Using Image Analysis Technique for Predicting Light Lamb Carcass Composition
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Carcasses
2.2. Acquisition of VIA Images and Measurements
2.3. Carcass Jointing and Dissection
2.4. Models and Statistical Analysis
3. Results
3.1. Prediction of Carcass Tissues Weight in the Carcass
3.2. Prediction of Carcass Tissues Percentage in the Carcass
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scholz, A.M.; Bünger, L.; Kongsro, J.; Baulain, U.; Mitchell, A.D. Non-invasive methods for the determination of body and carcass composition in livestock: Dual energy X-ray absorptiometry, computed tomography, magnetic resonance imaging and ultrasound: Invited review. Animal 2015, 9, 1250–1264. [Google Scholar] [CrossRef] [PubMed]
- Aalhus, J.L.; López-Campos, Ó.; Prieto, N.; Rodas-González, A.; Dugan, M.E.R.; Uttaro, B.; Juárez, M. Review: Canadian beef grading—Opportunities to identify carcass and meat quality traits valued by consumers. Can. J. Anim. Sci. 2014, 94, 545–556. [Google Scholar] [CrossRef]
- Allen, P. Recent developments in the objective measurement of carcass and meat quality for industrial application. Meat Sci. 2021, 181, 108601. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Pando, G.; Allen, P.; Troy, D.J.; McDonnell, C.K. Objective carcass measurement technologies: Latest developments and future trends. Trends Food Sci. Technol. 2021, 111, 771–782. [Google Scholar] [CrossRef]
- Rius-Vilarrasa, E.; Bünger, L.; Maltin, C.; Matthews, K.R.; Roehe, R. Evaluation of Video Image Analysis (VIA) technology to predict meat yield of sheep carcasses on-line under UK abattoir conditions. Meat Sci. 2009, 82, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Segura, J.; Aalhus, J.L.; Prieto, N.; Zawadski, S.; Scott, H.; López-Campos, O. Prediction of primal and retail cut weights, tissue composition and yields of youthful cattle carcasses using computer vision systems; whole carcass camera and/or ribeye camera. Meat Sci. 2023, 199, 109120. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, T.E.; Elam, N.A.; Miller, M.F.; Brooks, J.C.; Hilton, G.G.; VanOverbeke, D.L.; McKeith, F.K.; Killefer, J.; Montgomery, T.H.; Allen, D.M.; et al. Predicting red meat yields in carcasses from beef-type and calf-fed Holstein steers using the United States Department of Agriculture calculated yield grade. J. Anim. Sci. 2010, 88, 2139–2143. [Google Scholar] [CrossRef] [PubMed]
- López-Campos, Ó.; Larsen, I.L.; Prieto, N.; Juárez, M.; Dugan, M.E.R.; Aalhus, J.L. Evaluation of the Canadian and United States beef yield prediction equations and dua energy x-ray absorptiometry for a rapid, non-invasive yield prediction in beef. In Proceedings of the 61st International Congress of Meat Science and Technology Session VII-111, Clermont-Ferrand, France, 23–28 August 2015. [Google Scholar]
- Woerner, D.R.; Belk, K.-E. The History of Instrument Assessment of Beef—A Focus on the Last Ten Years; National Cattlemen’s Beef Association: Washington, DC, USA, 2008. [Google Scholar]
- Craigie, C.R.; Ross, D.W.; Maltin, C.A.; Purchas, R.W.; Bünger, L.; Roehe, R.; Morris, S.T. The relationship between video image analysis (VIA), visual classification, and saleable meat yield of sirloin and fillet cuts of beef carcasses differing in breed and gender. Livest. Sci. 2013, 158, 169–178. [Google Scholar] [CrossRef]
- Pabiou, T.; Fikse, W.F.; Amer, P.R.; Cromie, A.R.; Nasholm, A.; Berry, D.P. Genetic variation in wholesale carcass cuts predicted from digital images in cattle. Animal 2011, 5, 1720–1727. [Google Scholar] [CrossRef]
- Craigie, C.R.; Navajas, E.A.; Purchas, R.W.; Maltin, C.A.; Bünger, L.; Hoskin, S.O.; Ross, D.W.; Morris, S.T.; Roehe, R. A review of the development and use of video image analysis (VIA) for beef carcass evaluation as an alternative to the current EUROP system and other subjective systems. Meat Sci. 2012, 92, 307–318. [Google Scholar] [CrossRef]
- Allen, P. Evaluating video image analysis (VIA) systems for beef carcass classification. In The Science of Beef Quality, Proceedings of the Annual Meeting of the British Society of Animal Science, Bristol, UK, 18–19 May 2005; British Society of Animal Science: Midlothian, UK, 2005; pp. 9–12. [Google Scholar]
- Allen, P. New methods for grading beef and sheep carcasses. In Evaluation of Carcass and Meat Quality in Cattle and Sheep; Lazzaroni, C., Gigli, S., Gabiña, D., Eds.; EAAP publications No. 123; Wageningen Academic Publishers: Wageningen, The Netherlands, 2007; pp. 39–48. [Google Scholar]
- Commission Regulation (EC) No 1107/96 of 12 June 1996 on the Registration of Geographical Indications and Designations of Origin under the Procedure Laid down in Article 17 of Council Regulation (EEC) No 2081/92. Available online: https://www.legislation.gov.uk/eur/1996/1107 (accessed on 5 April 2024).
