Trends in Admissions and Outcomes at a British Wildlife Rehabilitation Centre over a Ten-Year Period (2012–2022)
Abstract
:Simple Summary
Abstract
1. Introduction
The United Kingdom Context
2. Materials and Methods
3. Results
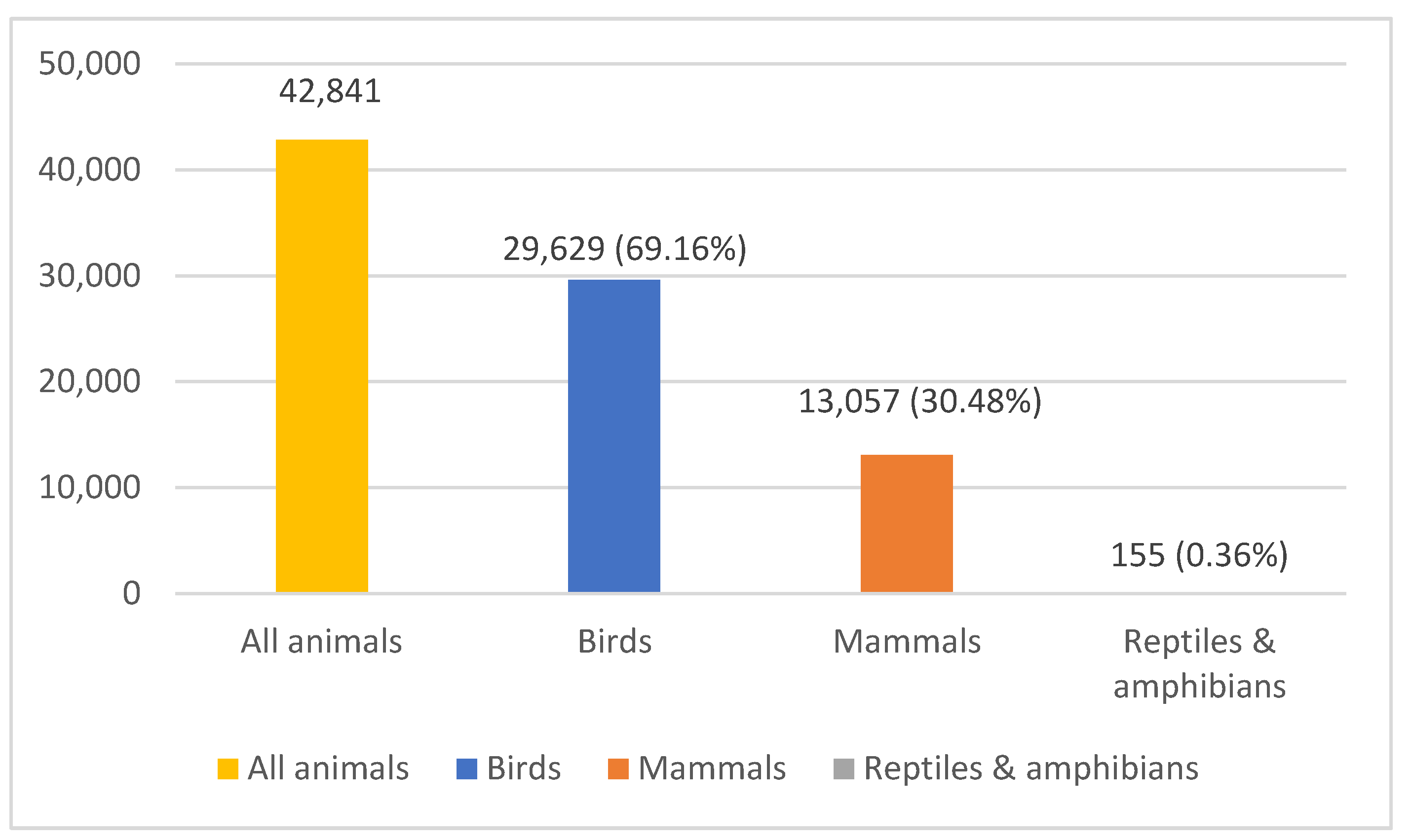
3.1. Total and Class of Animals Admitted
3.2. Changes in Total Admissions over the Study Period
3.3. Changes in the Class of Animal over the Study Period
3.4. Seasonal Variation in Admissions
3.5. Species Admitted
3.6. Sex of Animal
3.7. Age of Animal Admitted
3.8. Reason for Admission
3.9. Reason for Admission by Season
3.10. Overall and Animal Class Outcomes
3.11. Reason for Admission vs. Outcome
3.12. Outcomes over Time
3.13. Time in Captivity
4. Discussion
4.1. Total and Class of Animals Admitted
4.2. Changes in Admissions over the Study Period
4.3. Changes in Class of Animal over the Study Period
4.4. Seasonal Variation in Admissions
4.5. Species Admitted
4.6. Sex of Animal Admitted
4.7. Age of Animal Admitted
4.8. Reasons for Admission
4.8.1. Reasons for Admissions: ‘Injury’
4.8.2. Reasons for Admission: Poisoned/Polluted
4.8.3. Reasons for Admissions—Dog or Cat Attack
4.8.4. Reasons for Admissions—Anthropogenic
4.8.5. Reasons for Admission: ‘Orphaned’
4.8.6. Reasons for Admission: ‘Natural Causes’
4.8.7. Reason for Admission: ‘Unknown’
4.9. Reasons for Admissions by Season
4.10. Overall and Animal Class Outcomes
4.11. Reason for Admission vs. Outcome
4.12. Outcomes over Time
4.13. Time in Captivity
4.14. Importance of Findings and Opportunities for Further Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miller, E.A. (Ed.) Minimum Standards for Wildlife Rehabilitation, 4th ed.; National Wildlife Rehabilitators Association and International Wildlife Rehabilitation Council: St. Cloud, MN, USA, 2012; p. 9. [Google Scholar]
- Kwok, A.B.C.; Haering, R.; Travers, S.K.; Stathis, P. Trends in wildlife rehabilitation rescues and animal fate across a six-year period in New South Wales, Australia. PLoS ONE 2021, 16, e0257209. [Google Scholar] [CrossRef] [PubMed]
- Cope, H.R.; McArthur, C.; Dickman, C.R.; Newsome, T.M.; Gray, R.; Herbert, C.A. A systematic review of factors affecting wildlife survival during rehabilitation and release. PLoS ONE 2022, 17, e0265514. [Google Scholar] [CrossRef] [PubMed]
- Tribe, A.; Brown, P.R. The role of wildlife rescue groups in the care and rehabilitation of Australian fauna. Hum. Dimens. Wildl. 2000, 5, 69–85. [Google Scholar] [CrossRef]
- Englefield, B.; Blackman, S.A.; Starling, M.; McGreevy, P.D. A Review of Australian Animal Welfare Legislation, Regulation, Codes of Practice, and Policy, and Their Influence on Stakeholders Caring for Wildlife and the Animals for Whom They Care. Animals 2019, 9, 335. [Google Scholar] [CrossRef] [PubMed]
- Dubois, S.A. Survey of Wildlife Rehabilitation Goals, Impediments, Issues, and Success in British Columbia, Canada. Ph.D. Thesis, University of British Columbia, Vancouver, BC, Canada, 2003. Available online: https://open.library.ubc.ca/collections/ubctheses/831/items/1.0091066 (accessed on 7 July 2023).
- Haering, R.; Wilson, V.; Zhuo, A.; Stathis, P. Towards a more effective model of wildlife care and rehabilitation: A survey of volunteers in New South Wales, Australia. Australian Zoologist. Aust. Zool. 2020, 40, 605–627. [Google Scholar] [CrossRef]
