Mercury and Selenium Accumulation in the Tissues of Stranded Bottlenose Dolphins (Tursiops truncatus) in Northeast Florida, 2013–2021
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Sites
2.2. Field Collection
2.3. Sample Preparation
2.4. Mercury and Selenium Analysis
2.5. Data Analysis
3. Results
(3.048 × Mercury concentration (nmol/g)).
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Knap, A.H.; Cook, S.B.; Cook, C.B.; Simmons, J.A.; Jones, R.J.; Murray, A.E. Marine Environmental Studies to Determine the Impact of the Mass Burn Incinerator Proposed for Tynes Bay, Bermuda; Ministry of Works and Engineering, Bermuda Biological Station for Research: Hamilton, Bermuda, 1991. [Google Scholar]
- Guzman, H.M.; Garcia, E.M. Mercury levels in coral reefs along the Caribbean coast of Central America. Mar. Pollut. Bull. 2002, 44, 1415–1420. [Google Scholar] [CrossRef] [PubMed]
- Teng, H.; Altaf, A.R. Elemental mercury (Hg0) emission, hazards, and control: A brief review. J. Hazard. Mater. 2022, 5, 100049. [Google Scholar] [CrossRef]
- Yang, W.; Wang, Z.; Liu, Y. Review on magnetic adsorbents for removal of elemental mercury from flue gas. Energ. Fuel 2020, 34, 13474–13490. [Google Scholar] [CrossRef]
- Mason, R.P.; Choi, A.L.; Fitzgerald, W.F.; Hammerschmidt, C.R.; Lamborg, C.H.; Soerensen, A.L.; Sunderland, E.M. Mercury biogeochemical cycling in the ocean and policy implications. Environ. Res. 2012, 119, 101–117. [Google Scholar] [CrossRef] [PubMed]
- Pompe-Gotal, J.; Srebocan, E.; Gomercic, H.; Prevendar Crnic, A. Mercury concentrations in the tissues of bottlenose dolphins (Tursiops truncatus) and striped dolphins (Stenella coeruloalba) stranded on the Croatian Adriatic coast. Vet. Med. 2010, 54, 598–604. [Google Scholar] [CrossRef]
- Wang, S.L.; Xu, X.R.; Sun, Y.X.; Liu, J.L.; Li, H.B. Heavy metal pollution in coastal areas of South China: A review. Mar. Pollut. Bull. 2013, 76, 7–15. [Google Scholar] [CrossRef] [PubMed]
- McMeans, B.C.; Arts, M.T.; Fisk, A.T. Impacts of food web structure and feeding behavior on mercury exposure in Greenland sharks (Somniosus microcephalus). Sci. Total Environ. 2015, 509–510, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Durden, W.N.; Stolen, M.K.; Adams, D.H.; Stolen, E.D. Mercury and selenium concentrations in stranded bottlenose dolphins from the Indian River Lagoon system, Florida. Bull. Mar. Sci. 2007, 81, 37–54. [Google Scholar]
- Seixas, T.G.; Kehrig, H.A.; Costa, M.; Fillmann, G.; Di Beneditto, A.P.M.; Secchi, E.R.; Moreira, I. Total mercury, organic mercury and selenium in liver and kidney of a South American coastal dolphin. Environ. Pollut. 2008, 154, 98–106. [Google Scholar] [CrossRef]
- Stavros, H.W.; Stolen, M.; Durden, W.N.; McFee, W.; Bossart, G.D.; Fair, P.A. Correlation and toxicological inference of trace elements in tissues from stranded and free-ranging bottlenose dolphins (Tursiops truncatus). Chemosphere 2011, 82, 1649–1661. [Google Scholar] [CrossRef]
- Klinowska, M. Dolphins, Porpoises and Whales of the World: The IUCN Red Data Book; IUCN: Gland, Switzerland; Cambridge, UK, 1991; pp. 157–165. [Google Scholar]
- Frodello, J.P.; Romeo, M.; Viale, D. Distribution of mercury in the organs and tissues of five toothed-whale species of the Mediterranean. Environ. Pollut. 2000, 108, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Bilandžić, N.; Sedak, M.; Doki, M.; Gomercic, M.D.; Gomercic, T.; Zadravec, M.; Benic, M.; Crnic, A.P. Toxic element concentrations in the bottlenose (Tursiops truncatus), Striped (Stenella coeruleoalba) and Risso’s (Grampus griseus) dolphins stranded in Eastern Adriatic Sea. Bull. Environ. Contam. Toxicol. 2012, 89, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, M. Historical evidence of Tursiops truncatus exhibiting habitat preference and seasonal fidelity in northeast Florida. Aquat. Mamm. 2016, 42, 89–103. [Google Scholar] [CrossRef]
- Aguilar, A.; Borrel, A.; Pastor, T. Biological factors affecting variability of persistent pollutant levels in cetaceans. J. Cetac. Res. Manag. 1999, 1, 83–116. [Google Scholar] [CrossRef]
- Bossart, G.D. Marine mammals as sentinel species for oceans and human health. Oceanography 2006, 19, 44–47. [Google Scholar] [CrossRef]