- Rasband, W.S. ImageJ; U.S. National Institutes of Health: Bethesda, MD, USA, 2018. Available online: https://imagej.nih.gov/ij/ (accessed on 1 January 2019).
- Batista, A.C.; Santos, V.; Afonso, J.; Guedes, C.; Azevedo, J.; Teixeira, A.; Silva, S. Evaluation of an image analysis approach to predicting primal cuts and lean in light lamb carcasses. Animals 2021, 11, 1368. [Google Scholar] [CrossRef]
- Ngo, L.; Ho, H.; Hunter, P.; Quinn, K.; Thomson, A.; Pearson, G. Post-mortem prediction of primal and selected retail cut weights of New Zealand lamb from carcass and animal characteristics. Meat Sci. 2016, 112, 39–45. [Google Scholar] [CrossRef]
- Oliver, A.; Mendizabal, J.A.; Ripoll, G.; Alberti, P.; Purroy, A. Predicting meat yields and commercial meat cuts from carcasses of young bulls of Spanish breeds by the SEUROP method and an image analysis system. Meat Sci. 2010, 84, 628–633. [Google Scholar] [CrossRef]
- Rius-Vilarrasa, E.; Bünger, L.; Brotherstone, S.; Macfarlane, J.M.; Lambe, N.R.; Matthews, K.R.; Haresign, W.; Roehe, R. Genetic parameters for carcass dimensional measurements from Video Image Analysis and their association with conformation and fat class scores. Livest. Sci. 2010, 128, 92–100. [Google Scholar] [CrossRef]
- Santos, V.A.C.; Cabo, A.; Raposo, P.; Silva, J.A.; Azevedo, J.M.T.; Silva, S.R. The effect of carcass weight and sex on carcass composition and meat quality of “Cordeiro Mirandes”-Protected designation of origin lambs. Small Rumin. Res. 2015, 130, 136–140. [Google Scholar] [CrossRef]
- Panea, B.; Ripoll, G.; Albertí, P.; Joy, M.; Teixeira, A. Atlas of dissection of ruminant’s carcass. Inf. Tec. Econ. Agrar. 2012, 108, 3–105. [Google Scholar]
- Williams, P.C. Variables affecting near-infrared reflectance spectroscopic analysis. In Near-infrared Technology in the Agricultural and Food Industries; Williams, P., Norris, K., Eds.; American Association of Cereal Chemists: St. Paul, MN, USA, 1987; pp. 143–166. [Google Scholar]
- Araújo, J.C.; Santos, H.A.S.; Ribeiro, E.S.C.; Trindade, A.C.C.; Sousa, M.A.; Nunes, M.P.M.; Lima, A.C.S.; Daher, L.C.C.; Silva, A.G.M. Use of in vivo video image analysis as a substitute for manual biometric measurements on the prediction of qualitative and quantitative carcass characteristics of hair sheep lambs. Small Rumin. Res. 2022, 215, 106779. [Google Scholar] [CrossRef]
