The Ecological Separation of Deer and Domestic, Feral and Native Mammals in Tropical Northern Australia—A Review
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Review Process
2.2. Selection Criteria and Search Databases
2.3. Exclusion Criteria
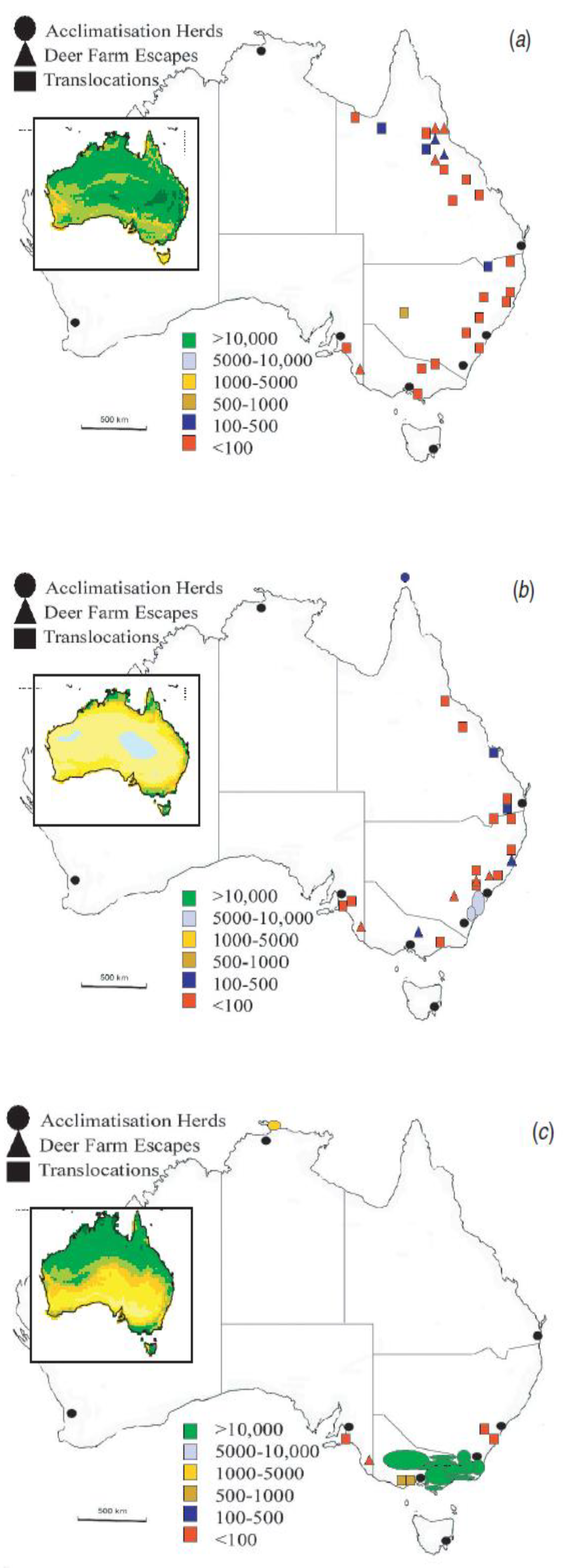
3. Results
- i.
- Between agile wallabies, antilopine wallaroos and common wallaroos, and where black wallaroos occur in the Northern Territory. These species have some level of dietary overlap (they are all grazers but may include other foods, e.g., fruits) but display habitat separation: black and common wallaroos are found in “rocky hills”, whereas the agile wallaby prefers open forest, and the antilopine wallaroo prefers tropical woodlands.
- ii.
- Agile and northern nail-tail wallabies, antilopine wallaroos, common and black wallaroos, black and whiptail wallabies and red and eastern grey kangaroos have similar diets. They are all grazers, except the black wallaby, which is primarily a browser, and the northern nail-tail wallaby, which mostly eats herbs but may also include other foods, e.g., succulents or fruit. Black wallabies will eat some exotic and poisonous plants.
- iii.
- Red and eastern grey kangaroos and the common wallaroo are predominantly grazers, although they utilise different habitats: respectively, open plains, open forest and rocky hills.
4. Discussion
5. Conclusions and Recommendations
- What the changes to plant community structure initiated or progressed by deer (grazing, browsing, the removal of seedlings, tree rubbing, etc.) are in different habitats, where populations of native and introduced herbivores are found.
- Whether deer individually or collectively compete with or negatively impact native herbivores or displace other introduced species.
- How frequently deer, individually or collectively, are predated by dingoes/wild dogs and crocodiles and whether this predation is a regulator of populations of both deer and dingo/wild dog populations.
- Whether deer, individually or collectively, will have their populations regulated by current climatic conditions and whether climate change, water sources, fire, floods and drought will be important regulators of deer populations in the future.
- What is the potential of deer, individually or collectively, to create local industries for meat, skins, velvet or hunting and how these might be engaged by First Nations people, as currently occurs in the Cobourg/Garig Gunak Barlu National Park in the Northern Territory.
- What the role of deer and other herbivores is as endozoochorous seed dispersers.
- What the role of deer and other herbivores is as potential reservoirs and vectors for parasites and infectious disease, to determine whether deer are different vectors of disease from other introduced ruminants; and
- Whether deer have impacts on smaller (<5 kg) native vertebrates and the wide range of invertebrates sharing the same habitats.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cook, G.D.; Bond, W.J.; February, E.C.; Williams, R.J. (Eds.) Savannas of Australia and New Guinea; Wiley: Hoboken, NJ, USA, 2019; pp. 51–75. [Google Scholar]
- The State of Queensland Department of Agriculture and Fisheries. Prickly Acacia Fact Sheet. Available online: https://www.daf.qld.gov.au/__data/assets/pdf_file/0007/73753/prickly-acacia.pdf (accessed on 21 February 2024).
