Role of Medaka (Oryzias latipes) Foxo3 in Resistance to Nervous Necrosis Virus Infection
Abstract
:Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Animals
2.2. Phylogenetic Analysis of Foxo3
2.3. Identification of the Coding Sequence of Foxo3
2.4. RT-PCR and RT-qPCR
2.5. Construction of Foxo3 Mutants
2.6. RGNNV Infection Experiment and Viral Quantitative Analysis
2.7. Statistical Analysis
3. Results
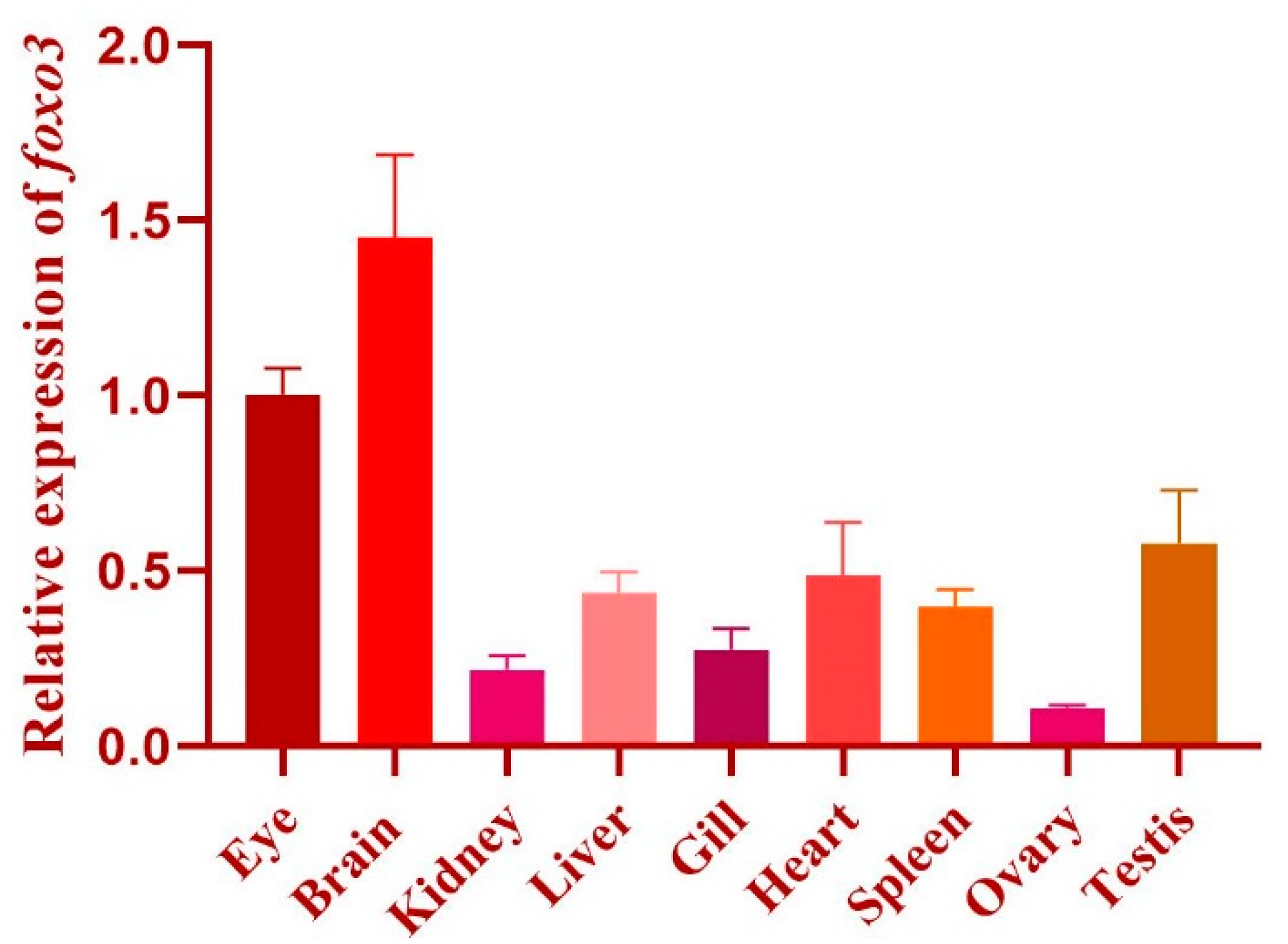
3.1. Characterization of Foxo3 in Medaka
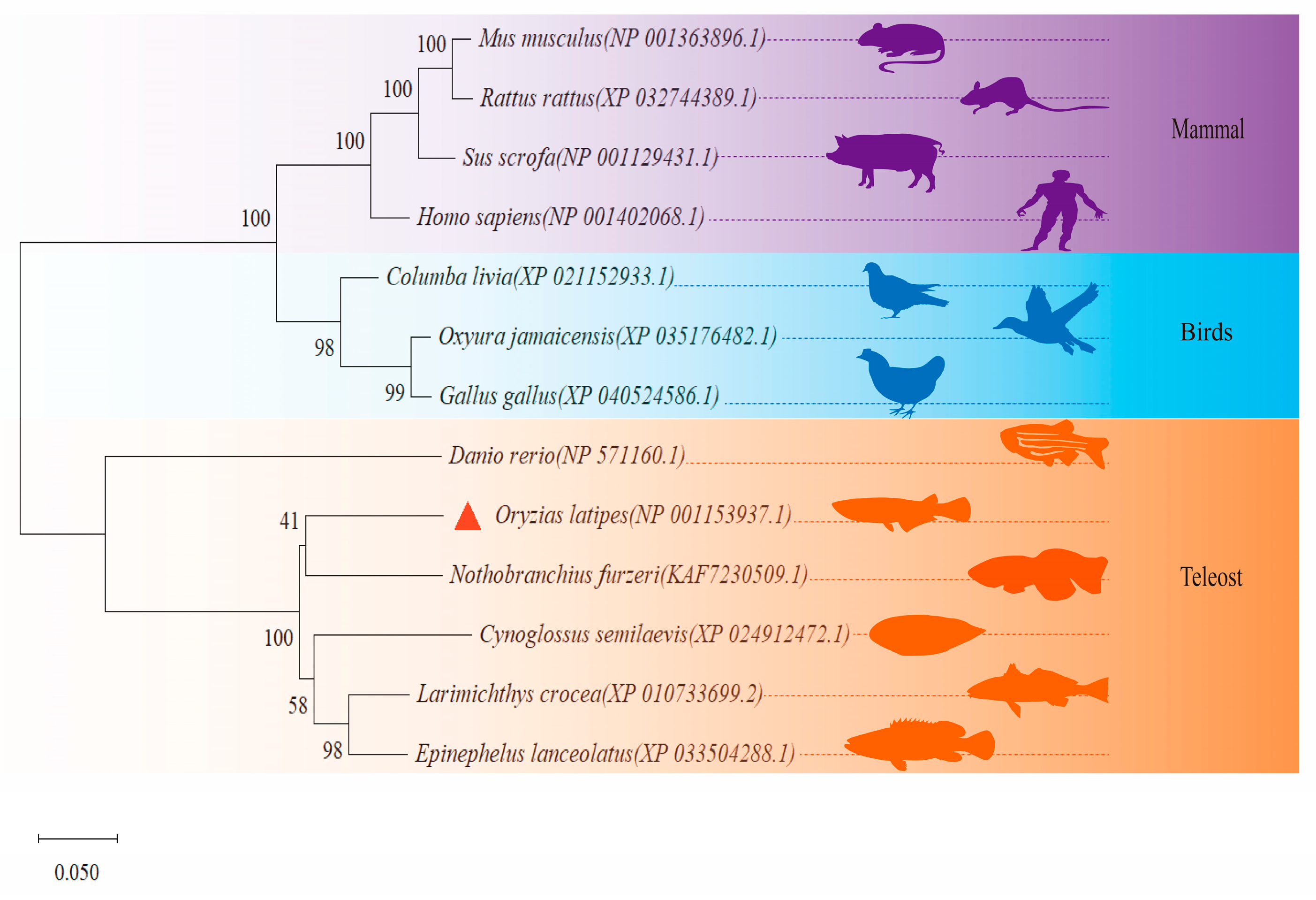
3.2. Phylogenetic Analyses of Foxo3
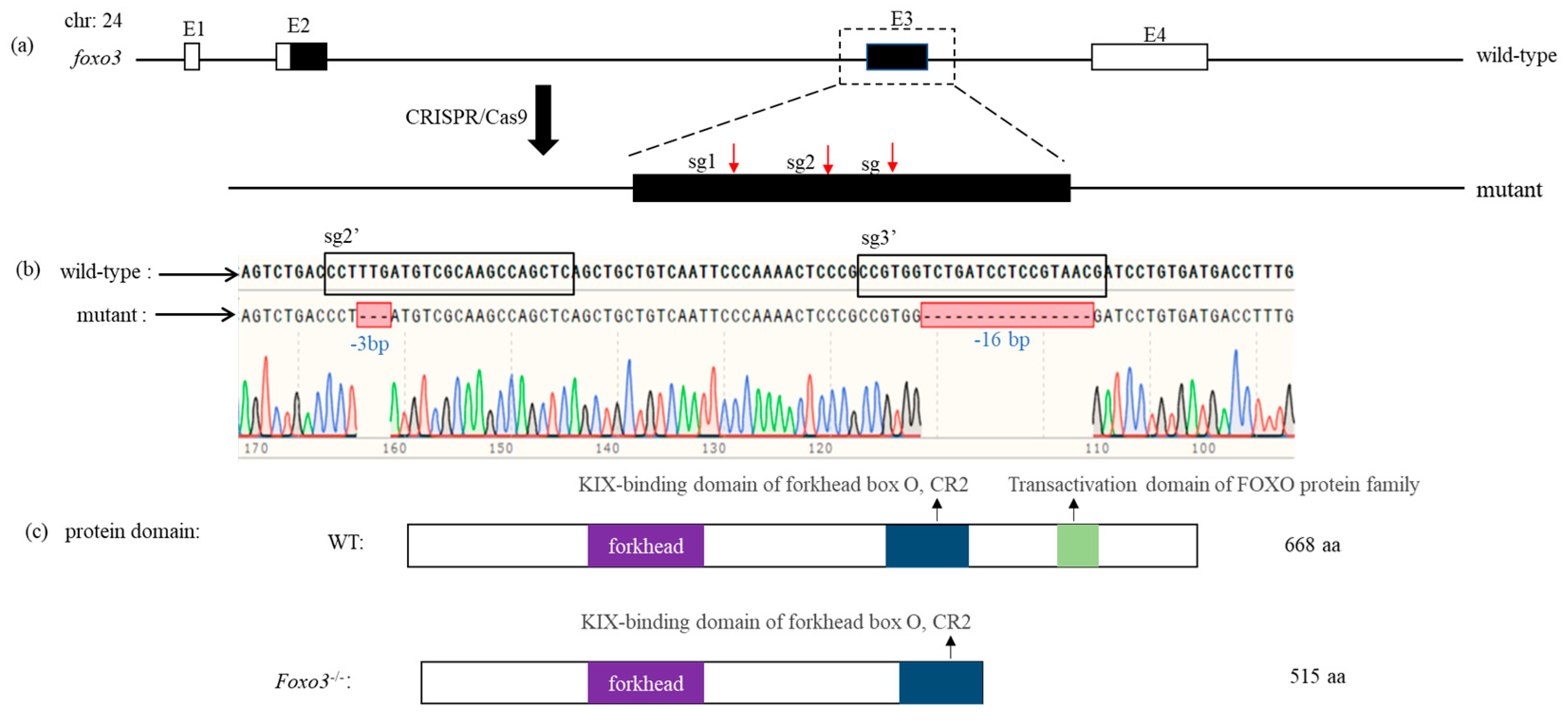
3.3. Construction of Foxo3 Mutant
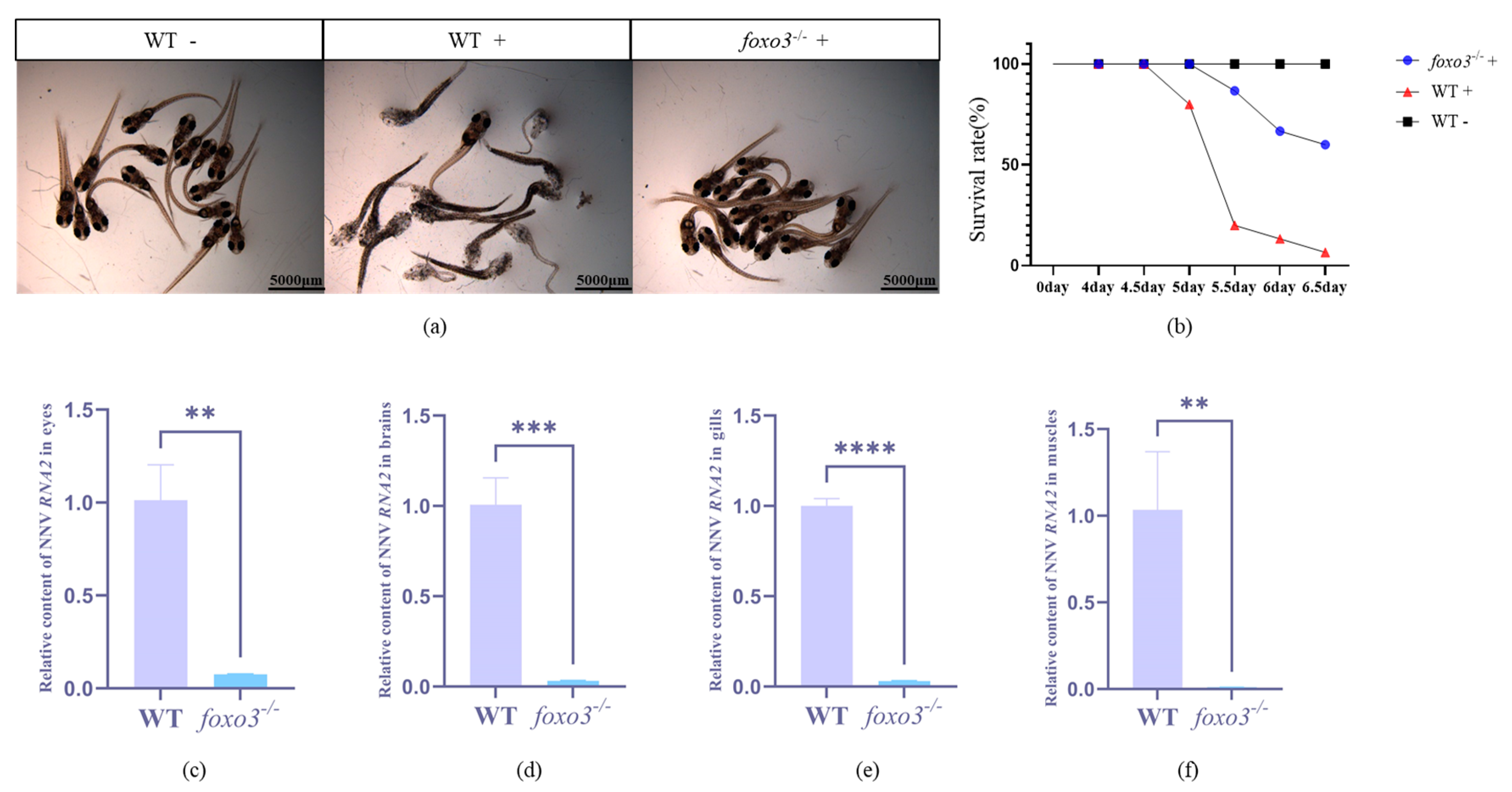
3.4. Loss of Foxo3 Enhanced the Resistance to RGNNV in Medaka
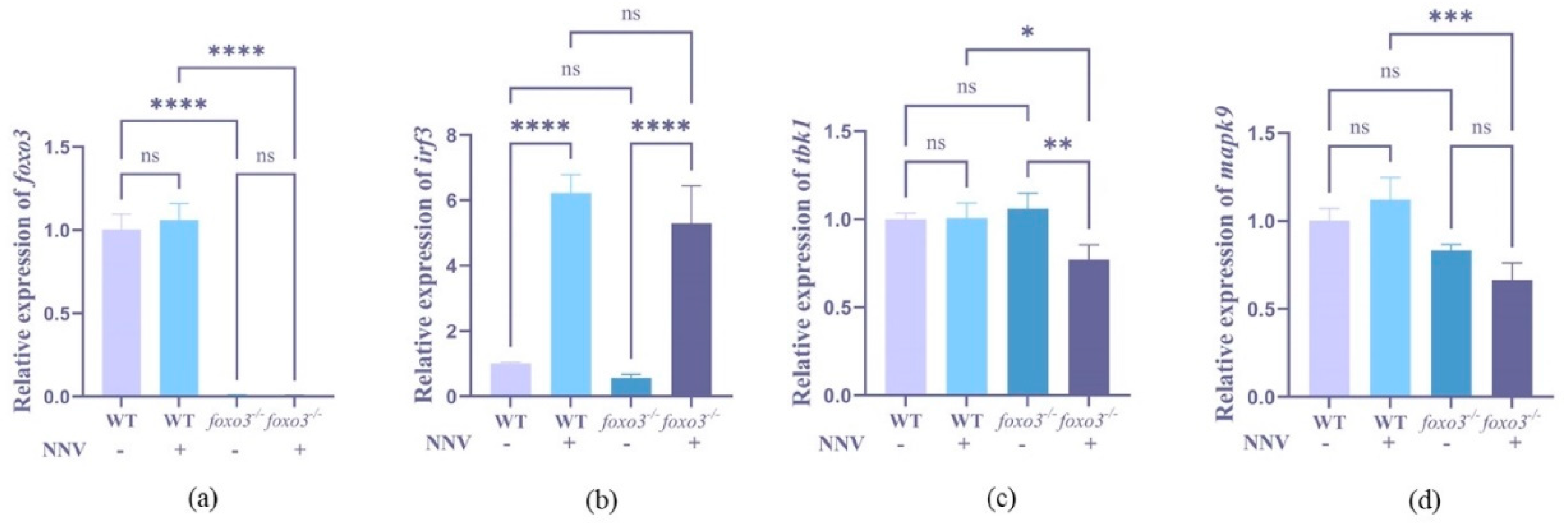
3.5. Detection of Interferon-Related Gene Expression after RGNNV Infection
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lü, L.; Zhou, S.Y.; Chen, C.; Weng, S.P.; Chan, S.M.; He, J.G. Complete genome sequence analysis of an iridovirus isolated from the orange-spotted grouper. Virology 2005, 339, 81–100. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, T.; Okinaka, Y.; Mise, K.; Mori, K.I.; Arimoto, M.; Okuno, T.; Nakai, T. Identification of host-specificity determinants in betanodaviruses by using reassortants between striped jack nervous necrosis virus and sevenband grouper nervous necrosis virus. J. Virol. 2004, 78, 1256–1262. [Google Scholar] [CrossRef] [PubMed]
- Gomez, D.K.; Baeck, G.W.; Kim, J.H.; Choresca, C.H., Jr.; Park, S.C. Molecular detection of betanodavirus in wild marine fish populations in Korea. J. Vet. Diagn. Investig. 