- Horgan, G.W.; Murphy, S.V.; Simm, G. Automatic assessment of sheep carcasses by image analysis. Anim. Sci. 1995, 60, 197–202. [Google Scholar] [CrossRef]
- Stanford, K.; Richmond, R.J.; Jones, S.D.M.; Robertson, W.M.; Price, M.A.; Gordon, A.J. Video image analysis for on-line classification of lamb carcasses. Anim. Sci. 1998, 67, 311–316. [Google Scholar] [CrossRef]
- Cunha, B.C.N.; Belk, K.E.; Scanga, J.A.; LeValley, S.B.; Tatum, J.D.; Smith, G.C. Development and validation of equations utilizing lamb vision system output to predict lamb carcass fabrication yields. J. Anim. Sci. 2004, 82, 2069–2076. [Google Scholar] [CrossRef]
- Brady, A.S.; Belk, K.E.; LeValley, S.B.; Dalsted, N.L.; Scanga, J.A.; Tatum, J.D.; Smith, G.C. An evaluation of the lamb vision system as a predictor of lamb carcass red meat yield percentage. J. Anim. Sci. 2003, 81, 1488–1498. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, D.L.; Safari, E.; Thompson, J.M.; Smith, C.R. Video image analysis in the Australian meat industry—Precision and accuracy of predicting lean meat yield in lamb carcasses. Meat Sci. 2004, 67, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Einarsson, E.; Eythorsdottir, E.; Smith, C.R.; Jonmundsson, J.V. The ability of video image analysis to predict lean meat yield and EUROP score of lamb carcasses. Animal 2014, 8, 1170–1177. [Google Scholar] [CrossRef] [PubMed]
- Rius-Vilarrasa, E.; Bünger, L.; Matthews, K.; Maltin, C.; Hinz, A.; Roehe, R. Evaluation of Video Image Analysis (VIA) technology to predict meat yield of sheep carcasses online under abattoir conditions. In Proceedings of the British Society of Animal Science, Southport, UK, 2–4 April 2007; p. 108. [Google Scholar]
- Viscarra Rossel, R.A.; McGlynn, R.N.; McBratney, A.B. Determining the composition of mineral-organic mixes using UV-vis-NIR diffuse reflectance spectroscopy. Geoderma 2006, 137, 70–82. [Google Scholar] [CrossRef]

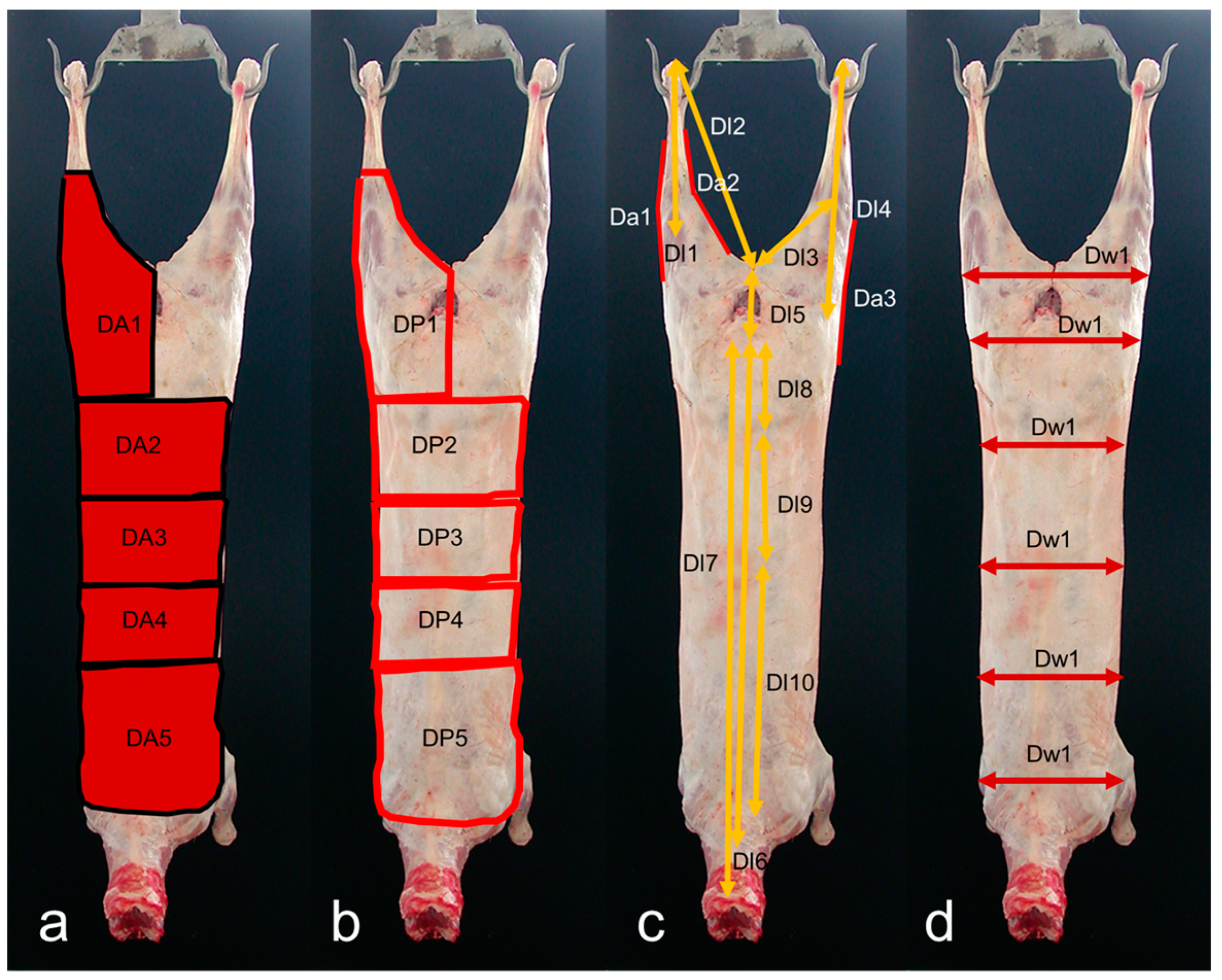
| Traits | Abbreviation | Description | Mean (sd) | Range | CV (%) |
|---|---|---|---|---|---|
| CCW (g) | 4522 (1336) | 2162–7622 | 29.6 | ||
| Carcass composition | |||||
| Carcass tissues (g) | M | Muscle | 1169.5 (341.4) | 546.9–1945.6 | 29.2 |
| SF | Subcutaneous fat | 131.0 (76.8) | 11.8–359.4 | 58.6 | |
| IF | Intermuscular fat | 236.4 (91.9) | 96.0–475.7 | 38.9 | |