- Williams, R.J.; Carter, J.; Duff, G.A.; Woinarski, J.C.Z.; Cook, G.D.; Farrer, S.L. Carbon Accounting, Land Management, Science and Policy Uncertainty in Australian Savanna Landscapes: Introduction and Overview. Aust. J. Bot. 2005, 53, 583–588. [Google Scholar] [CrossRef]
- Augustine, D.J.; Scogings, P.F.; Sankaran, M. Mesobrowser Abundance and Effects on Woody Plants in Savannas. In Savanna Woody Plants and Large Herbivores; Wiley: Hoboken, NJ, USA, 2019; pp. 551–583. [Google Scholar]
- Lehmann, C.E.R.; Anderson, T.M.; Sankaran, M.; Higgins, S.I.; Archibald, S.; Hoffmann, W.A.; Hanan, N.P.; Williams, R.J.; Fensham, R.J.; Felfili, J.; et al. Savanna Vegetation-Fire-Climate Relationships Differ among Continents. Science 2014, 343, 548–552. [Google Scholar] [CrossRef]
- Sankaran, M.; Ratnam, J.; Hanan, N. Woody Cover in African Savannas: The Role of Resources, Fire and Herbivory. Glob. Ecol. Biogeogr. 2008, 17, 236–245. [Google Scholar] [CrossRef]
- Sankaran, M.; Augustine, D.J.; Ratnam, J. Native Ungulates of Diverse Body Sizes Collectively Regulate Long-Term Woody Plant Demography and Structure of a Semi-Arid Savanna. J. Ecol. 2013, 101, 1389–1399. [Google Scholar] [CrossRef]
- Scholtz, R.; Kiker, G.A.; Smit, I.P.J.; Venter, F.J. Identifying Drivers That Influence the Spatial Distribution of Woody Vegetation in Kruger National Park, South Africa. Ecosphere 2014, 5, art71. [Google Scholar] [CrossRef]
- Scogings, P.F.; Sankaran, M. Woody Plants and Large Herbivores in Savannas. In Savanna Woody Plants and Large Herbivores; Wiley: Hoboken, NJ, USA, 2019; pp. 683–712. [Google Scholar]
- Bowman, D.M.J.S.; Brown, G.K.; Braby, M.F.; Brown, J.R.; Cook, L.G.; Crisp, M.D.; Ford, F.; Haberle, S.; Hughes, J.; Isagi, Y.; et al. Biogeography of the Australian Monsoon Tropics. J. Biogeogr. 2010, 37, 201–216. [Google Scholar] [CrossRef]
- Ritchie, E.G.; Bolitho, E.E. Australia’s Savanna Herbivores: Bioclimatic Distributions and an Assessment of the Potential Impact of Regional Climate Change. Physiol. Biochem. Zool. 2008, 81, 880–890. [Google Scholar] [CrossRef]
- Dawson, T.J. Kangaroos: Biology of the Largest Marsupials; University of New South Wales Press: Sydney, Australia, 1995. [Google Scholar]
- Woinarski, J.C.Z. The Illusion of Nature: Perception and the Reality of Natural Landscapes, as Illustrated by Vertebrate Fauna in the Northern Territory, Australia. Ecol. Manag. Restor. 2014, 15, 30–33. [Google Scholar] [CrossRef]
- Freeland, W.J. Large Herbivorous Mammals: Exotic Species in Northern Australia. J. Biogeogr. 1990, 17, 445–449. [Google Scholar] [CrossRef]
- Wroe, S.; Crowther, M.; Dortch, J.; Chong, J. The Size of the Largest Marsupial and Why It Matters. Proc. R. Soc. B 2004, 271 (Suppl. S3), S34–S36. [Google Scholar] [CrossRef]
- Rule, S.; Brook, B.W.; Haberle, S.G.; Turney, C.S.M.; Kershaw, A.P.; Johnson, C.N. The Aftermath of Megafaunal Extinction: Ecosystem Transformation in Pleistocene Australia. Science 2012, 335, 1483–1486. [Google Scholar] [CrossRef] [PubMed]