2008, 20, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Jimena, B.; Cherif, N.; Garcia-Rosado, E.; Infante, C.; Cano, I.; Castro, D.; Hammami, S.; Borrego, J.J.; Alonso, M.C. A combined RT-PCR and dot-blot hybridization method reveals the coexistence of SJNNV and RGNNV betanodavirus genotypes in wild meagre (Argyrosomus regius). J. Appl. Microbiol. 2010, 109, 1361–1369. [Google Scholar] [CrossRef] [PubMed]
- Thiery, R.; Cozien, J.; de Boisseson, C.; Kerbart-Boscher, S.; Nevarez, L. Genomic classification of new betanodavirus isolates by phylogenetic analysis of the coat protein gene suggests a low host-fish species specificity. J. Gen. Virol. 2004, 85, 3079–3087. [Google Scholar] [CrossRef] [PubMed]
- Nishizawa, T.; Mori, K.; Furuhashi, M.; Nakai, T.; Furusawa, I.; Muroga, K. Comparison of the coat protein genes of five fish nodaviruses, the causative agents of viral nervous necrosis in marine fish. J. Gen. Virol. 1995, 76 Pt 7, 1563–1569. [Google Scholar] [CrossRef] [PubMed]
- Bitchava, K.; Chassalevris, T.; Lampou, E.; Athanassopoulou, F.; Economou, V.; Dovas, C.I. Occurrence and molecular characterization of betanodaviruses in fish and invertebrates of the Greek territorial waters. J. Fish Dis. 2019, 42, 1773–1783. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.L.; Liu, S.; Li, J.; Xu, T.T.; Wang, X.H.; Fu, G.M.; Li, X.P.; Sang, S.W.; Bian, X.D.; Hao, J.W. Evidence for Cross-Species Transmission of Covert Mortality Nodavirus to New Host of Mugilogobius abei. Front. Microbiol. 2018, 9, 1447. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, X.; Liu, S.; Sang, S.; Zhang, Q. Preliminary study on the natural infection of Carassius auratus with covert mortality nodavirus (CMNV). Prog. Fish Sci. 2019, 40, 25–32. [Google Scholar]
