Increasing Risks to the Health of the Invertebrates—Balancing between Harm and Benefit
Abstract
:Simple Summary
Abstract
1. Introduction
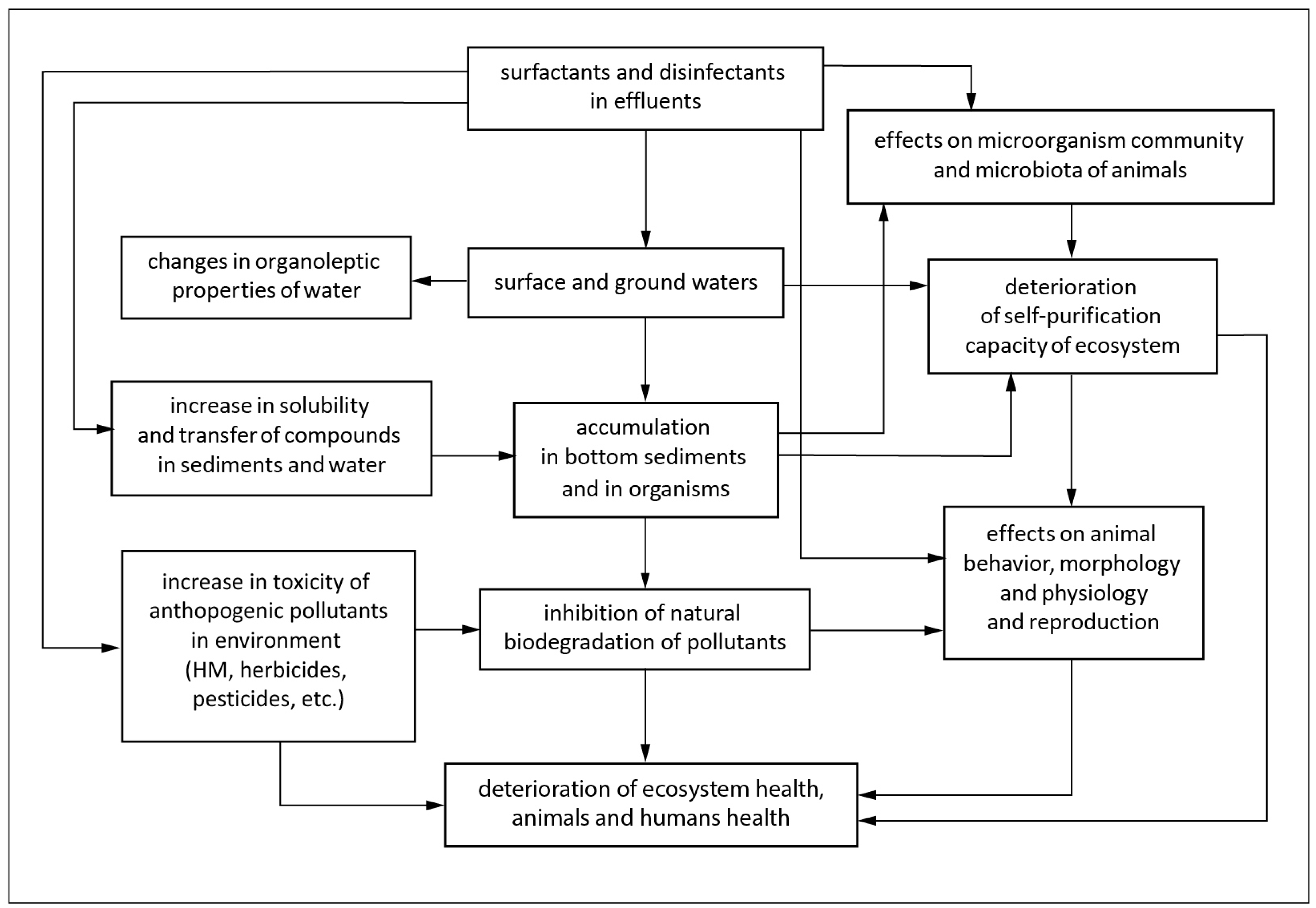
2. General Information on Disinfectants: Benefits and Harm
3. Use of the Invertebrates in Biomonitoring Studies
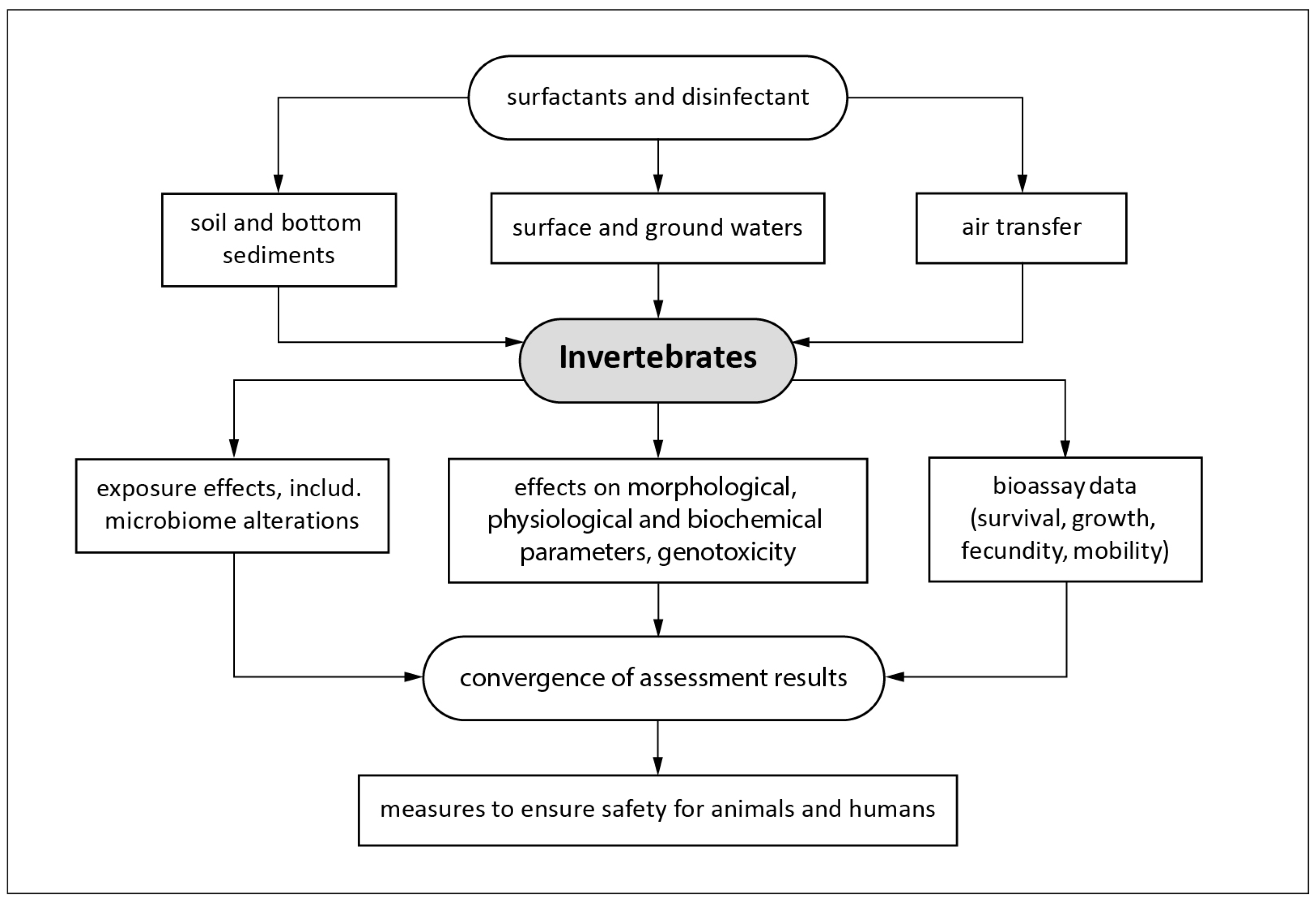
4. Use of the Invertebrates as Biosensors in Water Quality Monitoring Systems and as Test Organisms in Studying Disinfectants and Surfactants Actions
5. Possible Ways to Reduce Risks for Animals and Humans
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19); World Health Organization: Geneva, Switzerland, 2020; Available online: https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf (accessed on 23 February 2023).