- Perry, D.J.; Averka, J.P. Caring for the Circle of Life: Wildlife Rehabilitation and Sanctuary Care. Hum. Wildl. Interact. 2020, 14, 18. [Google Scholar] [CrossRef]
- Andrade, R.; Bateman, H.L.; Larson, K.L.; Herzog, C.; Brown, J.A. To the rescue—Evaluating the social-ecological patterns for bird intakes. Urban Ecosyst. 2022, 25, 179–192. [Google Scholar] [CrossRef]
- Loyd, K.; Hernandez, S.; McRuer, D. The role of domestic cats in the admission of injured wildlife at rehabilitation and rescue centers. Wildl. Soc. Bull. 2017, 41, 55–56. [Google Scholar] [CrossRef]
- Demezas, K.G.; Robinson, W.D. Characterizing the Influence of Domestic Cats on Birds with Wildlife Rehabilitation Center Data. Diversity 2021, 13, 322. [Google Scholar] [CrossRef]
- Vezyrakis, A.; Bontzorlos, V.; Rallis, G.; Ganoti, M. Two decades of wildlife rehabilitation in Greece: Major threats, admission trends and treatment outcomes from a prominent rehabilitation centre. J. Nat. Conserv. 2023, 73, 126372. [Google Scholar] [CrossRef]
- Sleeman, J. Use of wildlife rehabilitation centers as monitors of ecosystem health. Zoo Wild Anim. Med. 2008, 6, 97–104. [Google Scholar] [CrossRef]
- Dowding, C.V.; Shore, R.F.; Worgan, A.; Baker, P.J.; Harris, S. Accumulation of Anticoagulant Rodenticides in a Non-target Insectivore, the European hedgehog (Erinaceus europaeus). Environ. Pollut. 2010, 158, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Badry, A.; Krone, O.; Jaspers, V.L.B.; Mateo, R.; García-Fernández, A.; Leivits, M.; Shore, R.F. Towards harmonisation of chemical monitoring using avian apex predators: Identification of key species for pan-European biomonitoring. Sci. Total Environ. 2020, 731, 139198. [Google Scholar] [CrossRef] [PubMed]
- Taylor-Brown, A.; Booth, R.; Gillett, A.; Mealy, E.; Ogbourne, S.M.; Polkinghorne, A.; Conroy, G.C. The impact of human activities on Australian wildlife. PLoS ONE 2019, 14, e0206958. [Google Scholar] [CrossRef] [PubMed]
- Panter, C.T.; Allen, S.; Backhouse, N.; Mullineaux, E.; Rose, C.-A.; Amar, A. Causes, temporal trends, and the effects of urbanization on admissions of wild raptors to rehabilitation centers in England and Wales. Ecol. Evol. 2022, 12, e8856. [Google Scholar] [CrossRef] [PubMed]
- Sleeman, J.M.; Clark, E.E. Clinical Wildlife Medicine: A New Paradigm for a New Century. J. Avian Med. Surg. 2003, 17, 33–37. Available online: http://www.jstor.org/stable/40236483 (accessed on 20 November 2023). [CrossRef]
- Pacioni, C.; Rafferty, C.; Morley, K.; Stevenson, S.; Chapman, A.; Wickins, M.; Verney, T.; Deegan, G.; Trocini, S.; Spencer, P.B.S. Augmenting the conservation value of rehabilitated wildlife by integrating genetics and population modeling in the post-rehabilitation decision process. Curr. Zool. 2017, 64, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Pyke, G.H.; Szabo, J.K. Conservation and the 4 Rs, which are rescue, rehabilitation, release, and research. Conserv. Biol. 2018, 32, 50–59. [Google Scholar] [CrossRef]
- Paterson, J.E.; Carstairs, S.; Davy, C.M. Population-level effects of wildlife rehabilitation and release vary with life-history strategy. J. Nat. Conserv. 2021, 61, 125983. [Google Scholar] [CrossRef]
- Simpson, V.R. Health status of otters in southern and south west England 1996–2003. In Science Report: SCO010064/SR1; Bristol, Environment Agency: Bristol, UK, 2007. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/291660/scho0307bmkl-e-e.pdf (accessed on 20 November 2023).
