Lycium barbarum (Wolfberry) Branches and Leaves Enhance the Growth Performance and Improve the Rumen Microbiota in Hu Sheep
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Committee Approval
2.2. Animals Study Design and Diets
2.2.1. Determination Method of Nutrient Content
2.2.2. Growth Performance and Apparent Digestibility
2.2.3. Rumen Fermentation Parameters and Micro-Organisms
2.2.4. Extraction of DNA and Sequencing of 16S rDNA
2.2.5. Slaughter Performance and Meat Quality
2.3. Statistical Analysis
3. Results
3.1. Growth Performance and Apparent Digestibility
3.2. Rumen Fermentation Parameters
3.3. Effects of Abundance, Diversity, and Composition of Rumen Bacteria
3.4. Slaughter Performance, Meat Quality, and Organ Development
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gong, H.; Rehman, F.; Ma, Y.; A, B.; Zeng, S.; Yang, T.; Huang, J.; Li, Z.; Wu, D.; Wang, Y. Germplasm Resources and Strategy for Genetic Breeding of Lycium Species: A Review. Front. Plant Sci. 2022, 13, 802936. [Google Scholar] [CrossRef]
- Ma, R.; Zhang, X.; Ni, Z.; Thakur, K.; Wang, W.; Yan, Y.; Cao, Y.; Zhang, J.; Rengasamy, K.R.R.; Wei, Z. Lycium barbarum (Goji) as functional food: A review of its nutrition, phytochemical structure, biological features, and food industry prospects. Crit. Rev. Food Sci. 2023, 63, 10621–10635. [Google Scholar] [CrossRef]
- Vidović, B.B.; Milinčić, D.D.; Marčetić, M.D.; Djuriš, J.D.; Ilić, T.D.; Kostić, A.Ž.; Pešić, M.B. Health Benefits and Applications of Goji Berries in Functional Food Products Development: A Review. Antioxidants 2022, 11, 248. [Google Scholar] [CrossRef]
- Gao, Y.; Wei, Y.; Wang, Y.; Gao, F.; Chen, Z. Lycium barbarum: A Traditional Chinese Herb and a Promising Anti-Aging Agent. Aging Dis. 2017, 8, 778. [Google Scholar] [CrossRef]
- Xiao, X.; Ren, W.; Zhang, N.; Bing, T.; Liu, X.; Zhao, Z.; Shangguan, D. Comparative Study of the Chemical Constituents and Bioactivities of the Extracts from Fruits, Leaves and Root Barks of Lycium barbarum. Molecules 2019, 24, 1585. [Google Scholar] [CrossRef]
- Byambasuren, S.; Wang, J.; Gaudel, G. Medicinal value of wolfberry (Lycium barbarum L.). J. Med. Plants Stud. 2019, 7, 90–97. [Google Scholar]
- Zhao, J.; Li, H.; Xi, W.; An, W.; Niu, L.; Cao, Y.; Wang, H.; Wang, Y.; Yin, Y. Changes in sugars and organic acids in wolfberry (Lycium barbarum L.) fruit during development and maturation. Food Chem. 2015, 173, 718–724. [Google Scholar] [CrossRef]
- Zeng, W.; Chen, L.; Li, Y.; Ma, J.; Yang, R.; Ding, J.; Yang, J. The effect of in vitro digestion on the chemical and antioxidant properties of Lycium barbarum polysaccharides. Food Hydrocoll. 2023, 139, 108507. [Google Scholar] [CrossRef]
- Islam, T.; Yu, X.; Badwal, T.S.; Xu, B. Comparative studies on phenolic profiles, antioxidant capacities and carotenoid contents of red goji berry (Lycium barbarum) and black goji berry (Lycium ruthenicum). Chem. Cent. J. 2017, 11, 59. [Google Scholar] [CrossRef]
- Wang, Y.; Liang, X.; Guo, S.; Li, Y.; Zhang, B.Y.Y.; An, W.; Cao, Y.; Zhao, J. Evaluation of nutrients and related environmental factors for wolfberry (Lycium barbarum) fruits grown in the different areas of China. Biochem. Syst. Ecol. 2019, 86, 103916. [Google Scholar]