- Binesh, C.P. Mortality due to viral nervous necrosis in zebrafish and goldfish Carassius Auratus. Dis. Aquat. Organ. 2013, 104, 257–260. [Google Scholar] [CrossRef]
- Wang, C.; Liu, S.; Tang, K.F.J.; Zhang, Q.L. Natural infection of covert mortality nodavirus affects Zebrafish (Danio Rerio). J. Fish Dis. 2021, 44, 1315–1324. [Google Scholar] [CrossRef] [PubMed]
- Workenhe, S.T.; Rise, M.L.; Kibenge, M.J.T.; Kibenge, F.S.B. The fight between the teleost fish immune response and aquatic viruses. Mol. Immunol. 2010, 47, 2525–2536. [Google Scholar] [CrossRef]
- Chen, Y.M.; Wang, T.Y.; Chen, T.Y. Immunity to betanodavirus infections of marine fish. Dev. Comp. Immunol. 2014, 43, 174–183. [Google Scholar] [CrossRef]
- Ohta, T.; Ueda, Y.; Ito, K.; Miura, C.; Yamashita, H.; Miura, T.; Tozawa, Y. Anti-viral effects of interferon administration on sevenband grouper, Epinephelus septemfasciatus. Fish Shellfish. Immunol. 2011, 30, 1064–1071. [Google Scholar] [CrossRef]
- Ohtani, M.; Hikima, J.; Hwang, S.D.; Morita, T.; Suzuki, Y.; Kato, G.; Kondo, H.; Hirono, I.; Jung, T.S.; Aoki, T. Transcriptional regulation of type I interferon gene expression by interferon regulatory factor-3 in Japanese flounder, Paralichthys olivaceus. Dev. Comp. Immunol. 2012, 36, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.Y.; Nie, L.; Zhu, G.; Xiang, L.X.; Shao, J.Z. Advances in research of fish immune-relevant genes: A comparative overview of innate and adaptive immunity in teleosts. Dev. Comp. Immunol. 2013, 39, 39–62. [Google Scholar] [CrossRef]
- Robertsen, B. The interferon system of teleost fish. Fish Shellfish. Immunol. 2006, 20, 172–191. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Secombes, C.J. Teleost fish interferons and their role in immunity. Dev. Comp. Immunol. 2011, 35, 1376–1387. [Google Scholar] [CrossRef]
