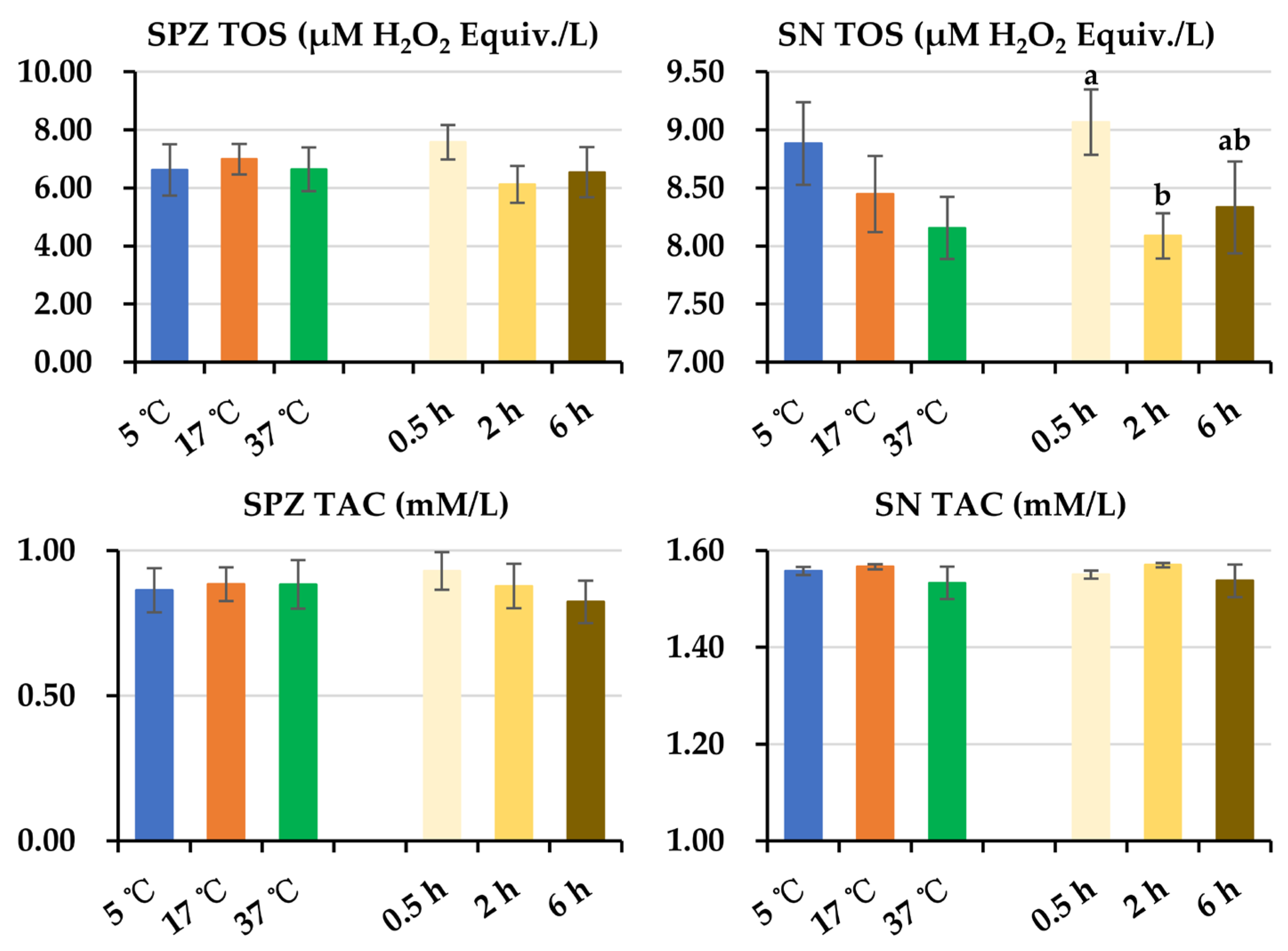
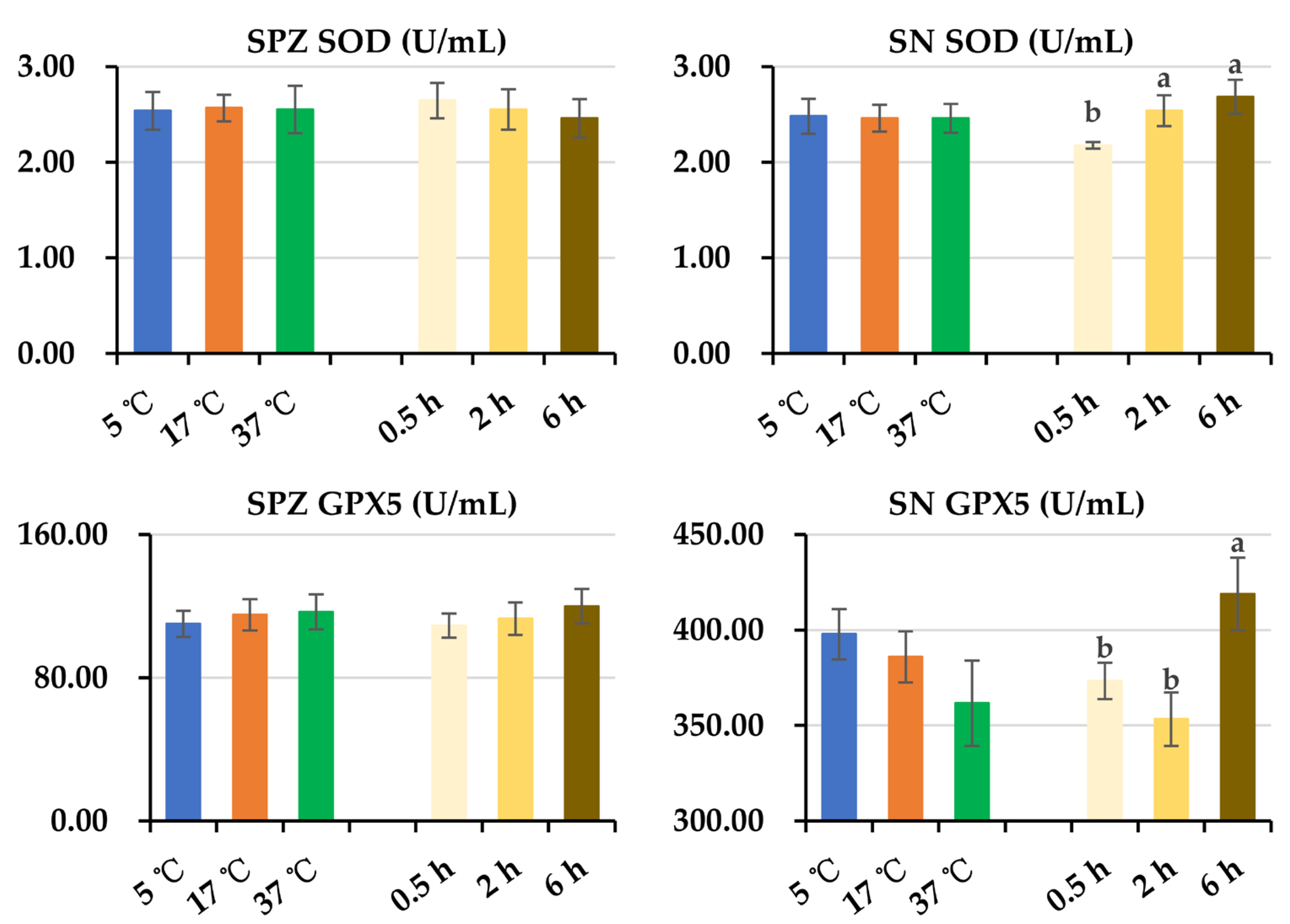
3.4. Effect of Post-Thaw Storage on Antioxidant and Oxidant Levels in Boar Sperm and Their Surrounding Environment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yánez-Ortiz, I.; Catalán, J.; Rodríguez-Gil, J.E.; Miró, J.; Yeste, M. Advances in sperm cryopreservation in farm animals: Cattle, horse, pig and sheep. Anim. Reprod. Sci. 2022, 246, 106904. [Google Scholar] [CrossRef]
- Knox, R.V. The fertility of frozen boar sperm when used for artificial insemination. Reprod. Domest. Anim. 2015, 50 (Suppl. S2), 90–97. [Google Scholar] [CrossRef] [PubMed]
- Yeste, M. Sperm cryopreservation update: Cryodamage, markers, and factors affecting the sperm freezability in pigs. Theriogenology 2016, 85, 47–64. [Google Scholar] [CrossRef] [PubMed]
- Masuda, Y.; Kheawkanha, T.; Nagahama, A.; Kawasaki, K.; Konno, T.; Yamanaka, K.; Tatemoto, H. Antifreeze protein type III addition to freezing extender comprehensively improves post-thaw sperm properties in Okinawan native Agu pig. Anim. Reprod. Sci. 2023, 252, 107232. [Google Scholar] [CrossRef] [PubMed]
- Pedrosa, A.C.; Andrade Torres, M.; Vilela Alkmin, D.; Pinzon, J.E.P.; Kitamura Martins, S.M.M.; Coelho da Silveira, J.; Furugen Cesar de Andrade, A. Spermatozoa and seminal plasma small extracellular vesicles miRNAs as biomarkers of boar semen cryotolerance. Theriogenology 2021, 174, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Prieto-Martínez, N.; Vilagran, I.; Morató, R.; Rivera Del Álamo, M.M.; Rodríguez-Gil, J.E.; Bonet, S.; Yeste, M. Relationship of aquaporins 3 (AQP3), 7 (AQP7), and 11 (AQP11) with boar sperm resilience to withstand freeze-thawing procedures. Andrology 2017, 5, 1153–1164. [Google Scholar] [CrossRef] [PubMed]
- Pinart, E.; Yeste, M.; Bonet, S. Acrosin activity is a good predictor of boar sperm freezability. Theriogenology 2015, 83, 1525–1533. [Google Scholar] [CrossRef]
- Vilagran, I.; Yeste, M.; Sancho, S.; Castillo, J.; Oliva, R.; Bonet, S. Comparative analysis of boar seminal plasma proteome from different freezability ejaculates and identification of Fibronectin 1 as sperm freezability marker. Andrology 2015, 3, 345–356. [Google Scholar] [CrossRef]
- Li, J.; Barranco, I.; Tvarijonaviciute, A.; Molina, M.F.; Martinez, E.A.; Rodriguez-Martinez, H.; Parrilla, I.; Roca, J. Seminal plasma antioxidants are directly involved in boar sperm cryotolerance. Theriogenology 2018, 107, 27–35. [Google Scholar] [CrossRef]