| TF | Total fat | 367.4 (166.0) | 107.9–834.9 | 45.2 | |
| B | Bone | 447.8 (102.5) | 259.6–683.3 | 22.9 | |
| Carcass tissues (%) | pM | Percentage muscle | 61.1 (2.4) | 56.4–69.7 | 4.0 |
| pSF | Percentage subcutaneous fat | 6.1 (2.1) | 1.1–10.7 | 34.2 | |
| pIF | Percentage intermuscular fat | 10.9 (1.7) | 5.9–13.9 | 15.2 | |
| pTF | Percentage total fat | 17.0 (3.5) | 7.0–24.7 | 20.4 | |
| pB | Percentage bone | 21.9 (2.1) | 17.4–26.0 | 9.7 | |
| Lateral view measurements | |||||
| Length (cm) | Ll1 | Leg length | 30.8 (3.4) | 23.0–38.0 | 11.0 |
| Ll2 | Lateral thoracolumbar length | 39.2 (4.3) | 29.7–47.0 | 10.9 | |
| Ll3 | Length between the calcaneus and the greater tubercle of humerus | 72.7 (6.9) | 55.3–86.4 | 9.5 | |
| Ll4 | Forearm length | 26.9 (3.2) | 18.9–32.4 | 12.1 | |
| Width (cm) | Lw1 | Thinnest leg width | 9.7 (1.1) | 7.2–12.0 | 11.6 |
| Lw2 | Largest leg width | 10.3 (1.3) | 7.7–13.0 | 12.7 | |
| Lw3 | Minimum waist width | 9.4 (1.2) | 7.1–12.7 | 12.6 | |
| Lw4 | Maximum waist width | 13.2 (1.7) | 9.2–16.6 | 13.0 | |
| Lw5 | Maximum thoracic width | 17.1 (2.1) | 13.1–20.9 | 12.1 | |
| Lw6 | Widest part of the chest | 17.4 (2.0) | 13.1–22.7 | 11.5 | |
| Angle (θ) | La1 | Lateral leg angle 1 | 142.6 (5.7) | 129.8–153.9 | 4.0 |
| La2 | Lateral leg angle 2 | 160.5 (5.6) | 149.2–172.8 | 3.5 | |
| La3 | Lateral leg angle 3 | 154.6 (9.5) | 136.0–178.9 | 6.1 | |
| Area (cm2) | LA1 | Lateral leg area | 185.0 (42.8) | 107.7–271.6 | 23.1 |
| LA2 | Loin area | 163.1 (39.8) | 97.8–270.6 | 24.4 | |
| LA3 | Forequarter area | 337.7 (67.5) | 204.2–471.3 | 20.0 | |
| LA4 | Lateral shoulder area | 140.3 (35.6) | 83.5–260.7 | 25.4 | |
| Perimeter (cm) | LP1 | Lateral leg perimeter | 58.6 (7.1) | 44.0–70.7 | 12.2 |
| LP2 | Loin perimeter | 50.8 (7.9) | 14.0–65.7 | 15.5 | |
| LP3 | Forequarter perimeter | 69.8 (10.4) | 15.3–83.2 | 14.9 | |
| LP4 | Lateral shoulder perimeter | 48.7 (8.2) | 11.4–67.5 | 16.8 | |
| Dorsal view measurements | - | ||||
| Length (cm) | Dl1 | Minimum leg length | 16.3 (3.8) | 8.6–27.0 | 23.3 |
| Dl2 | Length between the perineum and the tarsometatarsal joint | 19.3 (2.6) | 11.4–25.0 | 13.4 | |
| Dl3 | Length between the perineum and the femorotibial joint | 6.4 (0.8) | 4.9–8.8 | 12.5 | |
| Dl4 | Maximum leg length | 24.5 (3.8) | 6.1–33.8 | 15.5 | |
| Dl5 | Length between the basis of tail and the perineum | 9.1 (1.8) | 6.0–14.3 | 20.0 | |