- Zhang, Y.B.; Gui, J.F. Molecular regulation of interferon antiviral response in fish. Dev. Comp. Immunol. 2012, 38, 193–202. [Google Scholar] [CrossRef]
- Litvak, V.; Ratushny, A.V.; Lampano, A.E.; Schmitz, F.; Huang, A.C.; Raman, A.; Rust, A.G.; Bergthaler, A.; Aitchison, J.D.; Aderem, A. A FOXO3-IRF7 gene regulatory circuit limits inflammatory sequelae of antiviral responses. Nature 2012, 490, 421–425. [Google Scholar] [CrossRef]
- Hannenhalli, S.; Kaestner, K.H. The evolution of Fox genes and their role in development and disease. Nat. Rev. Genet 2009, 10, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Lam, E.W.; Brosens, J.J.; Gomes, A.R.; Koo, C.Y. Forkhead box proteins: Tuning forks for transcriptional harmony. Nat. Rev. Cancer 2013, 13, 482–495. [Google Scholar] [CrossRef] [PubMed]
- Dijkers, P.F.; Birkenkamp, K.U.; Lam, E.W.F.; Thomas, N.S.B.; Lammers, J.W.J.; Koenderman, L.; Coffer, P.J. FKHR-L1 can act as a critical effector of cell death induced by cytokine withdrawal: Protein kinase B-enhanced cell survival through maintenance of mitochondrial integrity. J. Cell Biol. 2002, 156, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Dejean, A.S.; Beisner, D.R.; Ch’en, I.L.; Kerdiles, Y.M.; Babour, A.; Arden, K.C.; Castrillon, D.H.; DePinho, R.A.; Hedrick, S.M. Transcription factor Foxo3 controls the magnitude of T cell immune responses by modulating the function of dendritic cells. Nat. Immunol. 2009, 10, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, J.A.; Kim, E.H.; Plisch, E.H.; Suresh, M. FOXO3 regulates the CD8 T cell response to a chronic viral infection. J. Virol. 2012, 86, 9025–9034. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, H.; Allen, P.; Peng, S.L. Inflammatory arthritis requires Foxo3a to prevent Fas ligand-induced neutrophil apoptosis. Nat. Med. 2005, 11, 666–671. [Google Scholar] [CrossRef]