- Key Messages and Actions for COVID-19 Prevention and Control in Schools; World Health Organization: Geneva, Switzerland, 2020; Available online: https://www.who.int/docs/defaultsource/coronaviruse/key-messages-and-actions-for-covid-19-prevention-and-controlin-schools-march-2020.pdf (accessed on 23 February 2023).
- List N: Disinfectants for Use against SARS-CoV-2. US EPA. 2020. Available online: https://www.epa.gov/pesticide-registration/list-ndisinfectants-use-against-SARS-CoV-2 (accessed on 9 March 2023).
- US EPA. Proposed Revisions to EPA’s Safer Choice Standard. Docket ID EPA-HQ-OPPT-2023-0520. 2023. Available online: https://www.epa.gov/system/files/documents/2023-11/proposed-changes-to-epas-safer-choice-standard.pdf (accessed on 23 February 2023).
- Escher, B.I.; Bramaz, N.; Ort, C. JEM spotlight: Monitoring the treatment efficiency of a full scale ozonation on a sewage treatment plant with a mode-of-action based test battery. J. Environ. Monit. 2009, 11, 1836–1846. [Google Scholar] [CrossRef]
- EU Council Directive 98/83/EC from 3 November 1998. On the Quality of Water Intended for Human Consumption. Available online: http://data.europa.eu/eli/dir/1998/83/oj (accessed on 23 February 2023).
- Jirova, G.; Wittlingerova, Z.; Zimova, M.; Vlkova, A.; Wittlerova, M.; Dvorakova, M.; Jirova, D. Bioindicators of wastewater ecotoxicity. Neuro Endocrinol. Lett. 2016, 37 (Suppl. S1), 17–24. [Google Scholar] [PubMed]
- US EPA. Technologies and Techniques for Early Warning Systems to Monitor and Evaluate Drinking Water Quality. A State-of-Art Review; Report EPA/600/R-05/156; US Environment Protection Agency Office of Water Office of Science and Technology, Health and Ecological Criteria: Wanshington, DC, USA, 2005; 236p. Available online: http://EPA%20report%202005.pdf (accessed on 26 May 2023).
- Pereira, S.S.P.; Oliveira, H.M.; de Turrini, R.N.T.; Lacerda, R.A. Disinfection with sodium hypochlorite in hospital environmental surfaces in the reduction of contamination and infection prevention: A systematic review. Rev. Esc. Enferm. USP 2015, 49, 0681–0688. [Google Scholar] [CrossRef]
- Köhler, A.T.; Rodloff, A.C.; Labahn, M.; Reinhardt, M.; Truyen, U.; Speck, S. Efficacy of sodium hypochlorite against multidrug-resistant Gram-negative bacteria. J. Hosp. Infect. 2018, 100, e40–e46. [Google Scholar] [CrossRef]
- Directive 2008/105/EC of the European Parliament and of the Council of 16 December 2008 on environmental quality standards in the field of water policy, amending and subsequently repealing Council Directives 82/176/EEC, 83/513/EEC, 84/156/EEC, 84/491/EEC, 86/280/EEC and amending Directive 2000/60/EC. Off. J. 2008, L 348, 84–97. Available online: http://eur-lex.europa.eu›eli/dir/2008/105 (accessed on 25 February 2023).
- The Order of the Federal Agency for Fisheries of 18.01.2010, № 20 «On approval of standards for water quality fishery water bodies, including the maximum permissible concentrations of harmful substances in the waters of fishery water bodies». Rossijskaja gazeta, 5 March 2010.
- Directive 2006/44/EC of the European Parliament and of the Council of 6 September 2006 on the quality of fresh waters needing protection or improvement in order to support fish life. Off. J. Eur. Union 2006, L 264, 20–35.