- Barranco, I.; Padilla, L.; Pérez-Patiño, C.; Vazquez, J.M.; Martínez, E.A.; Rodríguez-Martínez, H.; Roca, J.; Parrilla, I. Seminal plasma cytokines are predictive of the outcome of boar sperm preservation. Front. Vet. Sci. 2019, 6, 436. [Google Scholar] [CrossRef]
- Osman, R.; Lee, S.; Almubarak, A.; Han, J.I.; Yu, I.J.; Jeon, Y. Antioxidant effects of myo-inositol improve the function and fertility of cryopreserved boar semen. Antioxidants 2023, 12, 1673. [Google Scholar] [CrossRef]
- Ledwaba, M.R.; Mphaphathi, M.L.; Thema, M.A.; Pilane, C.M.; Nedambale, T.L. Investigation of the efficacy of dithiothreitol and glutathione on in vitro fertilization of cryopreserved Large White boar semen. Animals 2022, 12, 1137. [Google Scholar] [CrossRef]
- Winn, E.; Whitaker, B.D. Quercetin supplementation to the thawing and incubation media of boar sperm improves post-thaw sperm characteristics and the in vitro production of pig embryos. Reprod. Biol. 2020, 20, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Khophloiklang, V.; Chanapiwat, P.; Aunpad, R.; Kaeoket, K. Palm kernel meal protein hydrolysates enhance post-thawed boar sperm quality. Animals 2023, 13, 3040. [Google Scholar] [CrossRef] [PubMed]
- Balogun, K.B.; Nicholls, G.; Sokunbi, O.A.; Stewart, K.R. Cryoprotectant effects of natural honey on spermatozoa quality of pre-freezing and frozen-thawed boar semen. J. Anim. Sci. 2023, 101, skac384. [Google Scholar] [CrossRef] [PubMed]
- Fraser, L.; Zasiadczyk, Ł.; Strzeżek, J.; Strzeżek, R.; Karpiesiuk, K. Freezability and fertility of frozen-thawed boar semen supplemented with ostrich egg yolk lipoproteins. Pol. J. Vet. Sci. 2018, 21, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.T.; Wang, J.R.; Sun, L.Z.; Jin, X.H.; Shi, X.Y.; Lin, J.Y.; Yue, S.L.; Zhou, J.B. Effects of astaxanthin on plasma membrane function and fertility of boar sperm during cryopreservation. Theriogenology 2021, 164, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Alborcia, M.J.; Morrell, J.M.; Parrilla, I.; Barranco, I.; Vázquez, J.M.; Martinez, E.A.; Roca, J. Improvement of boar sperm cryosurvival by using single-layer colloid centrifugation prior freezing. Theriogenology 2012, 78, 1117–1125. [Google Scholar] [CrossRef] [PubMed]