| Dl6 | Length between the basis of the neck and the basis of tail | 49.7 (4.8) | 41.2–61.4 | 9.6 | |
| Dl7 | Length between the basis of tail and the atlas | 62.0 (5.7) | 48.9–77.1 | 9.2 | |
| Dl8 | Sacrum length | 8.6 (1.3) | 5.9–11.9 | 14.7 | |
| Dl9 | Lumbar spine length | 18.9 (3.0) | 10.2–26.3 | 15.9 | |
| Dl10 | Thoracic spine length | 29.5 (4.2) | 19.9–38.9 | 14.1 | |
| Width (cm) | Dw1 | Minimum leg width | 17.5 (1.5) | 14.2–21.8 | 8.4 |
| Dw2 | Maximum leg width | 13.0 (1.4) | 10.4–17.4 | 10.9 | |
| Dw3 | Minimum saddle width | 9.7 (1.2) | 7.9–13.0 | 12.7 | |
| Dw4 | Maximum chest width | 10.6 (1.4) | 7.6–14.3 | 13.3 | |
| Dw5 | Minimum chest width | 9.8 (1.1) | 7.0–12.0 | 11.2 | |
| Dw6 | Maximum shoulder width | 10.9 (1.3) | 7.6–13.3 | 11.9 | |
| Angle (θ) | Da1 | Dorsal leg angle 1 | 164.1 (5.6) | 150.2–174.0 | 3.4 |
| Da2 | Dorsal leg angle 2 | 122.3 (4.0) | 113.7–130.7 | 3.3 | |
| Da3 | Dorsal leg angle 3 | 167.7 (5.2) | 157.3–179.7 | 3.1 | |
| Area (cm2) | DA1 | Dorsal leg area | 131.0 (23.5) | 89.8–203.0 | 18.0 |
| DA2 | Lumbar area | 104.4 (26.8) | 55.7–199.8 | 25.7 | |
| DA3 | Thoracolumbar area | 105.1 (26.3) | 58.4–171.9 | 25.0 | |
| DA4 | Thoracic area | 101.9 (23.6) | 57.4–189.8 | 23.2 | |
| DA5 | Dorsal shoulder area | 136.8 (33.7) | 77.2–242.1 | 24.6 | |
| Perimeter (cm) | DP1 | Dorsal leg perimeter | 55.9 (4.9) | 45.3–71.8 | 8.8 |
| DP2 | Lumbar perimeter | 42.3 (5.2) | 31.9–60.2 | 12.3 | |
| DP3 | Thoracolumbar perimeter | 40.3 (4.9) | 29.8–53.1 | 12.2 | |
| DP4 | Thoracic perimeter | 40.1 (4.7) | 30.4–54.6 | 11.6 | |
| DP5 | Dorsal shoulder perimeter | 46.2 (5.7) | 35.6–62.1 | 12.3 | |
| Tissue | Dorsal + Lateral | (Model 1) | Dorsal | (Model 2) | Lateral | (Model 3) | |
|---|---|---|---|---|---|---|---|
| Muscle (g) | Intercept | −1570.57 | −152.25 | −73.05 | |||
| Independent variables | 0.128 | CCW | 0.23 | CCW | 0.197 | CCW | |
| −7.293 | Ll2 | 7.004 | DP3 | 1.891 | LA1 | ||
| 10.052 | Ll3 | ||||||
| 47.455 | Lw3 | ||||||
| 3.72 | La1 | ||||||
| −6.278 | Dl2 | ||||||
| 4.775 | Dl11 | ||||||
| 46.776 | Dw3 | ||||||
| 4.715 | DP2 | ||||||
| k-fold-R2 | 0.98 | 0.95 | 0.97 | ||||
| RMSE | 42.05 | 63.46 | 54.19 | ||||
| RPD | 8.12 | 5.38 | 6.3 | ||||
| IF (g) | Intercept | 406.761 | −205.125 | 347.615 | |||
| Independent variables | 0.058 | CCW | 0.033 | CCW | 0.052 | CCW | |