- Lundgren, E.J.; Ramp, D.; Rowan, J.; Middleton, O.; Schowanek, S.D.; Sanisidro, O.; Carroll, S.P.; Davis, M.; Sandom, C.J.; Svenning, J.C.; et al. Introduced Herbivores Restore Late Pleistocene Ecological Functions. Proc. Natl. Acad. Sci. USA 2020, 117, 7871–7878. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, C.; Jacobs, Z.; Marwick, B.; Fullagar, R.; Wallis, L.; Smith, M.; Roberts, R.G.; Hayes, E.; Lowe, K.; Carah, X.; et al. Human Occupation of Northern Australia by 65,000 Years Ago. Nature 2017, 547, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.N.; Rule, S.; Haberle, S.G.; Kershaw, A.P.; McKenzie, G.M.; Brook, B.W. Geographic Variation in the Ecological Effects of Extinction of Australia’s Pleistocene Megafauna. Ecography 2016, 39, 109–116. [Google Scholar] [CrossRef]
- Johnson, C.N.; Wallach, A.D. The Virtuous Circle: Predator-Friendly Farming Andecological Restoration in Australia. Restor. Ecol. 2016, 24, 821–826. [Google Scholar] [CrossRef]
- van der Kaars, S.; Miller, G.H.; Turney, C.S.M.; Cook, E.J.; Nürnberg, D.; Schönfeld, J.; Kershaw, A.P.; Lehman, S.J. Humans Rather Than Climate the Primary Cause of Pleistocene Megafaunal Extinction in Australia. Nat. Commun. 2017, 8, 14142. [Google Scholar] [CrossRef] [PubMed]
- Orians, G.H.; Milewski, A.V. Ecology of Australia: The Effects of Nutrient-Poor Soils and Intense Fires. Biol. Rev. 2007, 82, 393–423. [Google Scholar] [CrossRef] [PubMed]
- Reid, A.M.; Murphy, B.P.; Vigilante, T.; Barry, L.A.; Bowman, D.M.J.S.; Wunambal Gaambera Aboriginal Corp. Carbon Isotope Analysis Shows Introduced Bovines Have Broader Dietary Range than the Largest Native Herbivores in an Australian Tropical Savanna. Aust. Ecol. 2020, 45, 109–121. [Google Scholar] [CrossRef]
- Hempson, G.P.; Archibald, S.; Bond, W.J. A Continent-Wide Assessment of the Form and Intensity of Large Mammal Herbivory in Africa. Science 2015, 350, 1056–1061. [Google Scholar] [CrossRef]
- Legge, S.; Kennedy, M.S.; Lloyd, R.; Murphy, S.A.; Fisher, A. Rapid Recovery of Mammal Fauna in the Central Kimberley, Northern Australia, Following the Removal of Introduced Herbivores. Aust. Ecol. 2011, 36, 791–799. [Google Scholar] [CrossRef]
- Kutt, A.S.; Vanderduys, E.P.; O’Reagain, P. Spatial and Temporal Effects of Grazing Management and Rainfall on the Vertebrate Fauna of a Tropical Savanna. Rangel. J. 2012, 34, 173–182. [Google Scholar] [CrossRef]
- Fisher, D.O.; Johnson, C.N.; Lawes, M.J.; Fritz, S.A.; McCallum, H.; Blomberg, S.P.; VanDerWal, J.; Abbott, B.; Frank, A.; Legge, S.; et al. The Current Decline of Tropical Marsupials in Australia: Is History Repeating? Glob. Ecol. Biogeogr. 2014, 23, 181–190. [Google Scholar] [CrossRef]
- Woinarski, J.C.Z.; Legge, S.; Fitzsimons, J.A.; Traill, B.J.; Burbidge, A.A.; Fisher, A.; Firth, R.S.C.; Gordon, I.J.; Griffiths, A.D.; Johnson, C.N.; et al. The Disappearing Mammal Fauna of Northern Australia: Context, Cause, and Response. Conserv. Lett. 2011, 4, 192–201. [Google Scholar] [CrossRef]