- Liu, X.; Cai, X.L.; Zhang, D.W.; Xu, C.X.; Xiao, W.H. Zebrafish foxo3b negatively regulates antiviral response through suppressing the transactivity of irf3 and irf7. J. Immunol. 2016, 197, 4736–4749. [Google Scholar] [CrossRef] [PubMed]
- Wittbrodt, J.; Shima, A.; Schartl, M. Medaka—A model organism from the Far East. Nat. Rev. Genet 2002, 3, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Kasahara, M.; Naruse, K.; Sasaki, S.; Nakatani, Y.; Qu, W.; Ahsan, B.; Yamada, T.; Nagayasu, Y.; Doi, K.; Kasai, Y.; et al. The medaka draft genome and insights into vertebrate genome evolution. Nature 2007, 447, 714–719. [Google Scholar] [CrossRef]
- Wang, Y.D.; Kung, C.W.; Chen, J.Y. Antiviral activity by fish antimicrobial peptides of epinecidin-1 and hepcidin 1-5 against nervous necrosis virus in medaka. Peptides 2010, 31, 1026–1033. [Google Scholar] [CrossRef]
- Pan, Q.H.; Luo, J.Z.; Jiang, Y.W.; Wang, Z.; Lu, K.; Chen, T.S. Efficient gene editing in a medaka (Oryzias latipes) cell line and embryos by SpCas9/tRNA-gRNA. J. Zhejiang Univ. Sc. B 2022, 23, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, M.; Murata, K.; Naruse, K.; Tanaka, M. Medaka: Biology, Management, and Experimental Protocols; Wiley-Blackwell: Ames, Iowa, 2009. [Google Scholar]
- Chen, T.; Cavari, B.; Schartl, M.; Hong, Y. Identification and Expression of Conserved and Novel RNA Variants of Medaka pax6b Gene. J. Exp. Zool. Part B: Mol. Dev. Evol. 2017, 328, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11 Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Mali, P.; Esvelt, K.M.; Church, G.M. Cas9 as a versatile tool for engineering biology. Nat. Methods 2013, 10, 957–963. [Google Scholar] [CrossRef] [PubMed]