- Slye, J.L.; Kennedy, J.H.; Johnson, D.R.; Atkinson, S.F.; Dyer, S.D.; Ciarlo, M.; Stanton, K.; Sanderson, H.; Nielsen, A.M.; Price, B.B. Relationships between benthic macroinvertebrate community structure and geospatial habitat, in-stream water chemistry, and surfactants in the effluent-dominated Trinity River, Texas, USA. Environ. Toxicol. Chem. 2011, 30, 1127–1138. [Google Scholar] [CrossRef]
- Gagné, F.; Chantale, A.; Fortier, M.; Fournier, M. Immunotoxic potential of aeration lagoon effluents for the treatment of domestic and hospital wastewaters in the freshwater mussel Elliptio complanata. J. Environ. Sci. 2012, 24, 781–789. [Google Scholar] [CrossRef]
- Gilles, P. Cumulative impacts of urban runoff and municipal wastewater effluents on wild freshwater mussels (Lasmigona costata). Sci. Total Environ. 2012, 431, 348–356. [Google Scholar] [CrossRef]
- Messina, C.M.; Faggio, C.; Laudicella, V.A.; Sanfilippo, M.; Trischitta, F.; Santulli, A. Effect of sodium dodecyl sulfate (SDS) on stress response in the Mediterranean mussel (Mytilus galloprovincialis): Regulatory volume decrease (RVD) and modulation of biochemical markers related to oxidative stress. Aquat. Toxicol. 2014, 157, 94–100. [Google Scholar] [CrossRef]
- EN ISO 6341 Water Quality—Determination of the Inhibition of the Mobility of Daphnia magna Straus (Cladocera, Crustacea)—Acute Toxicity Test. ISO: Geneva, Switzerland, 1996.
- Moiseenko, T.I. Vodnaya Ekotoksikologiya: Teoreticheskie i Prikladnye Aspekty; Nauka: Moscow, Russia, 2009; 399p. (In Russian) [Google Scholar]
- Salánki, J.; Farkas, A.; Kamardina, T.; Rózsa, K.S. Molluscs in biological monitoring of water quality. Toxicol. Let. 2003, 140–141, 403–410. [Google Scholar] [CrossRef]
- Depledge, M.H.; Galloway, T.S. Healthy animals, healthy ecosystems. Front. Ecol. Environment. 2005, 3, 251–258. [Google Scholar] [CrossRef]
- Kuklina, I.; Kouba, A.; Kozák, P. Real-time monitoring of water quality using fish and crayfish as bio-indicators: A review. Environ. Monit. Assess. 2013, 185, 5043–5053. [Google Scholar] [CrossRef]
- Hook, S.E.; Gallagher, E.P.; Batley, G.E. The role of biomarkers in the assessment of aquatic ecosystem health. Integr. Environ. Assess. Manag. 2014, 10, 327–341. [Google Scholar] [CrossRef]
- Handy, R.D.; Depledge, M.H. Physiological Responses: Their measurement and use as environmental biomarkers in ecotoxicology. Ecotoxicology 1999, 8, 329–349. [Google Scholar] [CrossRef]
- Curtis, T.M.; Williamson, R.; Depledge, M.H. Simultaneous, long-term monitoring of valve and cardiac activity in the blue mussel Mytilus edulis exposed to copper. Mar. Biol. 2000, 136, 0837–0846. [Google Scholar] [CrossRef]
- Kuznetsova, T.V.; Sladkova, S.V.; Kholodkevich, S.V. Evaluation of functional state of crayfish Pontastacus leptodactylus in normal and toxic environment by characteristics of their cardiac activity and hemolymph biochemical parameters. J. Evol. Biochem. Physiol. 2010, 46, 241–250. [Google Scholar] [CrossRef]
- Widdows, J.; Donkin, P. Mussels and environmental contaminants: Bioaccumulation and physiological aspects. In The Mussel Mytilus: Ecology, Physiology, Genetics and Aquaculture; Gosling, E., Ed.; Elsevier: Amsterdam, The Netherlands, 1992; pp. 383–424. [Google Scholar]
- Gruber, D.; Frago, C.H.; Rasnake, W.J. Automated biomonitors—First line of defence. J. Aquat. Ecosyst. Health 1994, 3, 87–92. [Google Scholar] [CrossRef]