| −4.012 | Ll1 | 15.509 | Dl3 | −3.121 | La1 | ||
| −2.93 | La1 | 1.47 | DA1 | 0.619 | LA2 | ||
| 0.679 | LA2 | ||||||
| k-fold-R2 | 0.88 | 0.85 | 0.88 | ||||
| RMSE | 28.82 | 34.28 | 29.98 | ||||
| RPD | 3.19 | 2.68 | 3.07 | ||||
| SF (g) | Intercept | 45.155 | −239.455 | 8.721 | |||
| Independent variables | 0.044 | CCW | 0.016 | CCW | 0.063 | CCW | |
| −4.843 | Ll1 | −2.537 | Dl4 | −6.061 | Ll4 | ||
| −2.392 | Ll4 | 14.036 | Dw3 | ||||
| −3.775 | Dl7 | 1.722 | DA1 | ||||
| 11.523 | Dw3 | ||||||
| 1.699 | DA1 | ||||||
| k-fold-R2 | 0.83 | 0.85 | 0.84 | ||||
| RMSE | 24.07 | 27.61 | 29.5 | ||||
| RPD | 3.19 | 2.78 | 2.6 | ||||
| TF (g) | Intercept | 388.234 | −46.719 | 614.243 | |||
| Independent variables | 0.073 | CCW | 0.034 | CCW | 0.117 | CCW | |
| −12.861 | Ll1 | 24.227 | Dl3 | −9.676 | Ll1 | ||
| −3.456 | La1 | −5 | Dl4 | −4.361 | La1 | ||
| 0.799 | LA2 | 4.786 | Dl10 | 0.885 | LA2 | ||
| 0.363 | LA3 | 27.359 | Dw3 | ||||
| 2.171 | DA1 | −3.693 | Da1 | ||||
| 3.275 | DA1 | ||||||
| k-fold-R2 | 0.91 | 0.89 | 0.88 | ||||
| RMSE | 45.81 | 49.72 | 50.42 | ||||
| RPD | 3.62 | 3.34 | 3.29 | ||||
| Bone (g) | Intercept | −244.683 | −271.71 | −58.374 | |||
| Independent variables | 0.04 | CCW | 0.039 | CCW | 0.055 | CCW | |
| 22.367 | Lw1 | 5.022 | Dl7 | 26.264 | Lw1 | ||
| −16.285 | Dl3 | −20.496 | Dw4 | ||||
| 3.612 | Dl6 | 24.96 | Dw5 | ||||
| −17.319 | Dw4 | 5.14 | DP3 | ||||
| 19.553 | Dw5 | ||||||
| 5.189 | DP3 | ||||||
| k-fold-R2 | 0.95 | 0.91 | 0.92 | ||||
| RMSE | 21.76 | 28.2 | 27.63 | ||||
| RPD | 4.71 | 3.63 | 3.71 |
| Tissue | Dorsal + Lateral (Model 1) | Dorsal (Model 2) | Lateral (Model 3) | ||||
|---|---|---|---|---|---|---|---|
| Muscle | Intercept | −1259.646 | −1281.543 | −828.65 | |||
| Independent variables | 10.524 | DA2 | 18.335 | Dl7 | 66.034 | Lw6 | |
| 7.022 | Dw3 | 70.866 | Dw3 | 4.598 | LA1 | ||
| 6.932 | LA1 | 5.99 | DA2 | ||||
| 2.049 | Ll4 | ||||||
| 1.417 | Dl2 | ||||||
| 1.083 | Dl11 | ||||||
| K-fold-R2 | 0.98 | 0.94 | 0.88 | ||||
| RMSE | 56.642 | 84.222 | 122.901 | ||||
| RPD | 6.03 | 4.05 | 2.78 | ||||
| IF | Intercept | −164.563 | −455.011 | 97.734 | |||
| Independent variables | −2.22 | La1 | 16.103 | Dl3 | 5.573 | Ll2 | |
| 0.542 | LA2 | 4.975 | Dl7 | 16.602 | Lw6 | ||
| 4.236 | Dl7 | 2.139 | DA1 | −3.683 | La1 | ||
| 9.573 | Dw1 | 0.964 | LA2 | ||||