- Crespo-Félez, I.; Castañeda-Sampedro, A.; Sánchez, D.I.; Fernández-Alegre, E.; Álvarez-Rodríguez, M.; Domínguez, J.C.; Morrell, J.M.; Martínez-Pastor, F. Effect of Single Layer Centrifugation Porcicoll (70%, 80% and 90%) or supplementation with reduced glutathione, seminal plasma and bovine serum albumin on frozen-thawed boar sperm. Anim. Reprod. Sci. 2017, 187, 167–173. [Google Scholar] [CrossRef]
- Yeste, M.; Rodríguez-Gil, J.E.; Bonet, S. Artificial insemination with frozen-thawed boar sperm. Mol. Reprod. Dev. 2017, 84, 802–813. [Google Scholar] [CrossRef]
- Knox, R.V.; Ringwelski, J.M.; McNamara, K.A.; Aardsma, M.; Bojko, M. The effect of extender, method of thawing, and duration of storage on in vitro fertility measures of frozen-thawed boar sperm. Theriogenology 2015, 84, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhao, W.; Zhu, J.; Wang, S.; Ju, H.; Chen, S.; Basioura, A.; Ferreira-Dias, G.; Liu, Z. Temperature elevation during semen delivery deteriorates boar sperm quality by promoting apoptosis. Animals 2023, 13, 3203. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhao, W.; Zhu, J.; Ju, H.; Liang, M.; Wang, S.; Chen, S.; Ferreira-Dias, G.; Liu, Z. Antioxidants and oxidants in boar spermatozoa and their surrounding environment are associated with AMPK activation during liquid storage. Vet. Sci. 2023, 10, 214. [Google Scholar] [CrossRef]
- Guthrie, H.D.; Welch, G.R. Determination of intracellular reactive oxygen species and high mitochondrial membrane potential in Percoll-treated viable boar sperm using fluorescence-activated flow cytometry. J. Anim. Sci. 2006, 84, 2089–2100. [Google Scholar] [CrossRef] [PubMed]
- Erel, O. A new automated colorimetric method for measuring total oxidant status. Clin. Biochem. 2005, 38, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Parrilla, I.; Ortega, M.D.; Martinez, E.A.; Rodriguez-Martinez, H.; Roca, J. Post-thaw boar sperm motility is affected by prolonged storage of sperm in liquid nitrogen. A retrospective study. Cryobiology 2018, 80, 119–125. [Google Scholar] [CrossRef]
- Cremades, T.; Roca, J.; Rodriguez-Martinez, H.; Abaigar, T.; Vazquez, J.M.; Martinez, E.A. Kinematic changes during the cryopreservation of boar spermatozoa. J. Androl. 2005, 26, 610–618. [Google Scholar] [CrossRef]
- Broekhuijse, M.L.; Sostaric, E.; Feitsma, H.; Gadella, B.M. Application of computer-assisted semen analysis to explain variations in pig fertility. J. Anim. Sci. 2012, 90, 779–789. [Google Scholar] [CrossRef]