- Kramer, K.J.M.; Foekema, E.M. The “Musselmonitor®” as Biological Early Warning System. In Biomonitors and Biomarkers as Indicators of Environmental Change 2; Environmental Science Research; Butterworth, F.M., Gunatilaka, A., Gonsebatt, M.E., Eds.; Springer: Boston, MA, USA, 2001. [Google Scholar] [CrossRef]
- Nikinmaa, M. Bioindicators and Biomarkers. In An Introduction to Aquatic Toxicology; Elsevier: Amsterdam, The Netherlands; Academic Press: Cambridge, MA, USA, 2014; Chapter 12; pp. 147–155. ISBN 978-012-411574-3. [Google Scholar] [CrossRef]
- Koperski, P. Freshwater invertebrates—Neglected victims of biological monitoring: An Ethical View. Ethics Environ. 2022, 27, 29–57. [Google Scholar] [CrossRef]
- Ostroumov, S.A. Biological mechanism of self-purification in natural water bodies and streams: Theory and applications. Adv. Mod. Biol. 2004, 124, 429–442. [Google Scholar]
- Mather, J.A. Ethics and invertebrates: The problem is us. Animals 2023, 13, 2827. [Google Scholar] [CrossRef]
- Curpan, A.S.; Impellitteri, F.; Plavan, G.; Ciobica, A.; Faggio, C. Mytilus galloprovincialis: An essential, low-cost model organism for the impact of xenobiotics on oxidative stress and public health. Review. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2022, 256, 109302. [Google Scholar] [CrossRef]
- Bychkov, R.; Zhuravlev, V.; Kodirov, S.; Safonova, T. Cardiac inhibitory neurons in the snail Achatina fulica. J. Brain Res. 1997, 38, 263–278. [Google Scholar]
- Depledge, M.H.; Aagard, A.; Györkös, P. Assessment of trace metal toxicity using molecular, physiological and behavioural biomarkers. Mar. Pollut. Bull. 1995, 31, 19–27. [Google Scholar] [CrossRef]
- Depledge, M.H.; Lundeby, A.K.; Curtis, T.; Aagaar, A.; Andersen, B.B. Automated interpulse-duration assessment (AIDA): A new technique for detecting disturbances in cardiac activity in selected invertebrates. Mar. Biol. 1996, 126, 313–319. [Google Scholar] [CrossRef]
- Pautsina, A.; Kuklina, I.; Stys, D.; Cisăr, P.; Kozak, P. Noninvasive crayfish cardiac activity monitoring system. Limnol. Oceanogr. Meth. 2014, 12, 670–679. [Google Scholar] [CrossRef]
- Kholodkevich, S.V.; Kuznetsova, T.V.; Sharov, A.N.; Kurakin, A.S. Applicability of a bioelectronics cardiac monitoring system for the detection of biological effects of pollution in bioindicator species in the gulf of Finland. J. Mar. Syst. 2017, 171, 151–158. [Google Scholar] [CrossRef]
- Kozak, P.; Policar, T.; Fedotov, V.P.; Kuznetsova, T.V.; Buřič, M.; Kholodkevich, S.V. Effect of chloride content in water on heart rate in narrow-clawed crayfish (Astacus leptodactylus). Knowl. Manag. Aquatic Ecosyst. 2009, 394–395, 1–10. [Google Scholar] [CrossRef]
- Kuklina, I.; Sladkova, S.; Kouba, A.; Kholodkevich, S.; Kozák, P. Investigation of chloramine-T impact on crayfish Astacus leptodactylus (Esch., 1823) cardiac activity. Environ. Sci. Pollut. Res. 2014, 21, 10262–10269. [Google Scholar] [CrossRef]
- Kholodkevich, S.V.; Kuznetsova, T.V.; Sladkova, S.V.; Kurakin, A.S.; Ivanov, A.V.; Lyubimtsev, V.A.; Kornienko, E.L.; Fedotov, V.P. Industrial Operation of the Biological Early Warning System BioArgus for Water Quality Control Using Crayfish as a Biosensor. In Water Science and Sustainability; Sustainable Development Goals Series; Pandey, B.W., Anand, S., Cham, P., Eds.; Springer: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Vereycken, J.; Aldridge, D.S. Bivalve molluscs as biosensors of water quality: State water quality: State of the art and future directions. Hydrobiologia 2023, 850, 231–256. [Google Scholar] [CrossRef]