- Ding, H.; Yang, P.; Zhang, X.; Ma, Y. Efficacy of Pretreatment with Lycium barbarum Polysaccharide in Various Doses in Influencing Splenic Immunity and Prognosis of Sepsis in Rats. Evid.-Based Complement. Altern. Med. 2022, 2022, 9508603. [Google Scholar] [CrossRef]
- Liu, H.; Zhou, X.; Huang, S.; Yang, J.; Liu, R.; Liu, C. Lycium barbarum Polysaccharides and Wolfberry Juice Prevent DEHP-Induced Hepatotoxicity via PXR-Regulated Detoxification Pathway. Molecules 2021, 26, 859. [Google Scholar] [CrossRef]
- Bak, S.G.; Lim, H.; Won, Y.; Lee, S.; Cheong, S.H.; Lee, S.J.; Bae, E.Y.; Lee, S.W.; Lee, S.J.; Rho, M. Regulatory Effects of Lycium barbarum Extract and Isolated Scopoletin on Atopic Dermatitis-Like Skin Inflammation. BioMed Res. Int. 2022, 2022, 2475699. [Google Scholar] [CrossRef]
- Yu, C.; Hu, X.; Ahmadi, S.; Wu, D.; Xiao, H.; Zhang, H.; Ding, T.; Liu, D.; Ye, X.; Chen, S.; et al. Structure and In Vitro Fermentation Characteristics of Polysaccharides Sequentially Extracted from Goji Berry (Lycium barbarum) Leaves. J. Agric. Food Chem. 2022, 70, 7535–7546. [Google Scholar] [CrossRef]
- Niu, Y.; Liao, J.; Zhou, H.; Wang, C.C.; Wang, L.; Fan, Y. Flavonoids from Lycium barbarum Leaves Exhibit Anti-Aging Effects through the Redox-Modulation. Molecules 2022, 27, 4952. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, Y.; Wu, Z.; Mei, J.; Zhang, W.; Shang, Y.; Thakur, K.; Wei, Z. Exploring the effect of in vitro digestion on the phenolics and antioxidant activity of Lycium barbarum fruit extract. Food Biosci. 2023, 51, 102255. [Google Scholar] [CrossRef]
- Fan, Y.; Ma, M.; Chen, J.; Pei, Y.; Sun, X. Stability and antioxidant activity of flavonoids from Lycium barbarum L. leaves during digestion in vivo. Food Sci. Technol. 2022, 42, e87322. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Yao, G.W.; Luo, Y.; Du, M.; Yin, X.; Xu, X.G.; Zhang, G.J. Integrated Metabolomics and Transcriptome Revealed the Effect of Fermented Lycium barbarum Residue Promoting Ovis aries Immunity. J. Front Immunol. 2022, 13, 889436. [Google Scholar] [CrossRef]
- Cremonesi, P.; Curone, G.; Biscarini, F.; Cotozzolo, E.; Menchetti, L.; Riva, F.; Marongiu, M.L.; Castiglioni, B.; Barbato, O.; Munga, A.; et al. Dietary Supplementation with Goji Berries (Lycium barbarum) Modulates the Microbiota of Digestive Tract and Caecal Metabolites in Rabbits. Animals 2022, 12, 121. [Google Scholar] [CrossRef]
- Chen, J.; Long, L.; Jiang, Q.; Kang, B.; Li, Y.; Yin, J. Effects of dietary supplementation of Lycium barbarum polysaccharides on growth performance, immune status, antioxidant capacity and selected microbial populations of weaned piglets. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1106–1115. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International, 21st ed.; AOAC International: Gaithersburg, MD, USA, 2019. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhu, Z.Y.; Deng, S.Y.; Wang, M.G.; Bai, T.T.; Lu, D.; Guo, X.F. Fermented cotton by-product total mixed ration on growth performance, nutrient apparent digestibility and rumen fermentation parameters of karakul sheep. Anim. Nutr. 2024, 36, 1745–1755. [Google Scholar]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Duan, P.; Zhang, L.; Hu, L.; Xu, G. Biological functions and application in livestock and poultry production of Lycium barbarum and its extracts. Anim. Nutr. 2023, 35, 4207–4215. [Google Scholar]