- Lailson-Brito, J.; Cruz, R.; Dorneles, P.R.; Andrade, L.; Azevedo Ade, F.; Fragoso, A.B.; Vidal, L.G.; Costa, M.B.; Bisi, T.L.; Almeida, R.; et al. Mercury-selenium relationships in liver of Guiana dolphin: The possible role of Kupffer cells in the detoxification process by tiemannite formation. PLoS ONE 2012, 7, e42162. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, T.J. Environmental Contaminants and Marine Mammals. In Biology of Marine Mammals; Reynolds, J.M., Rommel, S.A., Eds.; Smithsonian Institution Press: Washington, DC, USA, 1999; pp. 485–564. [Google Scholar]
- Ruelas-Inzunza, J.R.; Horvat, M.; Perez-Cortes, H.; Paez-Osuna, F. Methylmercury and total mercury distribution in tissues of gray whales (Eschrichtius robustus) and spinner dolphins (Stenella longirostris) stranded along the lower Gulf of California, Mexico. Ciencias Marinas 2003, 29, 1–8. [Google Scholar] [CrossRef]
- De Moura, J.F.; Hacon Sde, S.; Vega, C.M.; Hauser-Davis, R.A.; de Campos, R.C.; Siciliano, S. Guiana dolphins (Sotalia guianensis, Van Benédén 1864) as indicators of the bioaccumulation of total mercury along the coast of Rio de Janeiro state, Southeastern Brazil. Bull. Environ. Contam. Toxicol. 2012, 88, 54–59. [Google Scholar] [CrossRef]
- Damseaux, F.; Kiszka, J.J.; Heithaus, M.R.; Scholl, G.; Eppe, G.; Thomé, J.P.; Lewis, J.; Hao, W.; Fontaine, M.C.; Das, K. Spatial variation in the accumulation of POPs and mercury in bottlenose dolphins of the Lower Florida Keys and the coastal Everglades (South Florida). Environ. Pollut. 2017, 220 Pt A, 577–587. [Google Scholar] [CrossRef]
- Lopez-Berenguer, G.; Peñalver, J.; Martínez-López, E. A critical review about neurotoxic effects in marine mammals of mercury and other trace elements. Chemosphere 2020, 246, 125688. [Google Scholar] [CrossRef]
- Cámara Pellissó, S.; Muñoz, M.J.; Carballo, M.; Sánchez-Vizcaíno, J.M. Determination of the immunotoxic potential of heavy metals on the functional activity of bottlenose dolphin leukocytes in vitro. Vet. Immunol. Immunopathol. 2008, 121, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Reif, J.; Schaefer, A.; Bossart, G. Atlantic bottlenose dolphins (Tursiops truncatus) as a sentinel for exposure to mercury in humans: Closing the loop. Vet. Sci. 2015, 2, 407–422. [Google Scholar] [CrossRef] [PubMed]
- Koeman, J.H.; Peeters, W.H.M.; Koudstaal-Hol, C.H.M.; Tjioe, P.S.; De Goeij, J.J.M. Mercury-selenium correlations in marine mammals. Nature 1973, 245, 385–386. [Google Scholar] [CrossRef] [PubMed]
- Ralston, N.V.C.; Unrine, J.; Wallschlager, D. Biogeochemistry and Analysis of Selenium and Its Species; North American Metals Council: Washington, DC, USA, 2008; Available online: https://www.researchgate.net/publication/228832815_Biogeochemistry_and_Analysis_of_Selenium_and_its_Species (accessed on 2 March 2024).
- Das, K.; Dupont, A.; De Pauw-Gillet, M.C.; Debier, C.; Siebert, U. Absence of selenium protection against methylmercury toxicity in harbour seal leucocytes in vitro. Mar. Pollut. Bull. 2016, 108, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Feng, X.; Chan, H.M.; Larssen, T. New insights into traditional health risk assessments of mercury exposure: Implications of selenium. Environ. Sci. Technol. 2014, 48, 1206–1212. [Google Scholar] [CrossRef] [PubMed]
- Decataldo, A.; Di Leo, A.; Giandomencio, S.; Cardellicchio, N. Association of metals (mercury, cadmium and zinc) with methallothionein-like proteins in storage organs of stranded dolphins from the Mediterranean Sea (Southern Italy). J. Environ. Monit. 2004, 6, 341–367. [Google Scholar] [CrossRef] [PubMed]
- Manhães, B.M.R.; Santos-Neto, E.B.; Tovar, L.R.; Guari, E.B.; Flach, L.; Kasper, D.; Galvão, P.M.A.; Malm, O.; Gonçalves, R.A.; Bisi, T.L.; et al. Changes in mercury distribution and its body burden in delphinids affected by a morbillivirus infection: Evidences of methylmercury intoxication in Guiana dolphin. Chemosphere 2021, 263, 128286. [Google Scholar] [CrossRef]
- Gales, N.; Woods, R.; Vogelnest, L. Marine mammal strandings and the role of the veterinarian. In Medicine of Australian Mammals; Woods, R., Vogelnest, L., Eds.; Csiro: Clayton South, Australia, 2008; pp. 39–55. [Google Scholar] [CrossRef]
- Dudhat, S.; Pande, A.; Nair, A.; Mondal, I.; Srinivasan, M.; Sivakumar, K. Spatio-temporal analysis identifies marine mammal stranding hotspots along the Indian coastline. Sci. Rep. 2002, 12, 4128. [Google Scholar] [CrossRef] [PubMed]
- Gulland, F.M.D.; Dierauf, L.A.; Whitman, K.L. CRC Handbook of Marine Mammal Medicine, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- NOAA Fisheries Marine Mammal Unusual Mortality Events. Available online: https://www.fisheries.noaa.gov/national/marine-mammal-protection/marine-mammal-unusual-mortality-events (accessed on 1 March 2024).