- Narayan, E.; Vanderneut, T. Physiological Stress in Rescued Wild Koalas Are Influenced by Habitat Demographics, Environmental Stressors, and Clinical Intervention. Front. Endocrinol. 2019, 10, 18. [Google Scholar] [CrossRef]
- Rasmussen, S.L.; Kalliokoski, O.; Dabelsteen, T.; Abelson, K. An exploratory investigation of glucocorticoids, personality and survival rates in wild and rehabilitated hedgehogs (Erinaceus europaeus) in Denmark. BMC Ecol. Evol. 2021, 21, 96. [Google Scholar] [CrossRef] [PubMed]
- Le Barzic, C.; Cmokova, A.; Denaes, C.; Arné, P.; Hubka, V.; Guillot, J.; Risco-Castillo, V. Detection and Control of Dermatophytosis in Wild European Hedgehogs (Erinaceus europaeus) Admitted to a French Wildlife Rehabilitation Centre. J. Fungi 2021, 7, 74. [Google Scholar] [CrossRef] [PubMed]
- Deem, S.L.; Karesh, W.B.; Weisman, W. Putting Theory into Practice: Wildlife Health in Conservation. Conserv. Biol. 2001, 15, 1224–1233. [Google Scholar] [CrossRef]
- Mullineaux, E.; Kidner, P. Managing public demand for badger rehabilitation in an area of England with endemic tuberculosis. Vet. Microbiol. 2011, 151, 205–208. [Google Scholar] [CrossRef] [PubMed]
- Baros Jorquera, C.; Moreno-Switt, A.I.; Sallaberry-Pincheira, N.; Munita, J.M.; Flores Navarro, C.; Tardone, R.; González-Rocha, G.; Singer, R.S.; Bueno, I. Antimicrobial resistance in wildlife and in the built environment in a wildlife rehabilitation center. One Health 2021, 13, 100298. [Google Scholar] [CrossRef] [PubMed]
- Fagre, A.C.; Cohen, L.E.; Eskew, E.A.; Farrell, M.; Glennon, E.; Joseph, M.B.; Frank, H.K.; Ryan, S.J.; Carlson, C.J.; Albery, G.F. Assessing the risk of human-to-wildlife pathogen transmission for conservation and public health. Ecol. Lett. 2022, 25, 1534–1549. [Google Scholar] [CrossRef] [PubMed]
- Barnes, E.; Farnworth, M.J. Perceptions of responsibility and capability for treating wildlife casualties in UK veterinary practices. Vet. Rec. 2017, 180, 197. [Google Scholar] [CrossRef]
- Orr, B.; Tribe, A. Animal welfare implications of treating wildlife in Australian veterinary practices. Aust. Vet. J. 2018, 96, 475–480. [Google Scholar] [CrossRef]
- Mo, M.; Oliver, R. Managing non-releasable animals following rehabilitation: The current management framework in New South Wales, recent trends and a stakeholder consultative review. Aust. Zool. 2020, 4, 58–73. [Google Scholar] [CrossRef]
- Hanson, M.; Hollingshead, N.; Schuler, K.; Siemer, W.F.; Martin, P.; Bunting, E.M. Species, causes, and outcomes of wildlife rehabilitation in New York State. PLoS ONE 2021, 16, e0257675. [Google Scholar] [CrossRef]
- Wimberger, K.; Downs, C.T.; Boyes, R.S. A survey of wildlife rehabilitation in South Africa: Is there a need for improved management? Anim. Welf. 2010, 19, 481–499. [Google Scholar] [CrossRef]
- Englefield, B.; Candy, S.; Starling, M.; McGreevy, P. The Demography and Practice of Australians Caring for Native Wildlife and the Psychological, Physical and Financial Effects of Rescue, Rehabilitation and Release of Wildlife on the Welfare of Carers. Animals 2019, 9, 1127. [Google Scholar] [CrossRef] [PubMed]
- Romero, F.; Espinoza, A.; Sallaberry-Pincheira, N.; Napolitano, C. A five-year retrospective study on patterns of casuistry and insights on the current status of wildlife rescue and rehabilitation centers in Chile. Rev. Chil. De Hist. Nat. 2019, 92, 6. [Google Scholar] [CrossRef]
- Haering, R.; Wilson, V.; Zhuo, A.; Stathis, P. A survey of veterinary professionals about their interactions with free-living native animals and the volunteer wildlife rehabilitation sector in New South Wales, Australia. Aust. Zool. 2021, 41, 254–282. [Google Scholar] [CrossRef]
- Heathcote, G.; Hobday, A.J.; Spaulding, M.; Gard, M.; Irons, G. Citizen reporting of wildlife interactions can improve impact-reduction programs and support wildlife carers. Wildl. Res. 2019, 46, 415–428. [Google Scholar] [CrossRef]
- Wimberger, K.; Downs, C. Annual intake trends of a large urban animal rehabilitation centre in South Africa: A case study. Anim. Welf. 2010, 19, 501–513. [Google Scholar] [CrossRef]
- Long, R.B.; Krumlauf, K.; Young, A.M. Characterizing trends in human-wildlife conflicts in the American Midwest using wildlife rehabilitation records. PLoS ONE 2020, 15, e0238805. [Google Scholar] [CrossRef] [PubMed]
- Help Wildlife. Available online: https://helpwildlife.co.uk (accessed on 20 November 2023).