- Winkler, C.; Vielkind, J.R.; Schartl, M. Transient expression of foreign DNA during embryonic and larval development of the medaka fish (Oryzias latipes). Mol. Gen. Genet 1991, 226, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Chen, T.; Pan, Q.; Wang, Q. Generation of albino medaka (Oryzias latipes) by CRISPR/Cas9. J. Exp. Zool. Part B 2018, 330, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.H.; Lu, K.; Luo, J.Z.; Jiang, Y.W.; Xia, B.L.; Chen, L.; Wang, M.Y.; Dai, R.G.; Chen, T.S. Japanese medaka Olpax6.1 mutant as a potential model for spondylo-ocular syndrome. Funct. Integr. Genomic 2023, 23, 168. [Google Scholar] [CrossRef] [PubMed]
- Stifter, S.A.; Feng, C.G. Interfering with immunity: Detrimental role of type I IFNs during infection. J. Immunol. 2015, 194, 2455–2465. [Google Scholar] [CrossRef] [PubMed]
- Jensen, S.; Thomsen, A.R. Sensing of RNA Viruses: A Review of Innate Immune Receptors Involved in Recognizing RNA Virus Invasion. J. Virol. 2012, 86, 2900–2910. [Google Scholar] [CrossRef]
- Randall, R.E.; Goodbourn, S. Interferons and viruses: An interplay between induction, signalling, antiviral responses and virus countermeasures. J. Gen. Virol. 2008, 89, 1–47. [Google Scholar] [CrossRef]
- Hu, Y.; Huang, Y.; Liu, J.; Zhang, J.; Qin, Q.; Huang, X. TBK1 from orange-spotted grouper exerts antiviral activity against fish viruses and regulates interferon response. Fish Shellfish. Immun. 2018, 73, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Zhang, W.; Huang, B.; Chen, X.; Huang, C. UBQLN2 Promotes the Production of Type I Interferon via the TBK1-IRF3 Pathway. Cells 2020, 9, 1205. [Google Scholar] [CrossRef] [PubMed]