- NOAA Fisheries 2013–2015 Bottlenose Dolphin Unusual Mortality Event in the Mid-Atlantic (Closed). Available online: https://www.fisheries.noaa.gov/national/marine-life-distress/2013-2015-bottlenose-dolphin-unusual-mortality-event-mid-Atlantic (accessed on 1 March 2024).
- Stephens, N.; Duignan, P.J.; Wang, J.; Bingham, J.; Finn, H.; Bejder, L.; Patterson, A.P.; Holyoake, C. Cetacean morbillivirus in coastal Indo-Pacific bottlenose dolphins, Western Australia. Emerg. Infect. Dis. 2014, 20, 666–670. [Google Scholar] [CrossRef]
- Van Bressem, M.F.; Duignan, P.J.; Banyard, A.; Barbieri, M.; Colegrove, K.M.; De Guise, S.; Di Guardo, G.; Dobson, A.; Domingo, M.; Fauquier, D.; et al. Cetacean morbillivirus: Current knowledge and future directions. Viruses 2014, 6, 5145–5181. [Google Scholar] [CrossRef]
- Pyati, R.; Bielmyer, G.K.; Chalk, S.; McCarthy, D.; McCarthy, H.; Pinto, G.; Sonnenberg, L.; Welsh, P. Case Study: St. Johns River Basin, USA; Fourth United Nations World Water Development Report, World Water Assessment Programme; UNESCO Publishing: Paris, France, 2012. [Google Scholar]
- Environmental Protection Agency, United States. Method 7473 (SW-846): Mercury in Solids and Solutions by Thermal Decomposition, Amalgamation, and Atomic Absorption Spectrophotometry. Revision 0. 1998. Available online: https://www.epa.gov/esam/epa-method-7473-sw-846-mercury-solids-and-solutions-thermal-decomposition-amalgamation-and (accessed on 2 March 2024).
- Bellante, A.; Sprovieri, M.; Buscaino, G.; Buffa, G.; Di Stefano, V.; Salvagio Manta, D.; Barra, M.; Filiciotto, F.; Bonanno, A.; Giacoma, C.; et al. Stranded cetaceans as indicators of mercury pollution in the Mediterranean Sea. Ital. J. Zool. 2012, 79, 151–160. [Google Scholar] [CrossRef]
- Cardellicchio, N.; Decataldo, A.; Di, L.A.; Misino, A. Accumulation and tissue distribution of mercury and selenium in striped dolphins (Stenella coeruleoalba) from the Mediterranean Sea (southern Italy). Environ. Pollut. 2002, 116, 265–271. [Google Scholar] [CrossRef] [PubMed]
- García-Alvarez, N.; Fernández, A.; Boada, L.D.; Zumbado, M.; Zaccaroni, A.; Arbelo, M.; Sierra, E.; Almunia, J.; Luzardo, O.P. Mercury and selenium status of bottlenose dolphins (Tursiops truncatus): A study in stranded animals on the Canary Islands. Sci. Total Environ. 2015, 536, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Squadrone, S.; Chiaravalle, E.; Gavinelli, S.; Monaco, G.; Rizzi, M.; Abete, M.C. Analysis of mercury and methylmercury concentrations, and selenium: Mercury molar ratios for a toxicological assessment of sperm whales (Physeter macrocephalus) in the most recent stranding event along the Adriatic coast (Southern Italy, Mediterranean Sea). Chemosphere 2015, 138, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Caceres-Saez, I.; Haro, D.; Blank, O.; Aguayo Lobo, A.; Dougnac, C.; Arredondo, C.; Cappozzo, H.L.; Ribeiro Guevara, S. High status of mercury and selenium in false killer whales (Pseudorca crassidens, Osen 1846) stranded on Southern South America: A possible toxicological concern. Chemosphere 2018, 199, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Moreira, I.; Seixas, T.G.; Kehrig, H.A.; Fillmann, G.; Di Beneditto, A.P.; Souza, C.M.; Malm, O. Selenium and mercury (total and organic) in tissues of a coastal small cetacean, Pontoporia blainvillei. J. Coast. Res. 2009, 56, 866–870. [Google Scholar]
- Sedak, M.; Bilandžić, N.; Đokić, M.; Đuras, M.; Gomerčić, T.; Benić, M. Body burdens and distribution of mercury and selenium in bottlenose, striped and Risso’s dolphins along the Adriatic coast: A 20-year retrospective. Mar. Pollut. Bull. 2002, 185 Pt A, 114298. [Google Scholar] [CrossRef]