- Grogan, A.; Kelly, A. A review of RSPCA research into wildlife rehabilitation. Vet. Rec. 2013, 172, 211. [Google Scholar] [CrossRef]
- Molony, S.; Baker, P.; Garland, L.; Cuthill, I.; Harris, S. Factors that can be used to predict release rates for wildlife casualties. Anim. Welf. 2007, 16, 361–367. [Google Scholar] [CrossRef]
- Mullineaux, E. Veterinary treatment and rehabilitation of indigenous wildlife. J. Small Anim. Pract. 2014, 55, 293–300. [Google Scholar] [CrossRef]
- Best, D. BWRC wildlife casualty recording scheme—The first five years. Rehabil. 1999, 28, 2–3. [Google Scholar]
- Kirkwood, J.K. Introduction: Wildlife casualties and the veterinary surgeon. In BSAVA Manual of Wildlife Casualties; Mullineaux, E., Best, D., Cooper, J.E., Eds.; BSAVA Publications: Quedgeley, UK, 2003; pp. 1–5. [Google Scholar]
- Mathews, F.; Kubasiewicz, L.M.; Gurnell, J.; Harrower, C.A.; McDonald, R.A.; Shore, R.F. A Review of the Population and Conservation Status of British Mammals. A Report by the Mammal Society under Contract to Natural England, Natural Resources Wales and Scottish Natural Heritage; Natural England: Peterborough, UK, 2018; ISBN 978-1-78354-494-3. [Google Scholar]
- Stanbury, A.J.; Eaton, M.A.; Aebischer, N.J.; Balmer, D.; Brown, A.F.; Douse, A.; Lindley, P.; McCulloch, N.; Noble, D.G.; Win, I. Birds of Conservation Concern 5. Br. Birds 2021, 114, 723–747. Available online: https://www.bto.org/sites/default/files/publications/bocc-5-a5-4pp-single-pages.pdf (accessed on 20 November 2023).
- Burroughes, N.D.; Dowler, J.; Burroughes, G. Admission and Survival Trends in Hedgehogs Admitted to RSPCA Wildlife Rehabilitation Centres. Proc. Zool. Soc. 2021, 74, 198–204. [Google Scholar] [CrossRef]
- Wildlife Aid Foundation 2023. Available online: https://www.wildlifeaid.org.uk (accessed on 20 November 2023).
- St Tiggywinkles 2023. Available online: https://www.sttiggywinkles.org.uk (accessed on 20 November 2023).
- Molina-López, R.A.; Manñosa, S.; Torres-Riera, A.; Pomarol, M.; Darwich, L. Morbidity, outcomes and cost-benefit analysis of wildlife rehabilitation in Catalonia (Spain). PLoS ONE 2017, 12, e0181331. [Google Scholar] [CrossRef] [PubMed]
- Montesdeoca, N.; Calabuig, P.; Corbera, J.A.; Cooper, J.E.; Orós, J. Causes of morbidity and mortality, and rehabilitation outcomes of birds in Gran Canaria Island, Spain. Bird Study 2017, 64, 523–534. [Google Scholar] [CrossRef]
- Al Zoubi, M.Y.; Hamidan, N.A.; Abu Baker, M.A.; Amr, Z. Causes of Raptor Admissions to Rehabilitation in Jordan. J. Raptor Res. 2020, 54, 273–278. [Google Scholar] [CrossRef]
- Adhikari, B.; Baral, K.; Bhandari, S.; Kunwar, R.M.; Subedi, S.C. Prevalence of mortality in mammals: A retrospective study from wildlife rescue center of Nepal. Conserv. Sci. Pract. 2022, 4, e12799. [Google Scholar] [CrossRef]
- Butkus, C.E.; Peyton, J.L.; Heeren, A.J.; Clifford, D.L. Prevalence, treatment and survival of burned wildlife presenting to rehabilitation facilities from 2015 TO 2018. J. Zoo Wildl. Med. 2021, 52, 555–563. [Google Scholar] [CrossRef]
- Parrott, M.L.; Wicker, L.V.; Lamont, A.; Banks, C.; Lang, M.; Lynch, M.; McMeekin, B.; Miller, K.A.; Ryan, F.; Selwood, K.E.; et al. Emergency Response to Australia’s Black Summer 2019–2020: The Role of a Zoo-Based Conservation Organisation in Wildlife Triage, Rescue, and Resilience for the Future. Animals 2021, 11, 1515. [Google Scholar] [CrossRef]