- Letts, G.A.; Bassingthwaite, A.; De Vos, W.E.L. Feral Animals in the Northern Territory: Report of the Board of Inquiry; National Library of Australia: Canberra, Australia, 1979. [Google Scholar]
- Bowman, D.M.J.S.; Panton, W.J. Sign and Habitat Impact of Banteng (Bos Javanicus) and Pig (Sus Scrofa), Cobourg Peninsula, Northern Australia. Aust. J. Ecol. 1991, 16, 15–17. [Google Scholar] [CrossRef]
- Lundgren, E.J.; Bergman, J.; Trepel, J.; Le Roux, E.; Monsarrat, S.; Kristensen, J.A.; Pedersen, R.Ø.; Pereyra, P.; Tietje, M.; Svenning, J.-C. Functional Traits-Not Nativeness-Shape the Effects of Large Mammalian Herbivores on Plant Communities. Science 2024, 383, 531–537. [Google Scholar] [CrossRef]
- Van Dyck, S.; Strahan, R. (Eds.) The Mammals of Australia, 3rd ed.; New Holland Publishers: Sydney, Australia, 2008. [Google Scholar]
- Lemcke, B.G. Prospects for Deer Farming in the Northern Territory; Technical Bulletin (Northern Territory. Department of Primary Production) No. 27—Agdex No 482/44; National Library of Australia: Canberra, Australia, 1979. [Google Scholar]
- Moriarty, A. The Liberation, Distribution, Abundance and Management of Wild Deer in Australia. Wildl. Res. 2004, 31, 291–299. [Google Scholar] [CrossRef]
- Davis, N.E.; Bennett, A.; Forsyth, D.M.; Bowman, D.; Lefroy, E.C.; Wood, S.W.; Woolnough, A.P.; West, P.; Hampton, J.O.; Johnson, C.N. A Systematic Review of the Impacts and Management of Introduced Deer (Family Cervidae) in Australia. Wildl. Res. 2016, 43, 515–532. [Google Scholar] [CrossRef]
- Schaller, G.B. The Deer and the Tiger. University of Chicago Press: Chicago, IL, USA, 1967; p. 370. [Google Scholar]
- Dinerstein, E. An Ecological Survey of the Royal Karnali-Bardia Wildlife Reserve, Nepal. Part I: Vegetation, Modifying Factors, and Successional Relationships. Biol. Conserv. 1979, 15, 127–150. [Google Scholar] [CrossRef]
- Khan, J.A.; Chellam, R.; Rodgers, W.A.; Johnsingh, A.J.T. Ungulate Densities and Biomass in the Tropical Dry Deciduous Forests of Gir, Gujarat, India. J. Trop. Ecol. 1996, 12, 149–162. [Google Scholar] [CrossRef]
- Madhusudan, M.D. Recovery of Wild Large Herbivores Following Livestock Decline in a Tropical Indian Wildlife Reserve. J. Appl. Ecol. 2004, 41, 858–869. [Google Scholar] [CrossRef]
- Ahrestani, F.S.; Heitkönig, I.M.A.; Prins, H.H.T. Diet and Habitat-Niche Relationships within an Assemblage of Large Herbivores in a Seasonal Tropical Forest. J. Trop. Ecol. 2012, 28, 385–394. [Google Scholar] [CrossRef]
- Bagchi, S.; Goyal, S.P.; Sankar, K. Niche Relationships of an Ungulate Assemblage in a Dry Tropical Forest. J. Mammal. 2003, 84, 981–988. [Google Scholar] [CrossRef]
- Hofmann, R.R. Endangered Tropical Herbivores—Their Nutritional Requirements and Habitat Demands. In Proceedings of the 3rd International Symposium of Nutrition of Herbivores, Pinang, Malaysia, 25–30 August 1991; pp. 27–34. [Google Scholar]
- Watter, K.; Baxter, G.; Brennan, M.; Pople, A.; Murray, P. Decline in Body Condition and High Drought Mortality Limit the Spread of Wild Chital Deer in North-East Queensland, Australia. Rangel. J. 2019, 41, 293–299. [Google Scholar] [CrossRef]