- Bubici, C.; Papa, S. JNK signalling in cancer: In need of new, smarter therapeutic targets. Brit. J. Pharmacol. 2014, 171, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Davis, R.J.; Flavell, R.A. MAP kinases in the immune response. Annu. Rev. Immunol. 2002, 20, 55–72. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Davis, R.J.; Flavell, R.A. Signaling by the JNK group of MAP kinases. J. Clin. Immunol. 2001, 21, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Constant, S.L.; Dong, C.; Yang, D.D.; Wysk, M.; Davis, R.J.; Flavell, R.A. JNK1 is required for T cell-mediated immunity against Leishmani major infection. J. Immunol. 2000, 165, 2671–2676. [Google Scholar] [CrossRef] [PubMed]
- Tong, W.Z.; Chen, X.X.; Song, X.; Chen, Y.Q.; Jia, R.Y.; Zou, Y.F.; Li, L.X.; Yin, L.Z.; He, C.L.; Liang, X.X.; et al. Resveratrol inhibits LPS-induced inflammation through suppressing the signaling cascades of TLR4-NF-κB/MAPKs/IRF3. Exp. Ther. Med. 2020, 19, 1824–1834. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Hron, J.D.; Peng, S.L. Regulation of NF-κB, Th activation, and autoinflammation by the forkhead transcription factor Foxo3a. Immunity 2004, 21, 203–213. [Google Scholar] [CrossRef]
- Zhao, C.; Sun, G.T.; Li, Y.X.; Kong, K.Y.; Li, X.D.; Kan, T.Y.; Yang, F.; Wang, L.; Wang, X.Q. Forkhead box O3 attenuates osteoarthritis by suppressing ferroptosis through inactivation of NF-κB/MAPK signaling. J. Orthop. Transl. 2023, 39, 147–162. [Google Scholar] [CrossRef]

| Primer Names | Primer Sequence (5′-3′) | Size (bp) |
|---|---|---|
| foxo3 CDS-F | TTTCAGGTGTCGTGAGGAATTCATGGCCGAGGCCCCGCTTCC | 2007 |
| foxo3 CDS-R | AACATCGTATGGGTAGGATCCGCCAGGTACCCAGCTCTGAG | |