- Warrington, M.H.; Schrimpf, M.B.; Des Brisay, P.; Taylor, M.E.; Koper, N. Avian behaviour changes in response to human activity during the COVID-19 lockdown in the United Kingdom. Proc. R. Soc. B 2022, 2740, 20212740. [Google Scholar] [CrossRef]
- Shoesmith, E.; Shahab, L.; Kale, D.; Mills, D.S.; Reeve, C.; Toner, P.; Santos de Assis, L.; Ratschen, E. The Influence of Human–Animal Interactions on Mental and Physical Health during the First COVID-19 Lockdown Phase in the U.K.: A Qualitative Exploration. Int. J. Environ. Res. Public Health 2021, 18, 76. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA); European Centre for Disease Prevention and Control; European Union Reference Laboratory for Avian Influenza; Adlhoch, C.; Fusaro, A.; Gonzales, J.L.; Kuiken, T.; Marangon, S.; Niqueux, E.; Staubach, C.; et al. Avian influenza overview December 2021–March 2022. EFSA J. 2022, 20, e07289. [Google Scholar] [CrossRef] [PubMed]
- Kelly, A.; Scivens, R.; Grogan, A. Post release survival of orphaned wild- born polecats Mustela putorius reared in captivity at a wildlife rehabilitation centre in England. Endanger. Species Res. 2010, 12, 107–115. [Google Scholar] [CrossRef]
- Kelly, A.; Halstead, C.; Hunter, D.; Leighton, K.; Grogan, A.; Harris, M. Factors affecting the likelihood of release of injured and orphaned woodpigeons (Columba palumbus). Anim. Welf. 2011, 20, 523–534. [Google Scholar] [CrossRef]
- Lukesova, G.; Voslarova, E.; Vecerek, V.; Vucinic, M. Trends in intake and outcomes for European hedgehog (Erinaceus europaeus) in the Czech rescue centers. PLoS ONE 2021, 16, e0248422. [Google Scholar] [CrossRef] [PubMed]
- Lukesova, G.; Voslarova, E.; Vecerek, V.; Vucinic, M. Causes of admission, length of stay and outcomes for common kestrels in rehabilitation centres in the Czech Republic. Sci. Rep. 2021, 11, 17269. [Google Scholar] [CrossRef]
- Kelly, T.R.; Sleeman, J.M. Morbidity and mortality of red foxes (Vulpes vulpes) and gray foxes (Urocyon cinereoargenteus) admitted to the Wildlife Center of Virginia, 1993–2001. J. Wildl. Dis. 2003, 39, 467–469. [Google Scholar] [CrossRef]
- Grogan, A.; Kelly, A. Rehabilitation and Release. In BSAVA Manual of Wildlife Casualties, 2nd ed.; Mullineaux, E., Keeble, E., Eds.; BSAVA Publications: Quedgeley, UK, 2016; pp. 81–92. [Google Scholar] [CrossRef]
- Molina-López, R.A.; Casal, J.; Darwich, L. Causes of Morbidity in Wild Raptor Populations Admitted at a Wildlife Rehabilitation Centre in Spain from 1995-2007: A Long Term Retrospective Study. PLoS ONE 2011, 6, e24603. [Google Scholar] [CrossRef]
- Cococcetta, C.; Coutant, T.; Collarile, T.; Vetere, A.; Di Ianni, F.; Huynh, M. Causes of Raptor Admission to the Wildlife Rehabilitation Centre in Abruzzo (Central Italy) from 2005–2016. Animals 2022, 12, 1916. [Google Scholar] [CrossRef]
- King, M.; Giacinti, J.; Dubois, S.; Lair, S.; Parmley, E.J.; Jardine, C.M. Using wildlife rehabilitation and postmortem data to identify key causes of morbidity and mortality impacting the health and welfare of free-living animals in Canada. J. Wildl. Dis. 2023, 59, 93–108. [Google Scholar] [CrossRef]
- Kratter, A.; Steadman, D.W. Mortality in birds from Florida wildlife rehabilitation clinics. Fla. Field Nat. 2020, 48, 147–166. Available online: https://sora.unm.edu/sites/default/files/FFN%2048.4%20pages%20147-166.pdf (accessed on 20 November 2023).