- Endo, T.; Kimura, O.; Hisamichi, Y.; Minoshima, Y.; Haraguchi, K.; Kakumoto, C.; Kobayashi, M. Distribution of total mercury, methyl mercury and selenium in pod of killer whales (Orcinus Orca) stranded in the northern area of Japan: Comparison of mature females with calves. Environ. Pollut. 2006, 144, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Hansen, A.M.; Bryan, C.E.; West, K.; Jensen, B.A. Trace element concentrations in liver of 16 species of cetaceans stranded on Pacific Islands from 1997 through 2013. Arch. Environ. Contam. Toxicol. 2016, 70, 75–95. [Google Scholar] [CrossRef]
- Kershaw, J.L.; Hall, A.J. Mercury in cetaceans: Exposure, bioaccumulation and toxicity. Sci. Total Environ. 2019, 694, 133683. [Google Scholar] [CrossRef]
- Roditi-Elasar, M.; Kerem, D.; Hornung, H.; Kress, N.; Shoham-Frider, E.; Goffman, O.; Spanier, E. Heavy metal levels in bottlenose and striped dolphins off the Mediterranean coast of Israel. Mar. Pollut. Bull. 2003, 46, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Martínez-López, E.; Peñalver, J.; Lara, L.; Garcia-Fernandez, A.J. Hg and Se in Organs of Three Cetacean Species from the Murcia Coastline (Mediterranean Sea). Bull. Environ. Contam. Toxicol. 2019, 103, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Wagemann, R.; Trebacz, E.; Boila, G.; Lockhart, W.L. Methylmercury and total mercury in tissues of arctic marine mammals. Sci. Total Environ. 1998, 218, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Rawson, A.J.; Patton, G.W.; Hofmann, S.; Pietra, G.G.; Johns, L. Liver Abnormalities Associated with Chronic Mercury Accumulation in Stranded Atlantic Bottlenosed Dolphins. Ecotoxicol. Environ. Saf. 1993, 25, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Meador, J.P.; Ernest, D.; Hohn, A.A.; Tilbury, K.; Gorzelany, J.; Worthy, G.; Stein, J.E. Comparison of elements in bottlenose dolphins stranded on the beaches of Texas and Florida in the Gulf of Mexico over a one-year period. Arch. Environ. Contam. Toxicol. 1999, 36, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Ralston, N.V.C.; Azenkeng, A.; Raymond, L.J. Mercury-Dependent Inhibition of Selenoenzymes and Mercury Toxicity. In Methylmercury and Neurotoxicity. Current Topics in Neurotoxicity; Ceccatelli, S., Aschner, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; Volume 2. [Google Scholar] [CrossRef]
- Nakazawa, E.; Ikemoto, T.; Hokura, A.; Terada, Y.; Kunito, T.; Tanabe, S.; Nakai, I. The presence of mercury selenide in various tissues of the striped dolphin: Evidence from μ-XRF-XRD and XAFS analyses. Metallomics 2011, 3, 719–725. [Google Scholar] [CrossRef]
- Marumoto, M.; Sakamoto, M.; Nakamura, M.; Marumoto, K.; Tsuruta, S. Organ-specific accumulation of selenium and mercury in Indo-Pacific bottlenose dolphins (Tursiops aduncus). Acta. Vet. Scand. 2022, 64, 1. [Google Scholar] [CrossRef]
- Pinto, G.; Bielmyer-Fraser, G.K.; Baynard, C.D.; Closmann, C.; Goldberg, N.; Jones, S.; Johnson, A.; Penwell, W.; Pyati, R.; Rosenblatt, A.; et al. State of the River Report for the Lower St. Johns River Basin, Florida: Water Quality, Fisheries, Aquatic Life, & Contaminants; Environmental Protection Board: Jacksonville, FL, USA, 2023. Available online: https://sjrreport.com (accessed on 15 June 2023).
- Marine Mammal Health and Stranding Response Program. Available online: https://www.fisheries.noaa.gov/national/marine-life-distress/marine-mammal-health-and-stranding-response-program (accessed on 1 March 2024).