| foxo3 RT-F | GACCTCCTCCCGCCGCAAC | 179 |
| foxo3 RT-R | AGTTCTTCCATCCCGCAGAG | |
| gRNA-R | AAAAGCACCGACTCGGTGCC | 20 |
| foxo3 D-F | ATGGACAGATTTCCGCTCTCG | 832 |
| foxo3 D-R | AGCCAGAGTATTAGCCTCGTT | |
| NNV RNA2 qRT-F | AAATGGTGGGAAAGCAGAACA | 167 |
| NNV RNA2 qRT-R | CGAACACTCCAGCGACACA | |
| foxo3 qRT-F | ACATCTTTCTCCAGCATCAGCA | 180 |
| foxo3 qRT-R | GTTACGGAGGATCAGACCACG | |
| tbk1 qRT-F | GCATCGGCGTTACCTTCTACCAC | 156 |
| tbk1 qRT-R | CAATCTTGCCGTTTTCGCTCT | |
| irf3 qRT-F | TCGACTCTGAAGCAGGAATCACA | 151 |
| irf3 qRT-R | TCAGGCAACCCAGTTTCACCG | |
| mapk9 qRT-F | CAGCCTCTGCCAGGTGATCCA | 183 |
| mapk9 qRT-R | CTTGCCAGCCCAAAGTCCA | |
| β-actin qRT-F | TATCATTCGCCTGAAACCGAT | 114 |
| β-actin qRT-R | CTTTGCACATGCCAGATCCG |
| Target Site | Location | Forward and Backward | Target Sequence 5′-3′ | PAM Sequence |
|---|---|---|---|---|
| sg1 | 24:7575244-7575266 | + | GGTACGATGAACTTGAATGA | TGG |
| sg2 | 24:7575643-7575665 | − | GAGCTGGCTTGCGACATCAA | AGG |
| sg3 | 24:7575693-7575715 | − | CGTTACGGAGGATCAGACCA | CGG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Wang, Z.; Liang, J.; Xia, B.; Chen, R.; Chen, T. Role of Medaka (Oryzias latipes) Foxo3 in Resistance to Nervous Necrosis Virus Infection. Animals 2024, 14, 1587. https://doi.org/10.3390/ani14111587
Li W, Wang Z, Liang J, Xia B, Chen R, Chen T. Role of Medaka (Oryzias latipes) Foxo3 in Resistance to Nervous Necrosis Virus Infection. Animals. 2024; 14(11):1587. https://doi.org/10.3390/ani14111587
Chicago/Turabian StyleLi, Wen, Zhi Wang, Jingjie Liang, Bilin Xia, Ruoxue Chen, and Tiansheng Chen. 2024. "Role of Medaka (Oryzias latipes) Foxo3 in Resistance to Nervous Necrosis Virus Infection" Animals 14, no. 11: 1587. https://doi.org/10.3390/ani14111587