- Zhang, D.; Zhang, X.; Li, F.; Li, C.; La, Y.; Mo, F.; Li, G.; Zhang, Y.; Li, X.; Song, Q.; et al. Transcriptome Analysis Identifies Candidate Genes and Pathways Associated With Feed Efficiency in Hu Sheep. Front. Genet. 2019, 10, 1183. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, G.; Li, F.; Zhang, D.; Yuan, L.; Zhao, Y.; Zhang, Y.; Li, X.; Song, Q.; Wang, W. Effect of feed efficiency on growth performance, body composition, and fat deposition in growing Hu lambs. Anim. Biotechnol. 2023, 34, 183–198. [Google Scholar] [CrossRef] [PubMed]
- Menchetti, L.; Vecchione, L.; Filipescu, I.E.; Petrescu, V.F.; Fioretti, B.; Beccari, T.; Ceccarini, M.R.; Codini, M.; Quattrone, A.; Trabalza-Marinucci, M.; et al. Effects of Goji berries supplementation on the productive performance of rabbit. Livest. Sci. 2019, 220, 123–128. [Google Scholar] [CrossRef]
- Hao, Z.; Li, Z.; Huo, J.; Chu, Y.; Li, J.; Yu, X.; Liu, F.; Yin, P. Effects of Chinese wolfberry and astragalus extracts on growth performance, pork quality, and unsaturated fatty acid metabolism regulation in Tibetan fragrant pigs. Anim. Sci. J. 2021, 92, e13581. [Google Scholar] [CrossRef]
- Zheng, M.H.; Xu, L.Y.; Xu, Z.H.; Niu, G.W.; Wang, Y.L.; Qiu, H.; Jing, T.; Shao, W. Effects of Lycium barbarum polysaccharide on growth performance, digestive level, immune function and antioxidant capacity of calves. Feed Res. 2023, 46, 1–5. [Google Scholar]
- Zhang, Y.; Wang, B.; Wei, D.; Guo, Y.; Du, M.; Zhang, G. Supplementation of Lycium barbarum residue improves the animal performance through the interaction of rumen microbiota and metabolome of Tan sheep. Res. Sq. 2022, 26. [Google Scholar] [CrossRef]
- Zhang, C.; Yu, Q.; Wang, J.; Yu, Y.; Zhang, Y.; Sun, Y. Effects of Dietary Supplementation with Clostridium butyricum on Growth Performance, Apparent Digestibility, Blood Metabolites, Ruminal Fermentation and Bacterial Communities of Fattening Goats. Front. Nutr. 2022, 9, 888191. [Google Scholar] [CrossRef]
- Tayengwa, T.; Chikwanha, O.C.; Raffrenato, E.; Dugan, M.E.R.; Mutsvangwa, T.; Mapiye, C. Comparative effects of feeding citrus pulp and grape pomace on nutrient digestibility and utilization in steers. Animal 2021, 15, 100020. [Google Scholar] [CrossRef]
- Jiang, B.; Zhou, Y.; Wang, T.; Li, F. Nutritive value and ruminal degradation of seven Chinese herbs as forage for Tan sheep. Bioengineered 2020, 11, 1159–1169. [Google Scholar] [CrossRef]
- Cao, C.; Wang, Z.; Gong, G.; Huang, W.; Huang, L.; Song, S.; Zhu, B. Effects of Lycium barbarum Polysaccharides on Immunity and Metabolic Syndrome Associated with the Modulation of Gut Microbiota: A Review. Foods 2022, 11, 3177. [Google Scholar] [CrossRef]
- Shao, W.; Zheng, M.; Xu, L.; Du, X.; Xu, Z.; Yang, H.; Ren, W. Mechanism and Application of Adding Lycium barbarum Polysaccharides to Improve Animal Microcirculation. China Feed. 2021, 6–11. [Google Scholar] [CrossRef]