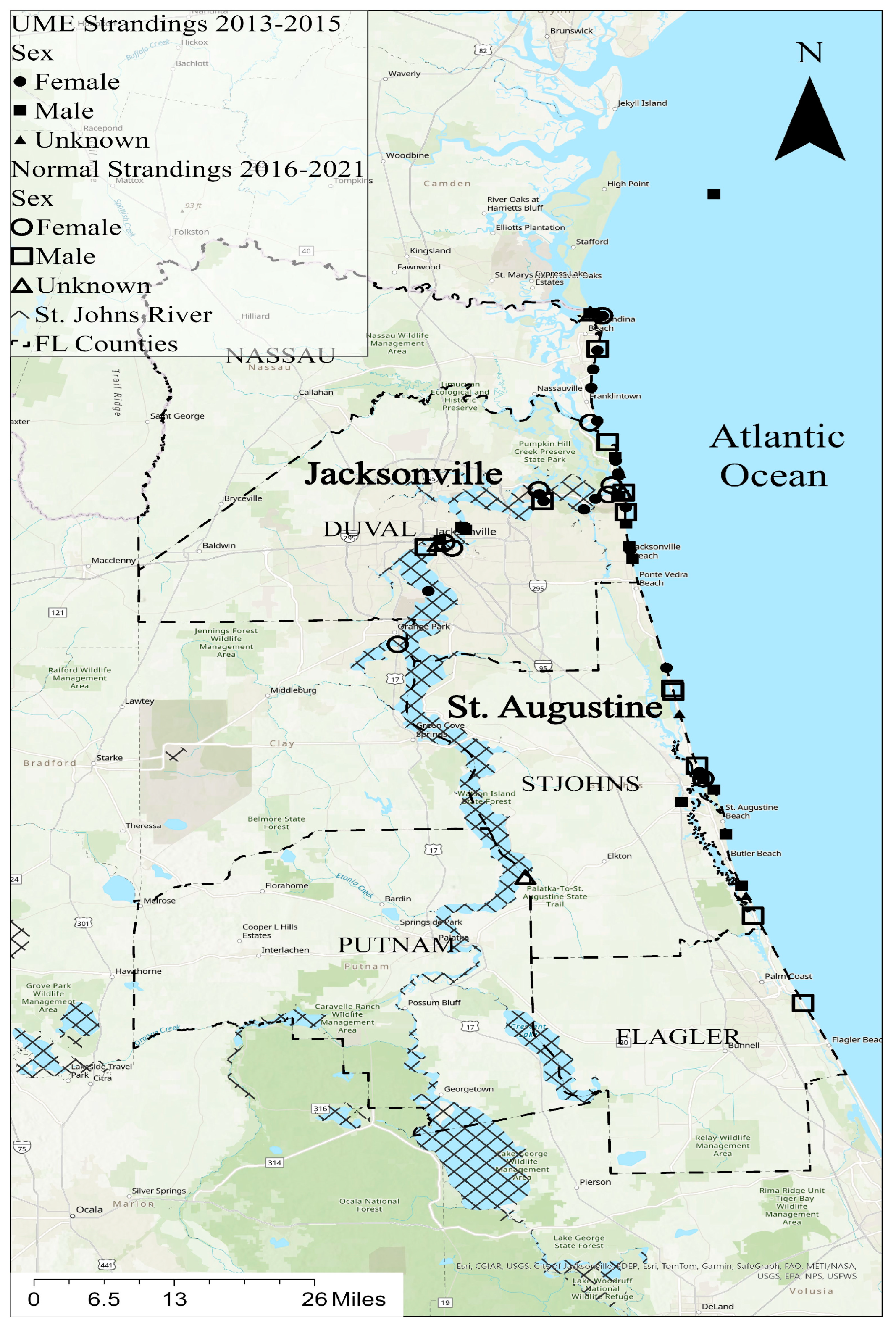

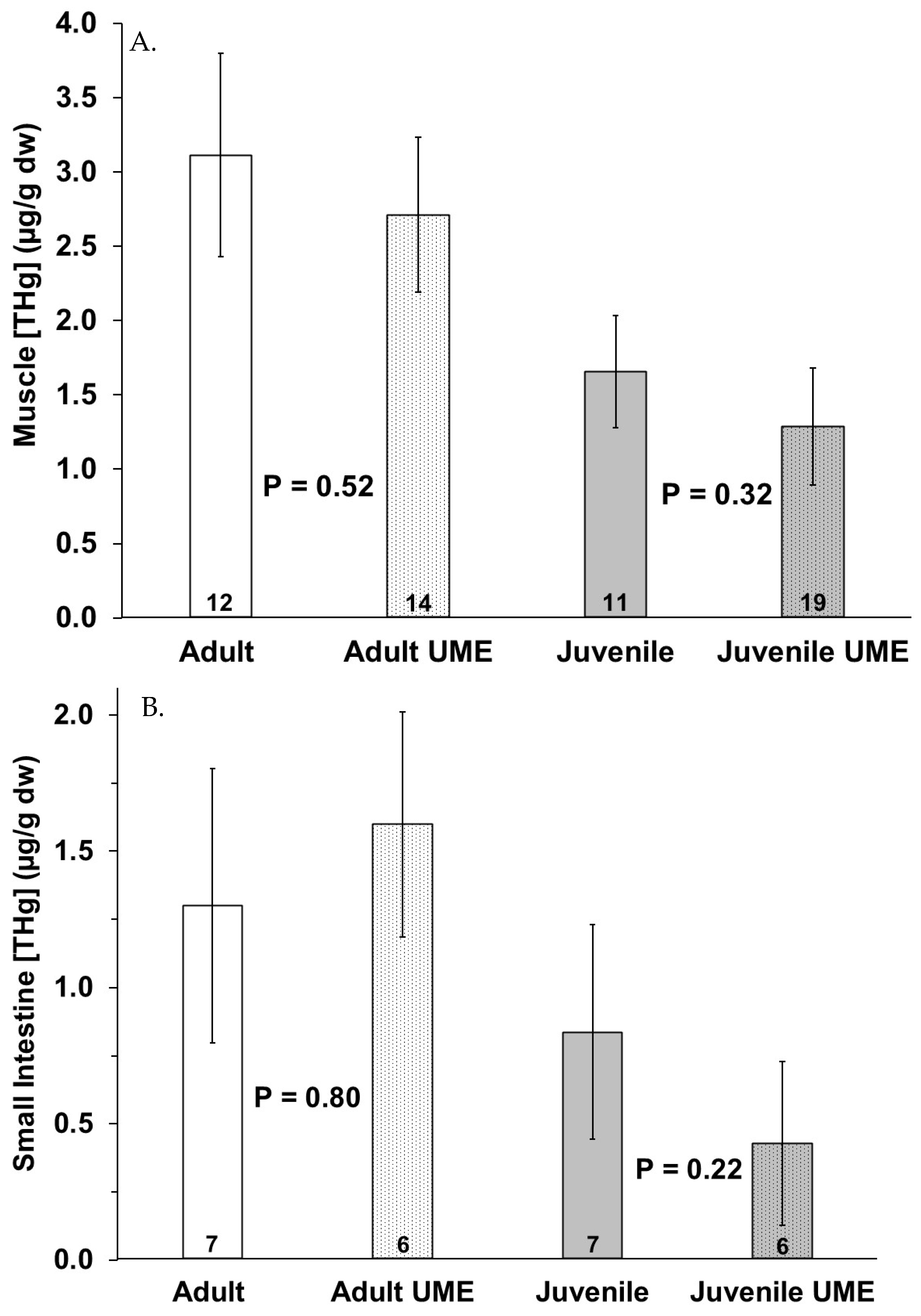

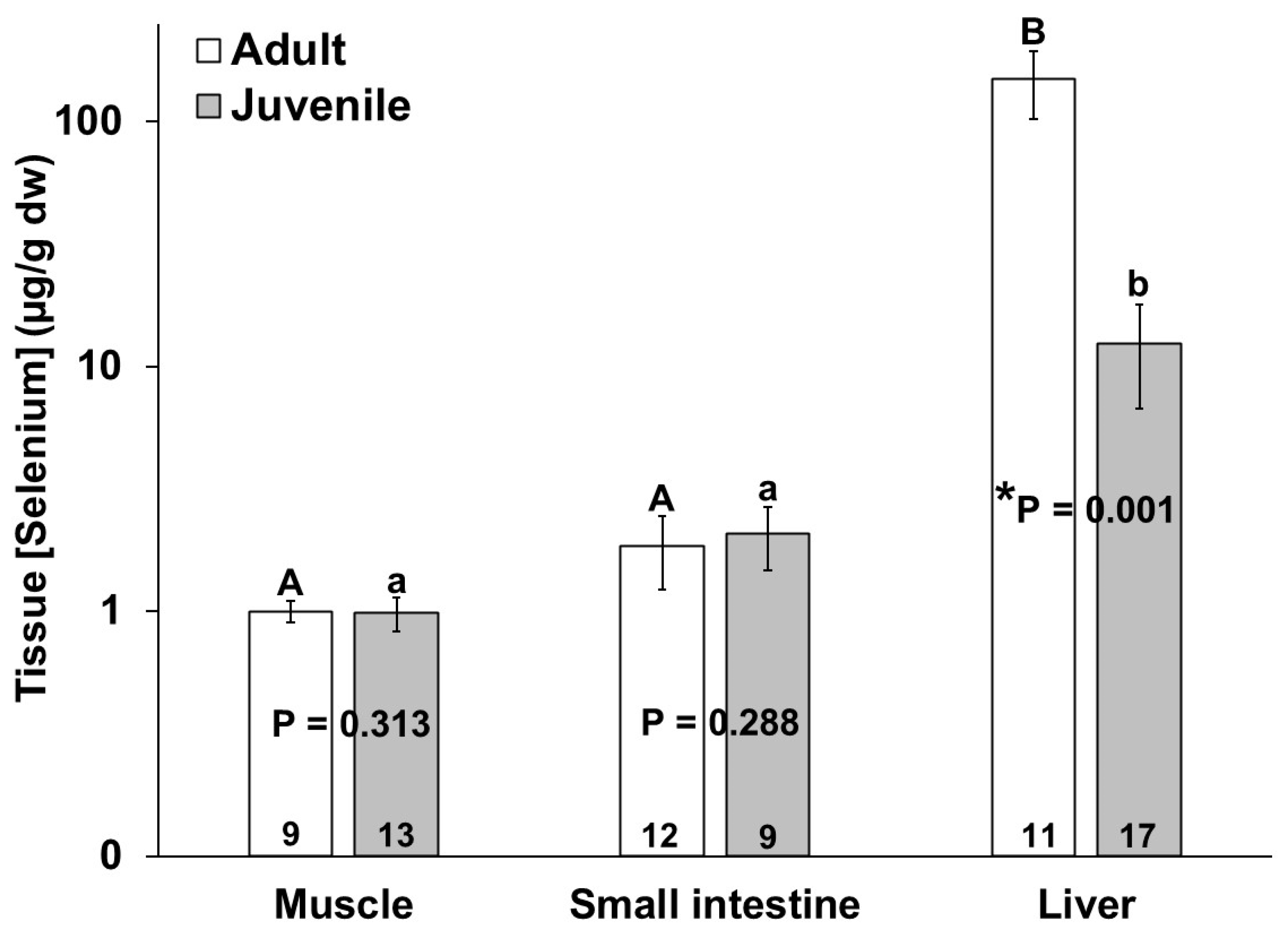


| Date | Sample ID | UME | Sex | Age Class | Total Length (cm) | Tissue | Element | Dec Code | Location |
|---|---|---|---|---|---|---|---|---|---|
| 3/21/2013 | TtNEFL1314 | N | M | Juvenile | 188 | L, M, SI L, M, SI | Se Hg | 3 | Coastal |
| 21/11/2013 | TtNEFL1345 | Y | M | Juvenile | 216 | M, SI M, SI | Se Hg | 3 | Coastal |
| 21/11/2013 | TtNEFL1346 | Y | M | Juvenile | 105 | M | Hg | 3 | Coastal |
| 21/11/2013 | TtNEFL1347 | Y | M | Juvenile | 136 | L, M | Hg | 3 | Coastal |
| 22/11/2013 | TtNEFL1348 | Y | F | Juvenile | 120 | M | Hg | 3 | Coastal |
| 24/11/2013 | TtNEFL1354 | Y | M | Adult | 257 | M | Hg | 3 | Coastal |
| 24/11/2013 | TtNEFL1356 | Y | UK | Juvenile | 110 | M | Hg | 3 | Coastal |
| 26/11/2013 | TtNEFL1361 | Y | F | Juvenile | 227 | M | Hg | 2 | Coastal |
| 27/11/2013 | TtNEFL1362 | Y | F | Adult | 252 | M | Hg | 2 | Coastal |
| 28/11/2013 | TtNEFL1363 | Y | F | Juvenile | 212 | M | Hg | 3 | Coastal |
| 1/12/2013 | TtNEFL1366 | Y | F | Adult | 249 | M | Hg | 3 | Coastal |
| 6/12/2013 | TtNEFL1373 | Y | F | Juvenile | 233 | SI SI | Se Hg | 3 | Coastal |
| 8/12/2013 | TtNEFL1376 | Y | F | Adult | 277 | M | Hg | 3 | Coastal |
| 19/12/2013 | TtNEFL1384 | Y | F | Juvenile | 214 | M | Hg | 3 | Coastal |
| 22/12/2013 | TtNEFL1385 | Y | F | Juvenile | UK | L, SI L, M | Se Hg | UK | Intra-coastal |
| 27/12/2013 | TtNEFL1387 | Y | M | Juvenile | 148 | M | Hg | 3 | Coastal |
| 24/3/2014 | TtNEFL1416 | Y | M | Juvenile | 143 | L, M | Hg | 3 | Coastal |
| 24/3/2014 | TtNEFL1417 | Y | M | Juvenile | 100 | M | Hg | 3 | Coastal |