| 1.522 | DA1 | ||||||
| K-fold-R2 | 0.89 | 0.86 | 0.82 | ||||
| RMSE | 31.416 | 35.577 | 40.7 | ||||
| RPD | 2.93 | 2.58 | 2.26 | ||||
| SF | Intercept | −32.639 | 55.649 | 113.112 | |||
| Independent variables | 10.248 | DA1 | 11.553 | DA1 | −6.682 | Ll1 | |
| 5.954 | Dw3 | 5.898 | Dw3 | 1.531 | LA1 | ||
| 5.123 | Ll1 | 3.438 | Da1 | 0.578 | LA2 | ||
| 4.052 | Da1 | 2.451 | Dl4 | 0.716 | LA3 | ||
| 2.455 | Lw3 | 1.935 | Dl9 | −6.75 | LP1 | ||
| 2.338 | Dl7 | 1.487 | Da3 | ||||
| 1.843 | DP5 | ||||||
| 1.501 | LP3 | ||||||
| 1.479 | Dl9 | ||||||
| K-fold-R2 | 0.94 | 0.91 | 0.74 | ||||
| RMSE | 20.598 | 23.978 | 41.358 | ||||
| RPD | 3.73 | 3.2 | 1.86 | ||||
| TF | Intercept | −134.537 | −839.286 | 41.391 | |||
| Independent variables | −10.169 | Ll1 | 12.691 | DA1 | −12.034 | Ll1 | |
| 1.926 | LP3 | 3.805 | Dw3 | 11.758 | Ll2 | ||
| −4.516 | Dl4 | 2.336 | Da1 | 44.735 | Lw6 | ||
| 24.446 | Dw1 | 1.81 | Dl4 | −5.603 | La1 | ||
| 30.785 | Dw5 | 1.674 | Dl9 | 1.582 | LA2 | ||
| −3.988 | Da1 | ||||||
| 5.482 | DA1 | ||||||
| K-fold-R2 | 0.92 | 0.91 | 0.80 | ||||
| RMSE | 49.017 | 52.401 | 77.904 | ||||
| RPD | 3.39 | 3.17 | 2.13 | ||||
| Bone | Intercept | −24.68 | −351.21 | −250.958 | |||
| Independent variables | 5.905 | Dl7 | 5.244 | Dl7 | 6.53 | Ll2 | |
| 4.241 | Ll2 | 2.668 | DP3 | 20.198 | Lw1 | ||
| 2.272 | DP3 | 2.213 | Dw5 | 0.906 | LA1 | ||
| 2.159 | Da2 | 2.1 | Dl12 | 0.486 | LA2 | ||
| 1.804 | LA1 | 1.523 | Dw4 | ||||
| 1.634 | Dl2 | ||||||
| K-fold-R2 | 0.94 | 0.92 | 0.89 | ||||
| RMSE | 25.864 | 30.175 | 34.997 | ||||
| RPD | 3.96 | 3.40 | 2.93 | ||||
| Tissue | Dorsal + Lateral (Model 1) | Dorsal (Model 2) | Lateral (Model 3) | ||||
|---|---|---|---|---|---|---|---|
| Muscle (%) | Intercept | 38.948 | 59.928 | 37.757 | |||
| Independent variables | 0.594 | Ll1 | 0.214 | Dl4 | 0.594 | Ll1 | |
| −0.385 | Ll2 | −0.179 | Dl10 | −0.385 | Ll2 | ||
| 0.171 | La1 | 0.733 | Dw6 | 0.171 | La1 | ||
| −0.026 | LA2 | −0.051 | DA1 | −0.026 | LA2 | ||
| k-fold-R2 | 0.46 | 0.16 | 0.41 | ||||
| RMSE | 1.77 | 2.177 | 1.776 | ||||
| RPD | 1.36 | 1.1 | 1.35 | ||||
| IF (%) | Intercept | 11.671 | 3.621 | 23.099 | |||
| Independent variables | −0.222 | Ll1 | 0.896 | Dl3 | 0.001 | CCW | |
| 0.216 | Ll2 | −0.129 | Dl4 | −0.231 | Ll1 | ||
| −0.09 | La1 | 0.161 | Dl10 | 0.165 | Ll2 | ||
| −0.033 | LA1 | −0.097 | La1 | ||||
| 0.024 | LA2 | −0.032 | LA1 | ||||