- Holt, C.; Holt, W.V.; Moore, H.D.; Reed, H.C.; Curnock, R.M. Objectively measured boar sperm motility parameters correlate with the outcomes of on-farm inseminations: Results of two fertility trials. J. Androl. 1997, 18, 312–323. [Google Scholar] [CrossRef]
- Vyt, P.; Maes, D.; Dejonckheere, E.; Castryck, F.; Van Soom, A. Comparative study on five different commercial extenders for boar semen. Reprod. Domest. Anim. 2004, 39, 8–12. [Google Scholar] [CrossRef]
- Pezo, F.; Romero, F.; Zambrano, F.; Sánchez, R.S. Preservation of boar semen: An update. Reprod. Domest. Anim. 2019, 54, 423–434. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Gong, Y.; He, B.; Zhao, R. Relationships between mitochondrial DNA content, mitochondrial activity, and boar sperm motility. Theriogenology 2017, 87, 276–283. [Google Scholar] [CrossRef] [PubMed]
- de Mercado, E.; Tomás-Almenar, C.; Gómez-Izquierdo, E. Improvement of the motility of boar sperm after cryopreservation. Anim. Reprod. Sci. 2020, 222, 106610. [Google Scholar] [CrossRef] [PubMed]
- Halo, M.; Tirpák, F.; Massányi, M.; Kováč, J.; Mlyneková, E.; Greń, A.; Massányi, P. The effects of caffeine on the motility and viability of stallion spermatozoa at different temperature conditions. Acta Vet. Hung. 2022, 70, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Einarsson, S.; Brandt, Y.; Lundeheim, N.; Madej, A. Stress and its influence on reproduction in pigs: A review. Acta Vet. Scand. 2008, 50, 48. [Google Scholar] [CrossRef] [PubMed]
- Cerolini, S.; Maldjian, A.; Surai, P.; Noble, R. Viability, susceptibility to peroxidation and fatty acid composition of boar semen during liquid storage. Anim. Reprod. Sci. 2000, 58, 99–111. [Google Scholar] [CrossRef]
- Casas, I.; Althouse, G.C. The protective effect of a 17 °C holding time on boar sperm plasma membrane fluidity after exposure to 5 °C. Cryobiology 2013, 66, 69–75. [Google Scholar] [CrossRef]
- Canvin, A.T.; Buhr, M.M. Effect of temperature on the fluidity of boar sperm membranes. J. Reprod. Fertil. 1989, 85, 533–540. [Google Scholar] [CrossRef]
- Henning, H.; Nguyen, Q.T.; Wallner, U.; Waberski, D. Temperature limits for storage of extended boar semen from the perspective of the sperm’s energy status. Front. Vet. Sci. 2022, 9, 953021. [Google Scholar] [CrossRef]