- Watter, K.; Baxter, G.; Brennan, M.; Pople, T.; Murray, P. Seasonal Diet Preferences of Chital Deer in the Northern Queensland Dry Tropics, Australia. Rangel. J. 2020, 42, 211–220. [Google Scholar] [CrossRef]
- Watter, K.; Baxter, G.S.; Pople, A.; Murray, P.J. Dietary Overlap between Cattle and Chital in the Queensland Dry Tropics. Rangel. J. 2020, 42, 221–225. [Google Scholar] [CrossRef]
- Watter, K.; Baxter, G.S.; Pople, T.; Murray, P.J. Effects of Wet Season Mineral Nutrition on Chital Deer Distribution in Northern Queensland. Wildl. Res. 2019, 46, 499–508. [Google Scholar] [CrossRef]
- Bhat, S.A.; Telang, S.; Wani, M.A.; Sheikh, K.A. Food Habits of Nilgai (Boselaphus Tragocamelus) in Van Vihar National Park, Bhopal, Madhya Pradesh, India. Biomed. Pharmacol. 2012, 5, 141–147. [Google Scholar] [CrossRef]
- Solanki, G.S.; Naik, R.M. Grazing Interactions between Wild and Domestic Herbivores. Small Rumin. Res. 1998, 27, 231–235. [Google Scholar] [CrossRef]
- Duncan, A.J.; Poppi, D.P. Nutritional Ecology of Grazing and Browsing Ruminants. In The Ecology of Browsing and Grazing; Gordon, I.J., Prins, H.H.T., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 89–116. [Google Scholar]
- Demment, M.W.; Van Soest, P.J. A Nutritional Explanation for Body-Size Patterns of Ruminant and Nonruminant Herbivores. Am. Nat. 1985, 125, 641–672. [Google Scholar] [CrossRef]
- Gordon, I.J. Browsing and Grazing Ruminants: Are They Different Beasts? For. Ecol. Manag. 2003, 181, 13–21. [Google Scholar] [CrossRef]
- Gordon, I.J.; Illius, A.W. The Functional Significance of the Browser-Grazer Dichotomy in African Ruminants. Oecologia 1994, 98, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, R.R. Evolutionary Steps of Ecophysiological Adaptation and Diversification of Ruminants: A Comparative View of Their Digestive System. Oecologia 1989, 78, 443–457. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, R.R. The Ruminant Stomach: Stomach Structure and Feeding Habits of East African Game Ruminants; East African Literature Bureau: Nairobi, Kenya, 1973; Volume 2, p. 354. [Google Scholar]
- Hofmann, R.R. Digestive Physiology of the Deer—Their Morphophysiological Specialisation and Adaptation. Biol. Deer Prod. R. Soc. N. Z. Bull. 1985, 22, 393–407. [Google Scholar]
- Hofmann, R.R.; Stewart, D.R.M. Grazer or Browser: A Classification Based on the Stomach-Structure and Feeding Habits of East African Ruminants. Mammalia 1972, 36, 226–240. [Google Scholar] [CrossRef]
- Clauss, M.; Frey, R.; Kiefer, B.; Lechner-Doll, M.; Loehlein, W.; Polster, C.; Rossner, G.E.; Streich, W.J. The Maximum Attainable Body Size of Herbivorous Mammals: Morphophysiological Constraints on Foregut, and Adaptations of Hindgut Fermenters. Oecologia 2003, 136, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Clauss, M.; Hume, I.D.; Hummel, J. Evolutionary Adaptations of Ruminants and Their Potential Relevance for Modern Production Systems. Animal 2010, 4, 979–992. [Google Scholar] [CrossRef] [PubMed]