| 0.203 | Dl7 | 0.022 | LA2 | ||||
| k-fold-R2 | 0.50 | 0.23 | 0.51 | ||||
| RMSE | 1.089 | 1.385 | 1.07 | ||||
| RPD | 1.56 | 1.23 | 1.50 | ||||
| SF (%) | Intercept | 10.3 | −1.698 | 8.06 | |||
| Independent variables | −0.328 | Ll1 | −0.178 | Dl4 | 0.002 | CCW | |
| −0.126 | Dl4 | 0.524 | DW3 | −0.308 | Ll1 | ||
| −0.239 | Dl5 | 0.053 | DA1 | ||||
| 0.737 | DW3 | ||||||
| −0.11 | Da1 | ||||||
| 0.078 | DA1 | ||||||
| −0.084 | DA4 | ||||||
| 0.403 | DP4 | ||||||
| 0.092 | DP5 | ||||||
| k-fold-R2 | 0.78 | 0.59 | 0.56 | ||||
| RMSE | 0.841 | 1.271 | 1.329 | ||||
| RPD | 2.5 | 1.65 | 1.64 | ||||
| TF (%) | Intercept | 51.799 | −18.547 | 34.396 | |||
| Independent variables | −0.496 | Ll1 | −0.329 | Dl4 | 0.003 | CCW | |
| −0.104 | La1 | 0.242 | Dl10 | −0.552 | Ll1 | ||
| −0.26 | Dl4 | 0.186 | Da2 | 0.925 | Lw6 | ||
| −0.317 | Dl5 | 0.105 | DA1 | −0.144 | La1 | ||
| 0.218 | Dl9 | −0.051 | LA1 | ||||
| 1.188 | Dw3 | ||||||
| −0.168 | Da1 | ||||||
| 0.126 | DA1 | ||||||
| k-fold-R2 | 0.63 | 0.49 | 0.58 | ||||
| RMSE | 1.827 | 2.264 | 2.073 | ||||
| RPD | 1.92 | 1.55 | 1.61 | ||||
| Bone (%) | Intercept | 44.53 | 32.965 | 23.029 | |||
| Independent variables | 0.001 | CCW | 0.119 | Dl4 | −0.002 | CCW | |
| 0.271 | Ll2 | −0.244 | DW3 | 0.161 | Ll2 | ||
| −0.011 | LA3 | −0.454 | Dw6 | ||||
| 0.127 | Dl4 | −0.051 | DA1 | ||||
| −0.358 | Dw1 | ||||||
| −0.612 | Dw6 | ||||||
| −0.107 | Da2 | ||||||
| −0.043 | DA1 | ||||||
| k-fold-R2 | 0.72 | 0.64 | 0.62 | ||||
| RMSE | 0.996 | 1.210 | 1.288 | ||||
| RPD | 2.11 | 1.74 | 1.71 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afonso, J.J.; Almeida, M.; Batista, A.C.; Guedes, C.; Teixeira, A.; Silva, S.; Santos, V. Using Image Analysis Technique for Predicting Light Lamb Carcass Composition. Animals 2024, 14, 1593. https://doi.org/10.3390/ani14111593
Afonso JJ, Almeida M, Batista AC, Guedes C, Teixeira A, Silva S, Santos V. Using Image Analysis Technique for Predicting Light Lamb Carcass Composition. Animals. 2024; 14(11):1593. https://doi.org/10.3390/ani14111593
Chicago/Turabian StyleAfonso, João J., Mariana Almeida, Ana Catharina Batista, Cristina Guedes, Alfredo Teixeira, Severiano Silva, and Virgínia Santos. 2024. "Using Image Analysis Technique for Predicting Light Lamb Carcass Composition" Animals 14, no. 11: 1593. https://doi.org/10.3390/ani14111593