- Montesdeoca, N.; Calabuig, P.; Corbera, J.A.; Orós, J. A long-term retrospective study on rehabilitation of seabirds in Gran Canaria Island, Spain (2003–2013). PLoS ONE 2017, 12, e0177366. [Google Scholar] [CrossRef] [PubMed]
- Kelly, A.; Bland, M. Admissions, diagnoses, and outcomes for Eurasian sparrowhawks (Accipiter nisus) brought to a wildlife rehabilitation centre in North-West England. J. Raptor Res. 2006, 40, 231–235. [Google Scholar] [CrossRef]
- Wendell, M.D.; Sleeman, J.M.; Kratz, G. Retrospective study of morbidity and mortality of raptors admitted to Colorado State University Veterinary Teaching Hospital during 1995 to 1998. J. Wildl. Dis. 2002, 38, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Komnenou, A.T.; Georgopoulou, I.; Savvas, I.; Dessiris, A. A retrospective study of presentation, treatment, and outcome of free-ranging raptors in Greece (1997–2000). J. Zoo Wildl. Med. 2005, 36, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, B.; Rodríguez, A.; Siverio, F.; Siverio, M. Causes of Raptor Admissions to a Wildlife Rehabilitation Center in Tenerife (Canary Islands). J. Raptor Res. 2010, 44, 30–39. [Google Scholar] [CrossRef]
- Molina–López, R.A.; Darwich, L. Causes of admission of little owl (Athene noctua) at a wildlife rehabilitation centre in Catalonia (Spain) from 1995 to 2010. Anim. Biodivers. Conserv. 2011, 34, 401–405. [Google Scholar] [CrossRef]
- Maphalala, M.I.; Monadjem, A.; Bildstein, K.L.; Hoffman, B.; Downs, C. Causes of admission to a raptor rehabilitation centre and factors that can be used to predict the likelihood of release. Afr. J. Ecol. 2021, 59, 510–517. [Google Scholar] [CrossRef]
- Movalli, P.; Krone, O.; Osborn, D.; Pain, D. Monitoring contaminants, emerging infectious diseases and environmental change with raptors, and links to human health. Bird Study 2018, 65, S96–S109. [Google Scholar] [CrossRef]
- Taggart, M.A.; Shore, R.F.; Pain, D.J.; Peniche, G.; Martinez-Haro, M.; Mateo, R.; Homann, J.; Raab, A.; Feldmann, J.; Lawlor, A.J.; et al. Concentration and origin of lead (Pb) in liver and bone of Eurasian buzzards (Buteo buteo) in the United Kingdom. Environ. Pollut. 2020, 267, 115629. [Google Scholar] [CrossRef]
- Garcês, A.; Soeiro, V.; Lóio, S.; Sargo, R.; Sousa, L.; Silva, F.; Pires, I. Outcomes, Mortality Causes, and Pathological Findings in European Hedgehogs (Erinaceus europeus, Linnaeus 1758): A Seventeen Year Retrospective Analysis in the North of Portugal. Animals 2020, 10, 1305. [Google Scholar] [CrossRef] [PubMed]
- Chilvers, B.L.; Morgan, K.J.; White, B.J. Sources and reporting of oil spills and impacts on wildlife 197–2018. Environ. Sci. Pollut. Res. 2021, 28, 754–762. [Google Scholar] [CrossRef] [PubMed]
- Dauphiné, N.; Cooper, R.J. Impacts of free-ranging domestic cats (Felis catus) on birds in the United States: A review of recent research with conservation and management recommendations. In Proceedings of the fourth international partners in flight conference: Tundra to tropics, McAllen, TX, USA, 13–16 February 2008; pp. 205–219. Available online: https://www.partnersinflight.org/wp-content/uploads/2017/03/Dauphine-N.-and-R.-J.-Cooper-p-205-219.pdf (accessed on 20 November 2023).
- Loss, S.; Will, T.; Marra, P. The impact of free-ranging domestic cats on wildlife of the United States. Nat. Commun. 2013, 4, 1396. [Google Scholar] [CrossRef] [PubMed]
- Onyejekwe, E. Improving the welfare of both wildlife and domestic cats. Vet. Nurs. J. 2022, 37, 20–25. [Google Scholar]
- Woods, M.; McDonald, R.A.; Harris, S. Predation of wildlife by domestic cats Felis catus in Great Britain. Mammal Rev. 2003, 33, 174. [Google Scholar] [CrossRef]
- Baker, P.; Thompson, R.; Grogan, A. Survival rates of cat-attacked birds admitted to RSPCA wildlife centres in the UK: Implications for cat owners and wildlife rehabilitators. Anim. Welf. 2018, 27, 305–318. [Google Scholar] [CrossRef]
- Dessalvi, G.; Borgo, E.; Galli, L. The contribution to wildlife conservation of an Italian Recovery Centre. Nat. Conserv. 2021, 44, 1–20. [Google Scholar] [CrossRef]
- Timm, M.; Kime, N.M. Effects of Cat and Dog Interactions on Urban Wildlife Admitted to a Wildlife Center in Wisconsin. J. Young Investig. 2020, 38, 61–66. Available online: https://static1.squarespace.com/static/5443d7c7e4b06e8b47de9a55/t/5ed7c9a29d9c001666a832c2/1591200164685/JYI_June2020_61-66_locked.pdf (accessed on 20 November 2023).