| 25/3/2014 | TtNEFL1418 | Y | F | Juvenile | 228 | L | Hg | 1,2 | Coastal |
| 4/4/2014 | TtNEFL1419 | Y | UK | UK | 253 | L, M, SI | Hg | 3 | Coastal |
| 11/4/2014 | TtNEFL1420 | Y | F | Juvenile | 197 | M | Hg | 2 | Coastal |
| 28/4/2014 | TtNEFL1423 | Y | M | Adult | 298 | L, M | Hg | 3 | Coastal |
| 25/5/2014 | TtNEFL1426 | Y | F | Juvenile | 185 | L, SI L, M, SI | Se Hg | 3 | SJR |
| 2/6/2014 | TtNEFL1428 | Y | F | Juvenile | 217 | L, M | Hg | 3 | SJR |
| 9/6/2014 | TtNEFL1429 | Y | F | Adult | 241 | SI M | Se Hg | 2 | Coastal |
| 17/6/2014 | TtNEFL1430 | Y | F | Adult | 238 | SI M, SI | Se Hg | 2 | Coastal |
| 296/2014 | TtNEFL1432 | Y | M | Juvenile | 198 | L | Hg | 3 | Coastal |
| 2/7/2014 | TtNEFL1433 | Y | M | Adult | 258 | L, SI M, SI | Se Hg | 3 | Coastal |
| 7/7/2014 | TtNEFL1435 | Y | M | Juvenile | 240 | M | Hg | 3 | SJR |
| 22/7/2014 | TtNEFL1440 | Y | F | Adult | 237 | L | Hg | 3 | SJR |
| 29/7/2014 | TtNEFL1444 | Y | F | Juvenile | 129 | L, SI L, M, SI | Se Hg | 3 | SJR |
| 7/8/2014 | TtNEFL1445 | Y | M | Adult | 254 | M | Hg | 2 | Coastal |
| 9/8/2014 | TtNEFL1446 | Y | M | Adult | 258 | M | Hg | 3 | Coastal |
| 19/8/2014 | TtNEFL1448 | Y | M | Juvenile | 148 | L, M, SI | Hg | 3 | Coastal |
| 22/8/2014 | TtNEFL1450 | Y | M | Adult | 247 | L, SI L, M, SI | Se Hg | 3 | Coastal |
| 29/8/2014 | TtNEFL1453 | Y | F | Juvenile | 113 | M | Hg | 3 | Coastal |
| 3/9/2014 | TtNEFL1454 | Y | M | Adult | 267 | L, SI L, M, SI | Se Hg | 3 | Coastal |
| 9/10/2014 | TtNEFL1458 | Y | M | Juvenile | 242 | L, M | Hg | 3 | SJR |
| 24/11/2014 | TtNEFL1462 | Y | M | Adult | 255 | SI M, SI | Se Hg | 3 | Coastal |