- Cheng, J.; Zhang, X.; Xu, D.; Zhang, D.; Zhang, Y.; Song, Q.; Li, X.; Zhao, Y.; Zhao, L.; Li, W.; et al. Relationship between rumen microbial differences and traits among Hu sheep, Tan sheep, and Dorper sheep. J. Anim. Sci. 2022, 100, skac261. [Google Scholar] [CrossRef]
- Song, J.; Ma, Y.; Zhang, H.; Wang, L.; Zhang, Y.; Zhang, G. Fermented Total Mixed Ration Alters Rumen Fermentation Parameters and Microbiota in Dairy Cows. Animals 2023, 13, 1062. [Google Scholar] [CrossRef]
- Farghaly, M.M.; Abdullah, M.A.M.; Youssef, I.M.I.; Abdel-Rahim, I.R.; Abouelezz, K. Effect of feeding hydroponic barley sprouts to sheep on feed intake, nutrient digestibility, nitrogen retention, rumen fermentation and ruminal enzymes activity. Livest. Sci. 2019, 228, 31–37. [Google Scholar] [CrossRef]
- Shi, M.J.; Ma, Z.X.; Tian, Y.J.; Ma, C.; Li, Y.D.; Zhang, X.W. Effects of corn straw treated with CaO on rumen degradation characteristics and fermentation parameters and their correlation with microbial diversity in rumen. Anim. Feed Sci. Technol. 2022, 292, 115403. [Google Scholar] [CrossRef]
- Wang, M.; Wang, H.; Zheng, H.; Uhrin, D.; Dewhurst, R.J.; Roehe, R. Comparison of HPLC and NMR for quantification of the main volatile fatty acids in rumen digesta. Sci. Rep. 2021, 11, 24337. [Google Scholar] [CrossRef]
- Paya, H.; Giannenas, I.; Taghizadeh, A.; Hosseinkhani, A.; Palangi, V.; Hasanpur, K.; Ayasan, T.; Montazerharzand, M.; Shirmohammadi, S.; Elmi, N. Effects of supplementary inulin on ewes milk composition and rumen fermentation parameters. J. Dairy Res. 2022, 89, 243–248. [Google Scholar] [CrossRef]
- Xia, W.; Li, X.; Su, L.; Khan, I.; Yin, L.; Su, L.; Leong, W.K.; Bian, X.; Su, J.; Huang, G.; et al. Lycium Berry Polysaccharides Strengthen Gut Microenvironment and Modulate Gut Microbiota of the Mice. Evid.-Based Complement. Altern. Med. 2020, 2020, 8097021. [Google Scholar] [CrossRef]
- Xing, B.; Han, Y.; Wang, X.C.; Wen, J.; Cao, S.; Zhang, K.; Li, Q.; Yuan, H. Persistent action of cow rumen microorganisms in enhancing biodegradation of wheat straw by rumen fermentation. Sci. Total Environ. 2020, 715, 136529. [Google Scholar] [CrossRef]
- Soltis, M.P.; Moorey, S.E.; Egert-McLean, A.M.; Voy, B.H.; Shepherd, E.A.; Myer, P.R. Rumen Biogeographical Regions and Microbiome Variation. Microorganisms 2023, 11, 747. [Google Scholar] [CrossRef]
- Li, F.; Li, C.; Chen, Y.; Liu, J.; Zhang, C.; Irving, B.; Fitzsimmons, C.; Plastow, G.; Guan, L.L. Host genetics influence the rumen microbiota and heritable rumen microbial features associate with feed efficiency in cattle. Microbiome 2019, 7, 92. [Google Scholar] [CrossRef]
- Loh, Z.H.; Ouwerkerk, D.; Klieve, A.V.; Hungerford, N.L.; Fletcher, M.T. Toxin Degradation by Rumen Microorganisms: A Review. Toxins 2020, 12, 664. [Google Scholar] [CrossRef]
- Newbold, C.J.; Ramos-Morales, E. Review: Ruminal microbiome and microbial metabolome: Effects of diet and ruminant host. Animal 2020, 14, s78–s86. [Google Scholar] [CrossRef]
- Yin, X.; Ji, S.K.; Duan, C.H.; Tian, P.Z.; Ju, S.S.; Yan, H.; Zhang, Y.J.; Liu, Y.Q. Age-Related Changes in the Ruminal Microbiota and Their Relationship With Rumen Fermentation in Lambs. Front Microbiol. 2021, 12, 679135. [Google Scholar] [CrossRef]