- Spicer, J.I.; Weber, R.E. Respiratory impairment in crustaceans and molluscs due to exposure to heavy metals. Comp. Biochem. Physiol. 1991, 100, 339–342. [Google Scholar] [CrossRef]
- Styrishave, B.; Rasmussen, A.D.; Depledge, M.H. The influence of bulk and trace metals on the circadian rhythm of heart rates in freshwater crayfish Astacus astacus. Mar. Pollut. Bull. 1995, 31, 87–92. [Google Scholar] [CrossRef]
- Kolupaev, B.I. Respiration of Hydrobionts in a Toxic Environment; Publishing House of Kazan University: Kazan, Russia, 1992. (In Russian) [Google Scholar]
- Martin, J.S.; Saker, M.L.; Teles, L.F.; Vasconcelos, V.M. Oxygen consumption by Daphnia magna Strauss as a marker of chemical stress in the aquatic environment. Environ. Toxicol. Chem. 2007, 26, 1987–1991. [Google Scholar] [CrossRef]
- Malinovska, V.; Ložek, F.; Kuklina, I.; Císăr, P.; Kozák, P. Crayfish as bioindicators for monitoring ClO2: A case study from a Brewery Water Treatment Facility. Water 2020, 12, 63. [Google Scholar] [CrossRef]
- World Health Organization. Chlorine Dioxide, Chlorite and Chlorate in Drinking-water. In Background Document for Development of WHO Guidelines for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 2016; p. 24. [Google Scholar]
- Ostroumov, S.A. An amphiphilic substance inhibits the mollusk capacity to filter out phytoplankton cells from water. Biol. Bull. 2001, 28, 95–102. [Google Scholar] [CrossRef]
- Ostroumov, S.A.; Widdows, J. Inhibition of mussel suspension feeding by surfactants of three classes. Hydrobiologia 2006, 556, 381–386. [Google Scholar] [CrossRef]
- Ryabuhina, E.V.; Botyazhova, O.A.; Nikiforova, J.A. Change of functional condition Ceriodaphnia affinis at influence of various factors of environment. In Current Problems of Physiology and Biochemistry of Aquatic Organisms: Proceedings of the III International Conference and Young Scientists School (22–26 June 2010); Karelian Research Centre of RAS: Petrozavodsk, Russia, 2010; pp. 161–163. [Google Scholar]
- Gostyukhina, O.L.; Soldatov, A.A.; Golovina, I.V. Influence of tetradecyltrimethyl ammonium bromide on the state of the enzymatic system of antioxidant defense of the tissues of the Black Sea mollusk Mytilus galloprovincialis Lam. Rep. Natl. Acad. Sci. Ukraine 2007, 11, 147–151. [Google Scholar]
- Trusevich, V.V.; Kuz’min, K.A.; Mishurov, V.J.; Zhuravskii, V.J.; Vyishkvarkova, E.V. Features of behavioral reactions of the Black Sea mussels Mytilus galloprovicialis in natural habitat. Biol. Inland Waters 2021, 1, 12–22. [Google Scholar] [CrossRef]
- Trusevich, V.V.; Kuzmin, K.A.; Mishurov, V.J. Biomonitoring of the surface water quality with use of freshwater bivalvia molluscs. Environ. Control Syst. 2017, 7, 83–93. (In Russian) [Google Scholar]
- Kuznetsova, T.; Kholodkevich, S. Comparative assessment of surface water quality through evaluation of physiological state of bioindicator species: Searching new biomarkers. In Proceedings of the 2015 4th Mediterranean Conference on Embedded Computing (MECO), Budva, Montenegro, 14–18 June 2015. [Google Scholar] [CrossRef]