| 5/2/2015 | TtNEFL1503 | Y | F | Adult | 255 | M, SI M, SI | Se Hg | 2 | Coastal |
| 1/3/2015 | TtNEFL1506 | N | UK | Adult | 248 | M | Hg | 4 | Coastal |
| 5/3/2015 | TtNEFL1507 | N | M | Juvenile | 183 | SI M, SI | Se Hg | 3 | Coastal |
| 29/3/2015 | TtNEFL1510 | N | F | Adult | 269 | M, SI | Hg | 3 | SJR |
| 6/1/2016 | TtNEFL1601 | N | M | Juvenile | 174 | L | Hg | 3 | Coastal |
| 19/2/2016 | TtNEFL1602 | N | M | Juvenile | 117 | M | Hg | 3 | Coastal |
| 7/4/2016 | TtNEFL1607 | N | M | Juvenile | 200 | L, M, SI | Hg | 3 | MR |
| 1/6/2016 | TtNEFL1608 | N | M | Adult | 261 | L, SI L, M, SI | Se Hg | 3 | Coastal |
| 6/6/2016 | TtNEFL1610 | N | F | Adult | 253 | M | Hg | 3 | SJR |
| 24/6/2016 | TtNEFL1613 | N | UK | UK | 253 | M | Hg | UK | UK |
| 18/6/2016 | TtNEFL1616 | N | F | Juvenile | 111 | L | Hg | 3 | Coastal |
| 25/8/2016 | TtNEFL1621 | N | F | Juvenile | 101 | SI SI | Se Hg | 3 | Coastal |
| 30/8/2016 | TtNEFL1624 | N | M | Juvenile | 212 | L, M | Hg | 3 | Coastal |
| 12/9/2016 | TtNEFL1626 | N | UK | Juvenile | 218 | M | Hg | 4 | Coastal |
| 27/12/2016 | TtNEFL1630 | N | M | Juvenile | 186 | M | Hg | 3 | SJR |
| 15/2/2019 | TtNEFL1904 | N | F | Adult | 247 | L, SI L, SI | Se Hg | 3 | SJR |
| 21/6/2019 | TtNEFL1914 | N | F | Adult | 247 | L, M, SI L, M, SI | Se Hg | 3 | SJR |
| 24/7/2019 | TtNEFL1916 | N | F | Juvenile | 170 | L, M, SI L, M, SI | Se Hg | 3 | SJR |
| 6/8/2019 | TtNEFL1918 | N | M | Adult | 271 | L, M, SI L, M, SI | Se Hg | 3 | SJR |
| 12/10/2019 | TtNEFL1922 | N | UK | Adult | 208 | L, M | Hg | 4 | SJR |
| 2/12/2019 | TtNEFL1923 | N | F | Juvenile | 162 | L, M, SI | Hg | 3 | Nassau Sound |