- Clauss, M.; Kaiser, T.; Hummel, J. The Morphophysiological Adaptations of Browsing and Grazing Mammals. In The Ecology of Browsing and Grazing; Gordon, I.J., Prins, H.H.T., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 47–88. [Google Scholar]
- Prins, H.H.T.; Olff, H. Species-Richness of African Grazer Assemblages: Towards a Functional Explanation. In Dynamics of Tropical Communities; Newbery, D.M., Prins, H.H.T., Brown, N.D., Eds.; Blackwell Science: Oxford, UK, 1998; pp. 449–490. [Google Scholar]
- Thompson, E.R.; Driscoll, D.A.; Venn, S.E.; Geary, W.L.; Ritchie, E.G. Interspecific Variation in the Diet of a Native Apex Predator and Invasive Mesopredator in an Alpine Ecosystem. Aust. Ecol. 2022, 47, 1260–1270. [Google Scholar] [CrossRef]
- Reid, A.M.; Murphy, B.P.; Vigilante, T.; Bowman, D.M.J.S.; Wunambal Gaambera Aboriginal Corporation (WGAC). Pyric Herbivory and the Nexus between Forage, Fire and Native and Introduced Large Grazing Herbivores in Australian Tropical Savannas. Ecosystems 2022, 26, 610–626. [Google Scholar] [CrossRef]
- Allred, B.W.; Fuhlendorf, S.D.; Engle, D.M.; Elmore, R.D. Ungulate Preference for Burned Patches Reveals Strength of Fire-Grazing Interaction. Ecol. Evol. 2011, 1, 132–144. [Google Scholar] [CrossRef]
- Hardin, G. The Competitive Exclusion Principle. Science 1960, 131, 1292–1297. [Google Scholar] [CrossRef]
- Pianka, E.R. Competition and Niche Theory. In Theoretical Ecology; May, R.M., Ed.; Blackwell Scientific Publications: Oxford, UK, 1976. [Google Scholar]
- Wiens, J.A. On Competition and Variable Environments: Populations May Experience “Ecological Crunches” in Variable Climates, Nullifying the Assumptions of Competition Theory and Limiting the Usefulness of Short-Term Studies of Population Patterns. Am. Sci. 1977, 65, 590–597. [Google Scholar]
- Werner, E.E.; Gilliam, J.F.; Hall, D.J.; Mittelbach, G.G. An Experimental Test of the Effects of Predation Risk on Habitat Use in Fish. Ecology 1983, 64, 1540–1548. [Google Scholar] [CrossRef]
- Jeffries, M.J.; Lawton, J.H. Enemy Free Space and the Structure of Ecological Communities. Biol. J. Linn. Soc. 2008, 23, 269–286. [Google Scholar] [CrossRef]
- Hutchinson, G.E. When Are Species Necessary? In Population Biology and Evolution; Lewontin, R.C., Ed.; Syracuse University Press: New York, NY, USA, 1968; pp. 177–186. [Google Scholar]
- Putman, R.J. Competition and Coexistence in a Multispecies Grazing System. Acta Theriol. 1986, 31, 271–291. [Google Scholar] [CrossRef]
- Schoener, T.W. Competition and Niche. In Biology of the Reptilia; Tinkle, C.G.D., Ed.; Academic Press: New York, NY, USA, 1977; Volume 7, pp. 35–136. [Google Scholar]
- Bentley, A. An Introduction to the Deer of Australia: With Special Reference to Victoria; Revised Edition; Koetong Trust: Melbourne, Australia, 1978. [Google Scholar]
- Smith-Jones, C. A Guide to Deer of the World; Quiller Publishing: Merrywalks, UK, 2022; p. 320. [Google Scholar]