- Wang, Y.; Nan, X.; Zhao, Y.; Jiang, L.; Wang, H.; Hua, D.; Zhang, F.; Wang, Y.; Liu, J.; Yao, J.; et al. Dietary supplementation with inulin improves lactation performance and serum lipids by regulating the rumen microbiome and metabolome in dairy cows. Anim. Nutr. 2021, 7, 1189–1204. [Google Scholar] [CrossRef]
- Mani, S.; Aiyegoro, O.A.; Adeleke, M.A. Characterization of Rumen Microbiota of Two Sheep Breeds Supplemented with Direct-Fed Lactic Acid Bacteria. Front. Vet. Sci. 2021, 7, 570074. [Google Scholar] [CrossRef] [PubMed]
- McKee, L.S.; La Rosa, S.L.; Westereng, B.; Eijsink, V.G.; Pope, P.B.; Larsbrink, J. Polysaccharide degradation by the Bacteroidetes: Mechanisms and nomenclature. Environ. Microbiol. Rep. 2021, 13, 559–581. [Google Scholar] [CrossRef]
- Seck, M.; Linton, J.A.V.; Allen, M.S.; Castagnino, D.S.; Chouinard, P.Y.; Girard, C.L. Apparent ruminal synthesis of B vitamins in lactating dairy cows fed diets with different forage-to-concentrate ratios. J. Dairy Sci. 2017, 100, 1914–1922. [Google Scholar] [CrossRef] [PubMed]
- Mouzaki, M.; Comelli, E.M.; Arendt, B.M.; Bonengel, J.; Fung, S.K.; Fischer, S.E.; McGilvray, I.D.; Allard, J.P. Intestinal microbiota in patients with nonalcoholic fatty liver disease. Hepatology 2013, 58, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Pinnell, L.J.; Reyes, A.A.; Wolfe, C.A.; Weinroth, M.D.; Metcalf, J.L.; Delmore, R.J.; Belk, K.E.; Morley, P.S.; Engle, T.E. Bacteroidetes and Firmicutes Drive Differing Microbial Diversity and Community Composition Among Micro-Environments in the Bovine Rumen. Front. Vet. Sci. 2022, 9, 897996. [Google Scholar] [CrossRef]
- Li, S.; Zeng, H.; Wang, C.; Han, Z. Effect of Methionine Hydroxy Analog on Hu Sheep Digestibility, Rumen Fermentation, and Rumen Microbial Community In Vitro. Metabolites 2023, 13, 169. [Google Scholar] [CrossRef]
- Hernández, R.; Chaib De Mares, M.; Jimenez, H.; Reyes, A.; Caro-Quintero, A. Functional and Phylogenetic Characterization of Bacteria in Bovine Rumen Using Fractionation of Ruminal Fluid. Front. Microbiol. 2022, 13, 813002. [Google Scholar] [CrossRef]
- Ma, Z.; Zhou, J.; Liu, T.; Chen, Z. Evaluation of the representative of using rumen fluid samples from lambs fed pelleted TMR for analysis of prokaryotic communities Frontiers in Microbiology. Front. Microbiol. 2023, 14, 1190253. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, X.; Chang, S.; Zhang, C.; Du, W.; Hou, F. Effect of Cistanche deserticola on Rumen Microbiota and Rumen Function in Grazing Sheep. Front. Microbiol. 2022, 13, 840725. [Google Scholar] [CrossRef]
- Ren, Z.; Yao, R.; Liu, Q.; Deng, Y.; Shen, L.; Deng, H.; Zuo, Z.; Wang, Y.; Deng, J.; Cui, H.; et al. Effects of antibacterial peptides on rumen fermentation function and rumen microorganisms in goats. PLoS ONE 2019, 14, e221815. [Google Scholar] [CrossRef]
- Fan, Q.; Wanapat, M.; Hou, F. Chemical Composition of Milk and Rumen Microbiome Diversity of Yak, Impacting by Herbage Grown at Different Phenological Periods on the Qinghai-Tibet Plateau. Animals 2020, 10, 1030. [Google Scholar] [CrossRef]
- Zhang, N.; Teng, Z.; Li, P.; Fu, T.; Lian, H.; Wang, L.; Gao, T. Oscillating dietary crude protein concentrations increase N retention of calves by affecting urea-N recycling and nitrogen metabolism of rumen bacteria and epithelium. PLoS ONE 2021, 16, e257417. [Google Scholar] [CrossRef]