| 7/2/2020 | TtNEFL2001 | N | M | Juvenile | 210 | L, M, SI L, M, SI | Se Hg | 2 | Coastal |
| 20/10/2020 | TtNEFL2013 | N | UK | UK | UK | L, M | Hg | 4 | SJR |
| 8/4/2021 | TtNEFL2109 | N | M | Adult | 267 | L, M, SI L, M, SI | Se Hg | 2 | Coastal |
| 3/5/2021 | TtNEFL2112 | N | F | Adult | UK | L, M, SI L, M, SI | Se Hg | 2 | SJR |
| Age Class | Muscle Mean Molar Se:Hg | SI Mean Molar Se:Hg | Liver Mean Molar Se:Hg |
|---|---|---|---|
| Adult Normal | 1.48 (±0.87) | 0.97 (±1.63) | 3.70 (±2.16) |
| Adult UME | 0.75 (±1.02) p = 0.11 | 6.36 (±5.20) p = 0.07 | 1.67 (±1.73) p = 0.17 |
| Juvenile Normal | 3.62 (±2.18) | 3.21 (±4.52) | 0.91 (±0.95) |
| Juvenile UME | 2.68 (±1.79) p = 0.44 | 6.80 (±5.66) p = 0.48 | 0.95 (±0.95) p = 0.63 |
| Adult vs. Juvenile | * p = 0.01 | p = 0.61 | * p < 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bielmyer-Fraser, G.K.; Courville, J.M.; Ward, A.; Hardie, M.M. Mercury and Selenium Accumulation in the Tissues of Stranded Bottlenose Dolphins (Tursiops truncatus) in Northeast Florida, 2013–2021. Animals 2024, 14, 1571. https://doi.org/10.3390/ani14111571
Bielmyer-Fraser GK, Courville JM, Ward A, Hardie MM. Mercury and Selenium Accumulation in the Tissues of Stranded Bottlenose Dolphins (Tursiops truncatus) in Northeast Florida, 2013–2021. Animals. 2024; 14(11):1571. https://doi.org/10.3390/ani14111571
Chicago/Turabian StyleBielmyer-Fraser, Gretchen K., Julia M. Courville, Ashlen Ward, and Mckenna M. Hardie. 2024. "Mercury and Selenium Accumulation in the Tissues of Stranded Bottlenose Dolphins (Tursiops truncatus) in Northeast Florida, 2013–2021" Animals 14, no. 11: 1571. https://doi.org/10.3390/ani14111571