- Olff, H.; Ritchie, M.E. Effects of Herbivores on Grassland Plant Diversity. Trends Ecol. Evol. 1998, 13, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Berman, D.M.; Pickering, J.; Smith, D.; Allen, B.L. Use of Density-Impact Functions to Inform and Improve the Environmental Outcomes of Feral Horse Management. Wildl. Biol. 2023, 2023, e01107. [Google Scholar] [CrossRef]
- Berman, D.M. The Ecology of Feral Horses in Central Australia. Ph.D. Thesis, University of New England, Armidale, Australia, 1991. [Google Scholar]
- Bomford, M.; O’Brien, P. Eradication or Control for Vertebrate Pests? Wildl. Soc. Bull. (1973–2006) 1995, 23, 249–255. [Google Scholar]
- Watter, K.; Thomas, E.; White, N.; Finch, N.; Murray, P.J. Reproductive Seasonality and Rate of Increase of Wild Sambar Deer (Rusa Unicolor) in a New Environment, Victoria, Australia. Anim. Reprod. Sci. 2020, 223, 11. [Google Scholar] [CrossRef]
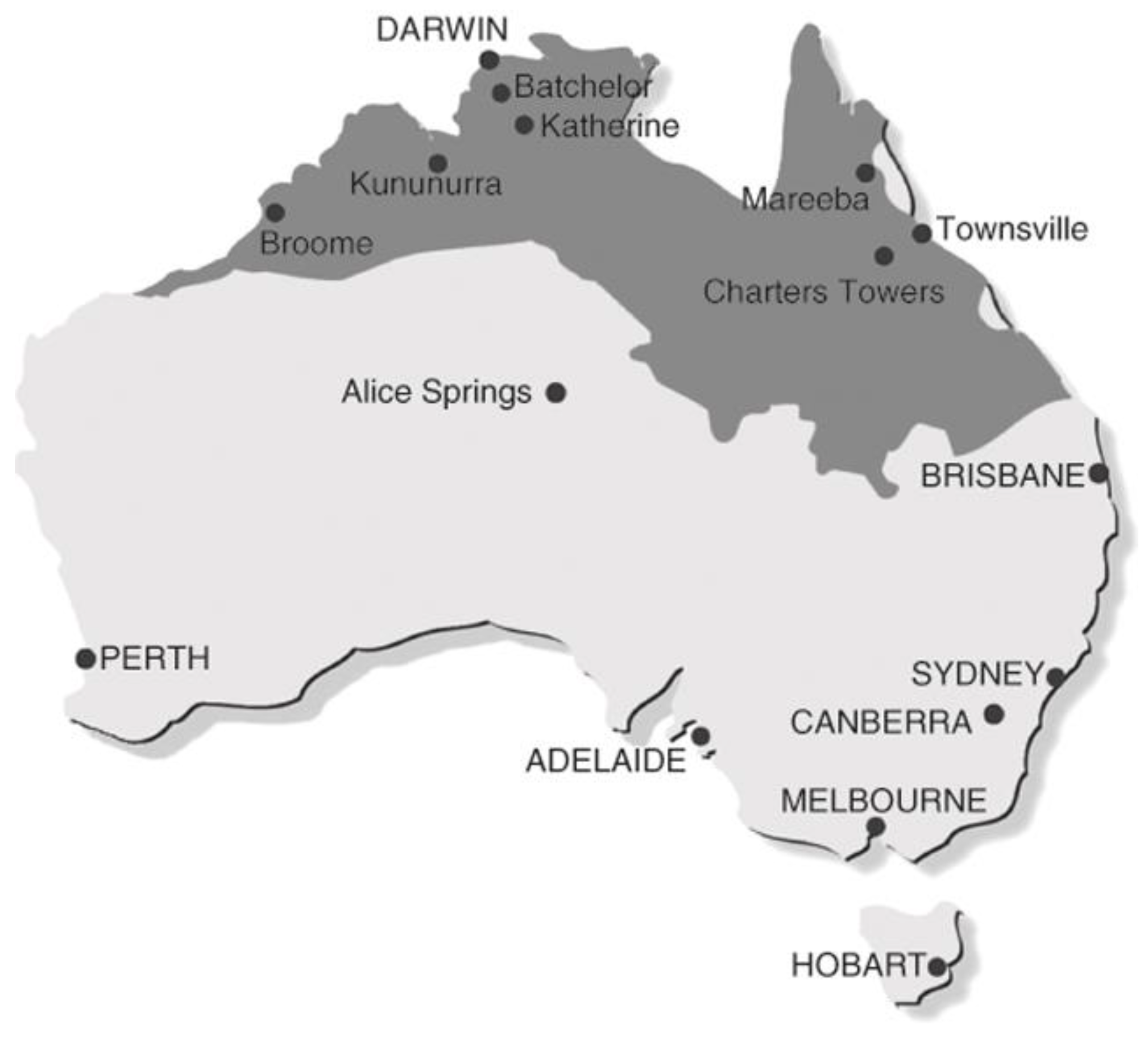
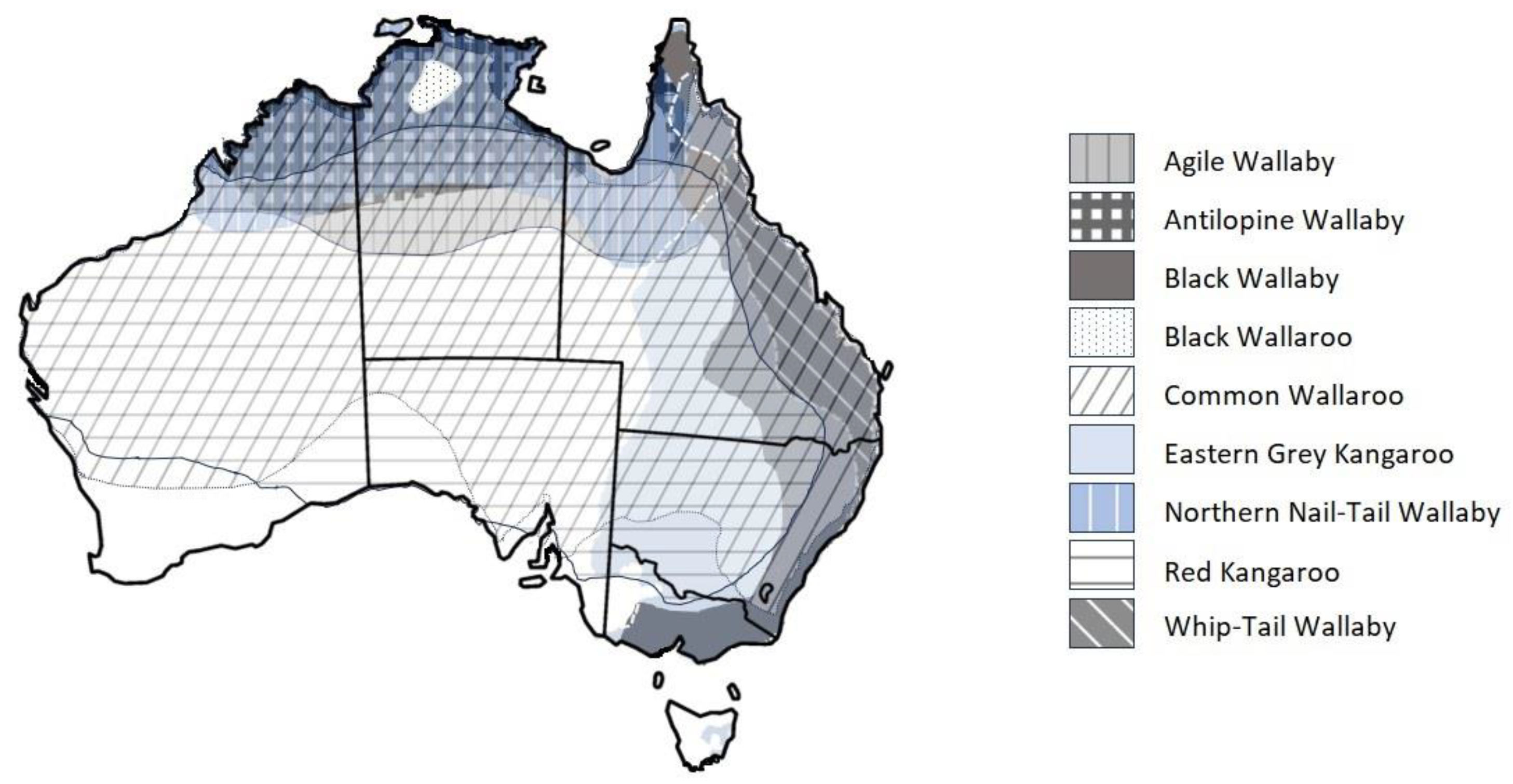
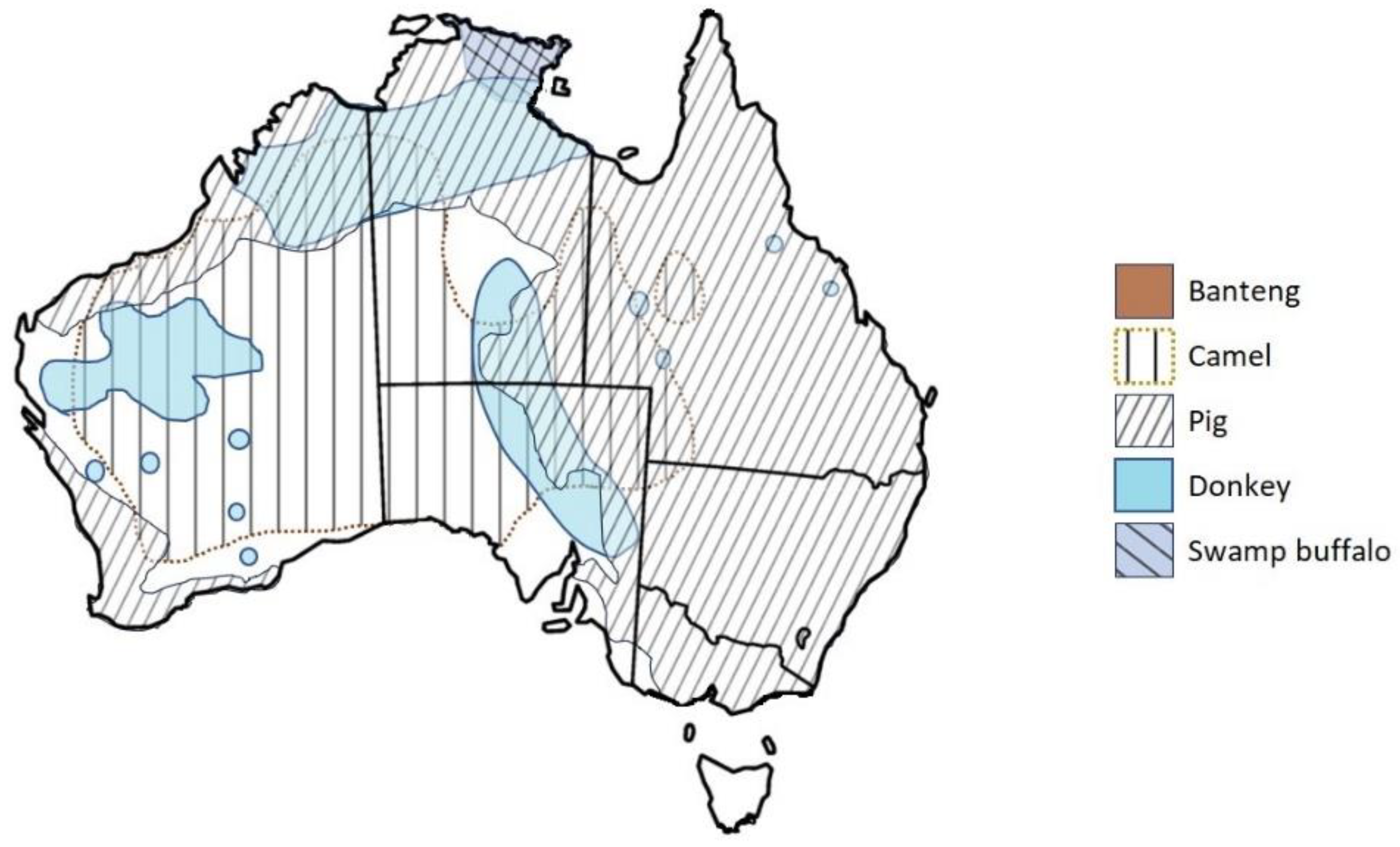
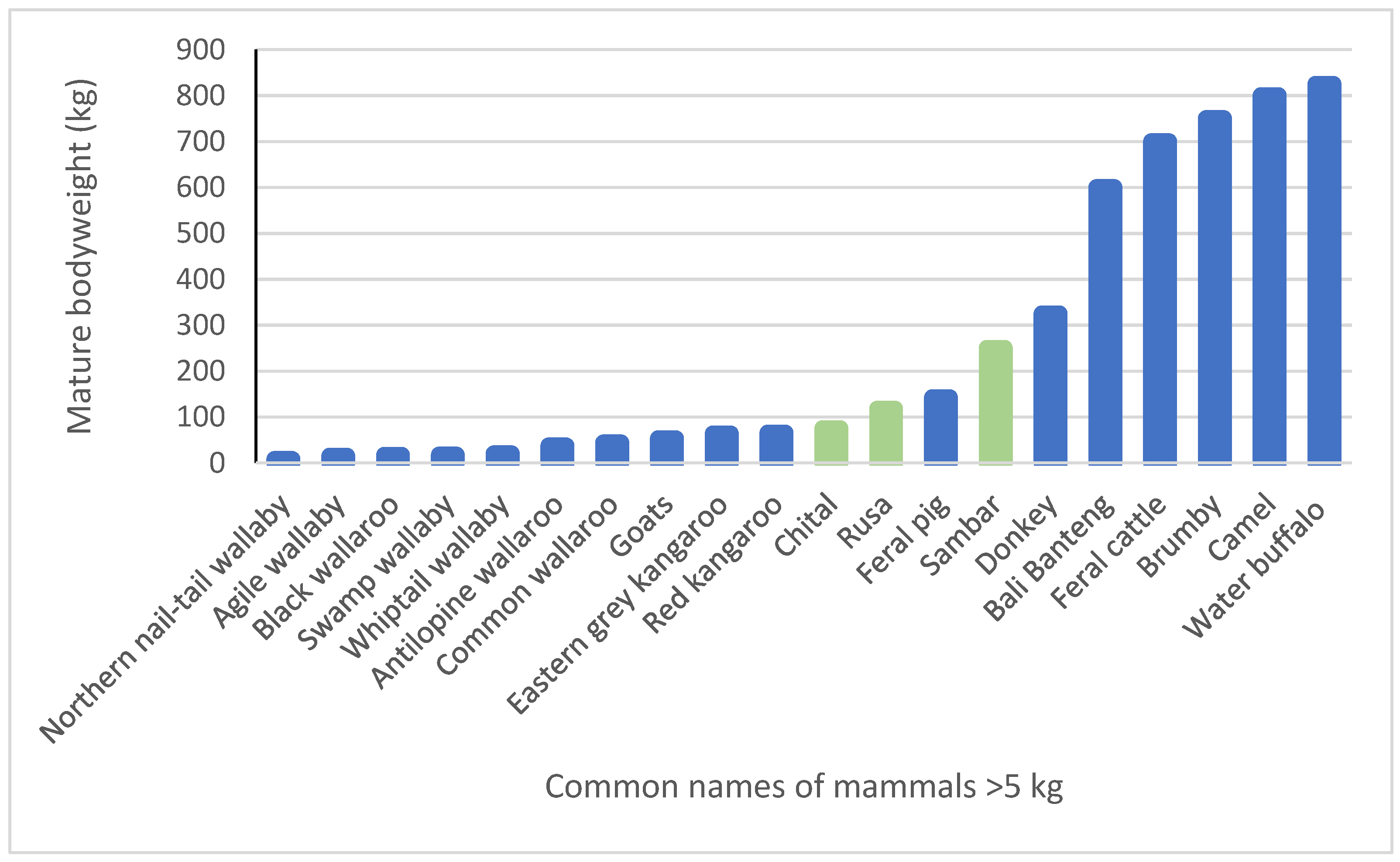
| Common Name (Mature Weight) | Species Name | Diet and Habitat Requirements |
|---|---|---|
| Common wallaroo (28–60 kg) | Macropus robustus | Preferential grazer, mainly of grasses; browser of some shrubs; lives in rocky hill country. |
| Agile wallaby (11–19 kg) | Macropus agilis | Most common macropod in tropical coastal Australia; feeds on native grasses, grass roots and some leaves, flowers and fruits. |
| Red kangaroo (39–92 kg) | Macropus rufus | Grazer and browser of grasses, forbs and shrubs; lives in open plains—savannas, open woodlands, arid and semi-arid regions. |
| Antilopine wallaroo (24–51 kg) | Macropus antilopinus | Grazes perennial grasses and some forbs in tropical grasslands with monsoonal eucalypts; at altitudes less than 500 m. |
| Black wallaroo (13–21 kg) | Macropus bernardus | Lives in Arnhem Land rocky escarpments and feeds on grasses, some leaves, flowers, fruits. |
| Black wallaby (15–20 kg) | Wallabia bicolor | Browser that eats shrubs, pasture grasses, agricultural crops, native and exotic vegetation; inhabits thick undergrowth in forests and woodlands, emerging at night to feed. |
| Whiptail wallaby (15–26 kg) | Macropus parryi | Grazer of grasses and monocots near creeks in grasslands and woodlands in central coastal eastern Qld and northern NSW. |
| Eastern grey kangaroo (42–85 kg) | Macropus giganteus | Specialised grazer of a wide variety of grasses across eastern Australia; adaptable but prefers open grassland habitats. |
| Northern nail-tail wallaby (7–9 kg) | Onychogalea unguifera | Feeds on a wide variety of herbs, fruits, succulent plants; will eat grass when herbs are not available; found in arid and sparsely treed plains with tussocks of tough grasses/low shrubs. |
| Common Name (DS) (Mature Weight) | Species Name | Diet and Habitat Requirements |
|---|---|---|
| Feral pig—O (110–175 kg) | Sus scrofa | Eats plants, small animals and carcasses; occupies 40% of mainland Australia, associated with most river systems and floodplains, inland drainages and thickly wooded habitats. |