- Zou, C.X.; Yang, Z.M. Evaluation on sperm quality of freshly ejaculated boar semen during in vitro storage under different temperatures. Theriogenology 2000, 53, 1477–1488. [Google Scholar] [CrossRef]
- Moazamian, R.; Polhemus, A.; Connaughton, H.; Fraser, B.; Whiting, S.; Gharagozloo, P.; Aitken, R.J. Oxidative stress and human spermatozoa: Diagnostic and functional significance of aldehydes generated as a result of lipid peroxidation. Mol. Hum. Reprod. 2015, 21, 502–515. [Google Scholar] [CrossRef]
- Basioura, A.; Tsakmakidis, I.A.; Martinez, E.A.; Roca, J.; Li, J.; Molina, M.F.; Theodoridis, A.; Boscos, C.M.; Parrilla, I. Effect of astaxanthin in extenders on sperm quality and functional variables of frozen-thawed boar semen. Anim. Reprod. Sci. 2020, 218, 106478. [Google Scholar] [CrossRef]
- Zhu, Z.; Li, R.; Fan, X.; Lv, Y.; Zheng, Y.; Hoque, S.A.M.; Wu, D.; Zeng, W. Resveratrol improves boar sperm quality via 5′AMP-activated protein kinase activation during cryopreservation. Oxid. Med. Cell Longev. 2019, 2019, 5921503. [Google Scholar] [CrossRef]
- He, W.H.; Zhai, X.H.; Duan, X.J.; Di, H.S. Effect of resveratrol treatment on apoptosis and apoptotic pathways during boar semen freezing. J. Zhejiang Univ. Sci. B 2020, 21, 485–494. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Li, J.; Wang, S.; Ju, H.; Chen, S.; Basioura, A.; Ferreira-Dias, G.; Liu, Z.; Zhu, J. Post-Thaw Storage Temperature Influenced Boar Sperm Quality and Lifespan through Apoptosis and Lipid Peroxidation. Animals 2024, 14, 87. https://doi.org/10.3390/ani14010087
Li J, Li J, Wang S, Ju H, Chen S, Basioura A, Ferreira-Dias G, Liu Z, Zhu J. Post-Thaw Storage Temperature Influenced Boar Sperm Quality and Lifespan through Apoptosis and Lipid Peroxidation. Animals. 2024; 14(1):87. https://doi.org/10.3390/ani14010087
Chicago/Turabian StyleLi, Junwei, Juncheng Li, Shuaibiao Wang, Huiming Ju, Shufang Chen, Athina Basioura, Graça Ferreira-Dias, Zongping Liu, and Jiaqiao Zhu. 2024. "Post-Thaw Storage Temperature Influenced Boar Sperm Quality and Lifespan through Apoptosis and Lipid Peroxidation" Animals 14, no. 1: 87. https://doi.org/10.3390/ani14010087
APA StyleLi, J., Li, J., Wang, S., Ju, H., Chen, S., Basioura, A., Ferreira-Dias, G., Liu, Z., & Zhu, J. (2024). Post-Thaw Storage Temperature Influenced Boar Sperm Quality and Lifespan through Apoptosis and Lipid Peroxidation. Animals, 14(1), 87. https://doi.org/10.3390/ani14010087