- Mcruer, D.L.; Gray, L.C.; Horne, L.A.; Clark, E.E. Free-roaming cat interactions with wildlife admitted to a wildlife hospital. J. Wildl. Manag. 2017, 81, 163–173. [Google Scholar] [CrossRef]
- Koenig, J.; Shine, R.; Shea, G. The Dangers of Life in the City: Patterns of Activity, Injury and Mortality in Suburban Lizards (Tiliqua scincoides). J. Herpetol. 2002, 36, 62–68. [Google Scholar] [CrossRef]
- Ancillotto, L.; Serangeli, M.T.; Russo, D. Curiosity killed the bat: Domestic cats as bat predators. Mamm. Biol. 2013, 78, 369–373. [Google Scholar] [CrossRef]
- Mühldorfer, K.; Speck, S.; Wibbelt, G. Diseases in free-ranging bats from Germany. BMC Vet. Res. 2011, 7, 61. [Google Scholar] [CrossRef] [PubMed]
- Legge, S.; Woinarski, J.C.Z.; Dickman, C.R.; Murphy, B.P.; Woolley, L.-A.; Calver, M.C. We need to worry about Bella and Charlie: The impacts of pet cats on Australian wildlife. Wildl. Res. 2020, 47, 523–539. [Google Scholar] [CrossRef]
- Lawson, B.; Best, D. Passerines and other small birds. In BSAVA Manual of Wildlife Casualties, 2nd ed.; Mullineaux, E., Keeble, E., Eds.; BSAVA Publications: Quedgeley, UK, 2016; pp. 421–438. [Google Scholar] [CrossRef]
- Schenk, A.N.; Souza, M.J. Major anthropogenic causes for and outcomes of wild animal presentation to a wildlife clinic in East Tennessee, USA, 2000–2011. PLoS ONE 2014, 9, e93517. [Google Scholar] [CrossRef] [PubMed]
- Lukesova, G.; Voslarova, E.; Vecerek, V. Juvenile European hedgehogs (Erinaceus europaeus) at rescue centers and their release rate depending on their weight on admission. PLoS ONE 2021, 16, e0258273. [Google Scholar] [CrossRef] [PubMed]
- Mazaris, A.; Mamakis, Y.; Kalpakis, S.; Poulopoulos, Y.; Matsinos, Y. Evaluating potential threats to birds in Greece: An analysis of a 10-year data set from a rehabilitation centre. Oryx 2008, 42, 408–414. [Google Scholar] [CrossRef]
- RSPB. Bird Crime. Exposing Bird of Prey Persecution in the UK. RSPB, the Lodge, Sandy, UK, 2019. Available online: https://www.rspb.org.uk/globalassets/downloads/documents/birds-and-wildlife/crime/birdcrime-report-2019.pdf (accessed on 20 November 2023).
- Kelly, G.; delBraco-Trillo, J. Importance of taxonomic group, life stage and circumstance of rescue upon wildlife rehabilitation in Ontario, Canada. J. Nat. Conserv. 2020, 67, 125897. [Google Scholar] [CrossRef]
- Drake, A.; Fraser, D. Admission trends and mortality correlates for mallard ducklings at wildlife rehabilitation facilities. J. Wildl. Rehabil. 2008, 29, 4–14. [Google Scholar]
- Saran, K.A.; Parker, G.; Parker, R.; Dickman, C.R. Rehabilitation as a conservation tool: A case study using the common wombat. Pac. Conserv. Biol. 2011, 17, 310–319. [Google Scholar] [CrossRef]
- Meredith, A. Wildlife triage and decision-making. In BSAVA Manual of Wildlife Casualties, 2nd ed.; Mullineaux, E., Keeble, E., Eds.; BSAVA Publications: Quedgeley, UK, 2016; pp. 27–36. [Google Scholar] [CrossRef]
- Mullineaux, E. Assessment, triage and first aid. In An Introduction to Wildlife Rescue, Rehabilitation and Release; Mullineaux, E., Ed.; Secret World Wildlife RescueL: Somerset, UK, 2021; pp. 85–130. [Google Scholar]
- Chardonnet, P.; des Clers, B.; Fischer, J.; Gerhold, R.; Jori, F.; Lamarque, F. The value of wildlife. Rev. Sci. Et Tech.-Off. Int. Des Épizooties 2002, 21, 15–52. [Google Scholar] [CrossRef]
- Molony, S.E.; Dowding, C.V.; Baker, P.J.; Cuthill, I.C.; Harris, S. The effect of translocation and temporary captivity on wildlife rehabilitation success: An experimental study using European hedgehogs (Erinaceus europaeus). Biol. Conserv. 2006, 130, 530–537. [Google Scholar] [CrossRef]
- Rodríguez Díez, C.; González, F.; López, I.; Suárez, L.; Moraleda, V.; Rodríguez, C. Pododermatitis in raptors admitted in a wildlife rehabilitation centre in central spain. Prev. Vet. Med. 2020, 175, 104875. [Google Scholar] [CrossRef] [PubMed]
- Blair, C.D.; Muller, L.I.; Clark, J.D.; Stiver, W.H. Survival and Conflict Behavior of American Black Bears after Rehabilitation. J. Wildl. Manag. 2020, 84, 75–84. [Google Scholar] [CrossRef]
- Loftin, R.W. The medical treatment of wild animals. Environ. Ethics 1985, 7, 231–239. [Google Scholar] [CrossRef]