- Soldatov, A.A.; Gostyukhina, O.L.; Golovina, I.V. Functional states of antioxidant enzymatic complex of tissues of Mytilus galloprovincialis Lam. under conditions of oxidative stress. J. Evol. Biochem. Physiol. 2014, 50, 206–214. [Google Scholar] [CrossRef]
- Chuiko, G.M. Biomarkers in Hydroecotoxicology: Principles, Methods and Methodology, Practice of Use. Ecol. Monitoring. Part VIII. Current Probl. of Monitoring Freshwater Ecosystems: A Study Guide; NNGU: Nizhnii Novgorod, Russia, 2014. (In Russian) [Google Scholar]
- Rahman, Z.; Singh, V.P. The relative impact of toxic heavy metals (THMs) (arsenic (As), cadmium (Cd), chromium (Cr)(VI), mercury (Hg), and lead (Pb)) on the total environment: An overview. Environ. Monit. Assess. 2019, 191, 419. [Google Scholar] [CrossRef]
- Water Frame Directive. Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 establishing a framework for Community action in the field of water policy. Off. J. 2000, L 327, 0001–0073. Available online: https://leap.unep.org/en (accessed on 23 February 2023).
- Horowitz, A. A Primer on Trace Metal-Sediment Chemistry, 2nd revised ed.; Lewis Publishers: Chelsea, MI, USA, 1991; 136p. [Google Scholar]
- Dash, S.; Borah, S.S.; Kalamdhad, A.S. Heavy metal pollution and potential ecological risk assessment for surficial sediments of Deepor Beel, India. Ecol. Indic. 2021, 122, 107265. [Google Scholar] [CrossRef]
- DeForest, D.K.; Brix, K.V.; Adams, W.J. Assessing metal bioaccumulation in aquatic environments: The inverse relationship between bioaccumulation factors, trophic transfer factors and exposure concentration. Aquat. Toxicol. 2007, 84, 236–246. [Google Scholar] [CrossRef]
- Strode, E.; Balode, M. Toxico-resistance of Baltic amphipod species to heavy metals. Crustaceana 2013, 86, 1007–1024. [Google Scholar] [CrossRef]
- Banaee, M.; Zeidi, A.; Mikušková, N.; Faggio, K. Assessing metal toxicity on crustaceans in aquatic ecosystems: A comprehensive review. Biol. Trace Elem. Res. 2024, 202, 1–21. [Google Scholar] [CrossRef]
- Nel, A.; Xia, T.; Madler, L.; Li, N. Toxic potential of materials at the nanolevel. Science 2006, 311, 622–627. [Google Scholar] [CrossRef]
- Du Laing, G.; Rinklebe, J.; Vandecasteele, B.; Meers, E.; Tack, F.M.G. Trace metal behavior in estuarine and riverine floodplain soils and sediments: A review. Sci. Total Environ. 2009, 407, 3972–3985. [Google Scholar] [CrossRef]
- Gadd, G.M. Microbial influence on metal mobility and application for bioremediation. Geoderma 2004, 122, 109–119. [Google Scholar] [CrossRef]
- Kováčová, S.; Šturdík, E. Interactions between microorganisms and heavy metals including radionuclides. Biol.-Sect. Cell. Mol. Biol. 2002, 57, 651–663. [Google Scholar]
- Levit, R.L.; Kudriavtseva, V.A.; Shigaeva, T.G. The effects of the main cations of natural aquatic media on zinc(II), cadmium(II), lead(II) and copper(II) sorption by aluminium oxide and kaolin. Interdiscip. Sci. Appl. J. “Biosph.” 2014, 6, 382–387. [Google Scholar]
- Qian, G.; Ao, W.; Yu, L. Combined effect of co-existing heavy metals and organophosphate pesticide on adsorption of atrazine to river sediments. Korean J. Chem. Eng. 2011, 28, 1200–1206. [Google Scholar] [CrossRef]