| Water buffalo—R (450–1200 kg) | Bubalus bubalis | Feeds on aquatic grasses, grass-like wetland plants, plus dryland grasses, herbs, pandanus leaves; in the main, a grazing animal on subcoastal plains and river basins between Darwin and Arnhem Land. |
| Banteng—R (400–800 kg) | Bos javanicus | A grazer for c. 200 years in the Cobourg/Garig Gunak Barlu National Park, under First Nations Management; preferred habitat of monsoon forest and associated coastal plain, with freshwater lagoons. |
| Feral cattle—R (500–900 kg) | Bos taurus/Bos indicus | A grazer in a wide range of habitats from forest to semi-desert wetlands. |
| Goat—R (27–79 kg) | Capra aegagrus hircus | A preferential browser that eats leaves, twigs, bark, flowers, fruit, roots and most plant types in pastoral regions, consuming vegetation avoided by sheep or cattle. |
| Chital—R (50–100 kg) | Axis axis | Mainly a grazer but also an intermediate mixed feeder. Populations north of Charters Towers and near Townsville, Barcaldine and Texas in Queensland. |
| Rusa—R (75–160 kg) | Rusa timorensis | Intermediate mixed feeder; will browse depending on season and availability; prefers grassy plains bordered by dense brush or woodlands. Reports from Murulag, Boigu and Saibai islands in the Torres Strait, central Cape York Peninsula, Groote Eylandt, the Gulf Savannah region, around Townsville and Rockhampton and in southern Queensland near Stanthorpe. |
| Sambar—R (150–350 kg) | Rusa unicolor | Intermediate mixed feeder eating a wide variety of grasses, shrubs and tree foliage; prefers forested mountain country and also inhabits open forest with suitable understory cover with gullies. Naturalised under First Nations management in the Cobourg/Garig Gunak Barlu National Park and Western Arnhem Land. |
| Camel—PR (600–1000 kg) | Camelus dromedarius | Preferential browser but will eat most plants and has extraordinary drought tolerance; widely distributed in bushland and sand plains over the arid and semi-arid regions of central Australia. |
| Donkey—HF (300–350 kg) | Equus asinus | Eats grasses, shrubs and tree bark; drought-tolerant, found in the NT and northern and northwest WA. |
| Feral horse—HF (600–900 kg) | Equus caballus | Prefers grassland where drinking water is relatively available; also eats other plants, including tree bark. Occupies over half of Australia, absent from most desert regions and intensively farmed land. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murray, P.J.; Nevard, T.D. The Ecological Separation of Deer and Domestic, Feral and Native Mammals in Tropical Northern Australia—A Review. Animals 2024, 14, 1576. https://doi.org/10.3390/ani14111576
Murray PJ, Nevard TD. The Ecological Separation of Deer and Domestic, Feral and Native Mammals in Tropical Northern Australia—A Review. Animals. 2024; 14(11):1576. https://doi.org/10.3390/ani14111576
Chicago/Turabian StyleMurray, Peter J., and Timothy D. Nevard. 2024. "The Ecological Separation of Deer and Domestic, Feral and Native Mammals in Tropical Northern Australia—A Review" Animals 14, no. 11: 1576. https://doi.org/10.3390/ani14111576