- Pyke, G.H.; Szabo, J.K. What can we learn from untapped wildlife rescue databases? The masked lapwing as a case study. Pac. Conserv. Biol. 2018, 24, 148–156. [Google Scholar] [CrossRef]
- Martínez, J.C.; Rosique, A.I.; Royo, M.S. Causes of admission and final dispositions of hedgehogs admitted to three Wildlife Rehabilitation Centers in eastern Spain. Hystrix Ital. J. Mammal. 2014, 25, 107–110. [Google Scholar] [CrossRef]
- Ruszkowski, J.J.; Hetman, M.; Turlewicz-Podbielska, H.; Pomorska-Mól, M. Hedgehogs as a Potential Source of Zoonotic Pathogens—A Review and an Update of Knowledge. Animals 2021, 11, 1754. [Google Scholar] [CrossRef]
- Wells, K.; Butterworth, A.; Richards, N. A review of secondary pentobarbital poisoning in scavenging wildlife, companion animals and captive carnivores. J. Vet. Forensic Sci. 2020, 1, 1–15. [Google Scholar] [CrossRef]
- Shine, R.; Koenig, J. Snakes in the garden: An analysis of reptiles “rescued” by community-based wildlife carers. Biol. Conserv. 2001, 102, 271–283. [Google Scholar] [CrossRef]
- Yeung, P.; White, B.; Ziccardi, M.; Chilvers, B.L. What Helps Oiled Wildlife Responders Care for Animals While Minimizing Stress and Compassion Fatigue. Animals 2021, 11, 1952. [Google Scholar] [CrossRef]








| Year-to-Year Transition | % Change on Previous Year | |
|---|---|---|
| Birds | Mammals | |
| 2012–2013 | 17% | −14% |
| 2013–2014 | 4% | 13% |
| 2014–2015 | 16% | 7% |
| 2015–2016 | – | 6% |
| 2016–2017 | −2% | – |
| 2017–2018 | 12% | −7% |
| 2018–2019 | −23% | 4% |
| 2019–2020 | −28% | −27% |
| 2020–2021 | 13% | 13% |
| Year-to-Year Transition | Birds | Mammals | ||
|---|---|---|---|---|
| Change | Z Statistic | Change | Z Statistic | |
| 2012–2013 | +7% | 6.58 * | –7% | 6.38 * |
| 2013–2014 | −1% | 1.58 | +2% | 1.66 |
| 2014–2015 | +1% | 1.80 | –2% | 1.77 |
| 2015–2016 | –1% | 1.21 | +1% | 1.16 |
| 2016–2017 | - | 0.54 | +1% | 0.06 |
| 2017–2018 | +3% | 4.07 * | –4% | 4.04 * |
| 2018–2019 | –7% | 7.29 * | +7% | 6.90 * |
| 2019–2020 | - | 0.27 | +1% | 0.31 |
| 2020–2021 | - | 0.02 | - | 0.03 |
| Season-to-Season Transition | ||
|---|---|---|
| Change | Z Statistic | |
| Autumn to Winter | +13% | 12.30 * |
| Winter to Spring | +5% | 5.25 * |
| Spring to Summer | +6% | 12.36 * |
| Summer to Autumn | −24% | 40.30 * |
| Common Name | Latin Name | UK Conservation Status | Number |
|---|---|---|---|
| Hedgehog-European | Erinaceus europaeus | Mammal Society Red List 1 | 5972 |
| Pigeon-Wood | Columba palumbus | BTO Amber List 2 | 3737 |
| Gull-Herring | Larus atgentatus | BTO Red List 2 | 3659 |
| Pigeon-Feral/Domestic/Racing | Columba livia domestica | BTO Green List 2 | 3115 |
| Blackbird-Common | Turdus merula | BTO Green List 2 | 2340 |
| Sparrow-House | Passer domesticus | BTO Red List 2 | 1703 |
| Duck Mallard | Anas platyrhynchos | BTO Amber List 2 | 1436 |
| Dove-Collard | Streptoppelia decaocto | BTO Green List 2 | 1334 |
| Rabbit-European | Oryctolagus cuniculus | Mammal Society Green List 1 | 1303 |
| All Classes | Birds | Mammals | Reptiles/Amphibians | |
|---|---|---|---|---|
| Injured | 10,909 (25.5%) | 8484 (28.7%) | 2346 (18.0%) | 69 (44.5%) |
| Poisoned/polluted | 89 (0.2%) | 54 (0.2%) | 35 (0.3%) | 0 |
| Orphaned | 12,124 (28.3%) | 7974 (26.9%) | 4147 (31.8%) | 3 (1.9%) |
| Natural causes | 1131 (2.6%) | 619 (2.1%) | 507 (3.9%) | 5 (3.2%) |
| Other | 9772 (22.8%) | 5841 (19.7%) | 3887 (29.8%) | 44 (28.4%) |
| Caught by cat | 4159 (9.7%) | 3486 (11.8%) | 656 (5.0%) | 17 (11.0%) |
| Caught by dog | 429 (1.0%) | 184 (0.6%) | 242 (1.9%) | 3 (1.9%) |
| Unknown | 4228 (9.9%) | 2977 (10.0%) | 1237 (9.5%) | 14 (9.0%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mullineaux, E.; Pawson, C. Trends in Admissions and Outcomes at a British Wildlife Rehabilitation Centre over a Ten-Year Period (2012–2022). Animals 2024, 14, 86. https://doi.org/10.3390/ani14010086
Mullineaux E, Pawson C. Trends in Admissions and Outcomes at a British Wildlife Rehabilitation Centre over a Ten-Year Period (2012–2022). Animals. 2024; 14(1):86. https://doi.org/10.3390/ani14010086
Chicago/Turabian StyleMullineaux, Elizabeth, and Chris Pawson. 2024. "Trends in Admissions and Outcomes at a British Wildlife Rehabilitation Centre over a Ten-Year Period (2012–2022)" Animals 14, no. 1: 86. https://doi.org/10.3390/ani14010086