- Mendoza-Carranza, M.; Sepulveda-Lozada, A.; Dias-Ferreira, C.; Geissen, V. Distribution and bioconcentration of heavy metals in a tropical aquatic food web: A case study of a tropical estuarine lagoon in SE Mexico. Environ. Poll. 2016, 210, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.T.; Depledge, M.H. Metabolism of Trace Metals in Aquatic Organisms; Bebianno, M.J., Langston, W.J., Eds.; Chapman & Hall: London, UK, 1998. [Google Scholar]
- Brown, R.J.; Galloway, T.S.; Lowe, D.; Browne, M.A.; Dissanayake, A.; Jones, M.B.; Depledge, M.H. Differential sensitivity of three marine invertebrates to copper assessed using multiple biomarkers. Aquat. Toxicol. 2004, 66, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Gundacker, C. Comparison of heavy metal bioaccumulation in freshwater molluscs of urban river habitats in Vienna. Environ. Pollut. 2000, 110, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Zarykhta, V.V.; Zhang, Z.; Kholodkevich, S.V.; Kuznetsova, T.V.; Sharov, A.N.; Zhang, Y.; Sun, K.; Lv, M.; Feng, Y. Comprehensive assessments of ecological states of Songhua River using chemical analysis and bivalves as bioindicators. Environ. Sci. Pollut. Res. 2019, 26, 33341–33350. [Google Scholar] [CrossRef] [PubMed]
- Kudryavtseva, V.; Shigaeva, T.; Alekseeva, N. Heavy metals in the bottom sediments of the coastal zone of the eastern part of the Gulf of Finland. E3S Web Conf. 2021, 265, 02015. [Google Scholar] [CrossRef]
- Lisco, S.N.; Acquafredda, P.; Gallicchio, S.; Sabato, L.; Bonifazi, A.; Cardone, F.; Corriero, G.; Gravina, M.F.; Pierri, C.; Moretti, M. The sedimentary dynamics of Sabellaria alveolata bioconstructions (Ostia, Tyrrhenian Sea, central Italy). J. Palaeogeogr. 2020, 9, 1–18. Available online: https://www.researchgate.net/publication/338548583 (accessed on 14 May 2024). [CrossRef]
- HELCOM 2014. BASE Project 2012–2014: Preparation of Biodiversity and Hazardous Substances Indicators with Targets That Reflect Good Environmental Status for HELCOM (Including the HELCOM CORESET Project) and Improvement of Russian Capacity to Participate in Operationalization of Those Indicators; Baltic Marine Environment Protection Commission HELCOM: Helsinki, Finland, 2014. [Google Scholar]
- SETAC Europe Annual Meeting, 2022, Copenhagen, Denmark, Discussion. Available online: https://www.oecd.org/chemicalsafety/risk-assessment/guiding-principles-and-key-elements-for-establishing-a-weight-of-evidence-for-chemical-assessment.pdf (accessed on 6 May 2022).
- Recommendations for Reducing Micropollutants in Wastewater; German Environment Agency. Section II 2.1 General Aspects of Water and Soil; Helmecke, M. (Ed.) 2018; 60p, Available online: https://www.umweltbundesamt.de/sites/default/files/medien/1410/publikationen/180709_uba_pos_mikroverunreinigung_en_bf.pdf (accessed on 26 May 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuznetsova, T.V.; Kudryavtseva, V.A.; Kapranova, L.L. Increasing Risks to the Health of the Invertebrates—Balancing between Harm and Benefit. Animals 2024, 14, 1584. https://doi.org/10.3390/ani14111584
Kuznetsova TV, Kudryavtseva VA, Kapranova LL. Increasing Risks to the Health of the Invertebrates—Balancing between Harm and Benefit. Animals. 2024; 14(11):1584. https://doi.org/10.3390/ani14111584
Chicago/Turabian StyleKuznetsova, Tatiana V., Valentina A. Kudryavtseva, and Larisa L. Kapranova. 2024. "Increasing Risks to the Health of the Invertebrates—Balancing between Harm and Benefit" Animals 14, no. 11: 1584. https://doi.org/10.3390/ani14111584