- Conte, G.; Dimauro, C.; Daghio, M.; Serra, A.; Mannelli, F.; McAmmond, B.M.; Van Hamme, J.D.; Buccioni, A.; Viti, C.; Mantino, A.; et al. Exploring the relationship between bacterial genera and lipid metabolism in bovine rumen. Animal 2022, 16, 100520. [Google Scholar] [CrossRef]
- Zhang, C.; Ma, T.; Tu, Y.; Ma, S.; Diao, Q. Effects of Circadian Rhythm and Feeding Modes on Rumen Fermentation and Microorganisms in Hu Sheep. Microorganisms 2022, 10, 2308. [Google Scholar] [CrossRef]
- Ren, Y.; Zhaxi, Y.; Ciwang, R.; Wang, Z.; Liu, M. Responses of rumen microorganisms and metabolites to different roughage of domesticated Tibetan sheep. Front. Microbiol. 2023, 14, 1247609. [Google Scholar] [CrossRef]
- Li, S.; Zeng, H.; Wang, C.; Han, Z. Effect of methionine hydroxy analog feed supplements: Significant alteration and enrichment of rumen microbiota and metabolome in Hu sheep. Front. Vet. Sci. 2022, 9, 999726. [Google Scholar] [CrossRef]
- Qu, X.; Raza, S.H.A.; Zhao, Y.; Deng, J.; Ma, J.; Wang, J.; Alkhorayef, N.; Alkhalil, S.S.; Pant, S.D.; Lei, H.; et al. Effect of Tea Saponins on Rumen Microbiota and Rumen Function in Qinchuan Beef Cattle. Microorganisms 2023, 11, 374. [Google Scholar] [CrossRef]
- Atsbha, K.; Gebremariam, T.; Aregawi, T. Slaughter performance and meat quality of Begait breed lambs fattened under different diets. Heliyon 2021, 7, e6935. [Google Scholar] [CrossRef]
- Yu, Q.P.; Feng, D.Y.; Xia, M.H.; He, X.J.; Liu, Y.H.; Tan, H.Z.; Zou, S.G.; Ou, X.H.; Zheng, T.; Cao, Y.; et al. Effects of a traditional Chinese medicine formula supplementation on growth performance, carcass characteristics, meat quality and fatty acid profiles of finishing pigs. Livest. Sci. 2017, 202, 135–142. [Google Scholar] [CrossRef]
- Lien, T.; Liao, C.; Lin, K. Effects of supplemental Chinese traditional herbal medicine complex on the growth performance, carcass characteristics, and meat quality of male Holstein calves. J. Appl. Anim. Res. 2013, 42, 222–227. [Google Scholar] [CrossRef]
- Castrica, M.; Menchetti, L.; Balzaretti, C.M.; Branciari, R.; Ranucci, D.; Cotozzolo, E.; Vigo, D.; Curone, G.; Brecchia, G.; Miraglia, D. Impact of Dietary Supplementation with Goji Berries (Lycium barbarum) on Microbiological Quality, Physico-Chemical, and Sensory Characteristics of Rabbit Meat. Foods 2020, 9, 1480. [Google Scholar] [CrossRef] [PubMed]
- Menchetti, L.; Brecchia, G.; Branciari, R.; Barbato, O.; Fioretti, B.; Codini, M.; Bellezza, E.; Trabalza-Marinucci, M.; Miraglia, D. The effect of Goji berries (Lycium barbarum) dietary supplementation on rabbit meat quality. Meat Sci. 2020, 161, 108018. [Google Scholar] [CrossRef] [PubMed]
- Ju, H.; Hao, X.; Liu, S.; Zhang, H.; Xiang, B.; Zhang, J. Effects of dietary Lycium barbarum polysaccharides on growth performance, slaughter performance and meat quality of lambs. Anim. Nutr. 2022, 34, 1777–1788. [Google Scholar]
- Liu, Y.L.; Yin, R.Q.; Liang, S.S.; Duan, Y.L.; Yao, J.H.; Duan, Y.L.; Yang, X.J. Effect of dietary Lycium barbarum polysaccharide on growth performance and immune function of broilers. J. Appl. Poult. Res. 2017, 26, 200–208. [Google Scholar] [CrossRef]
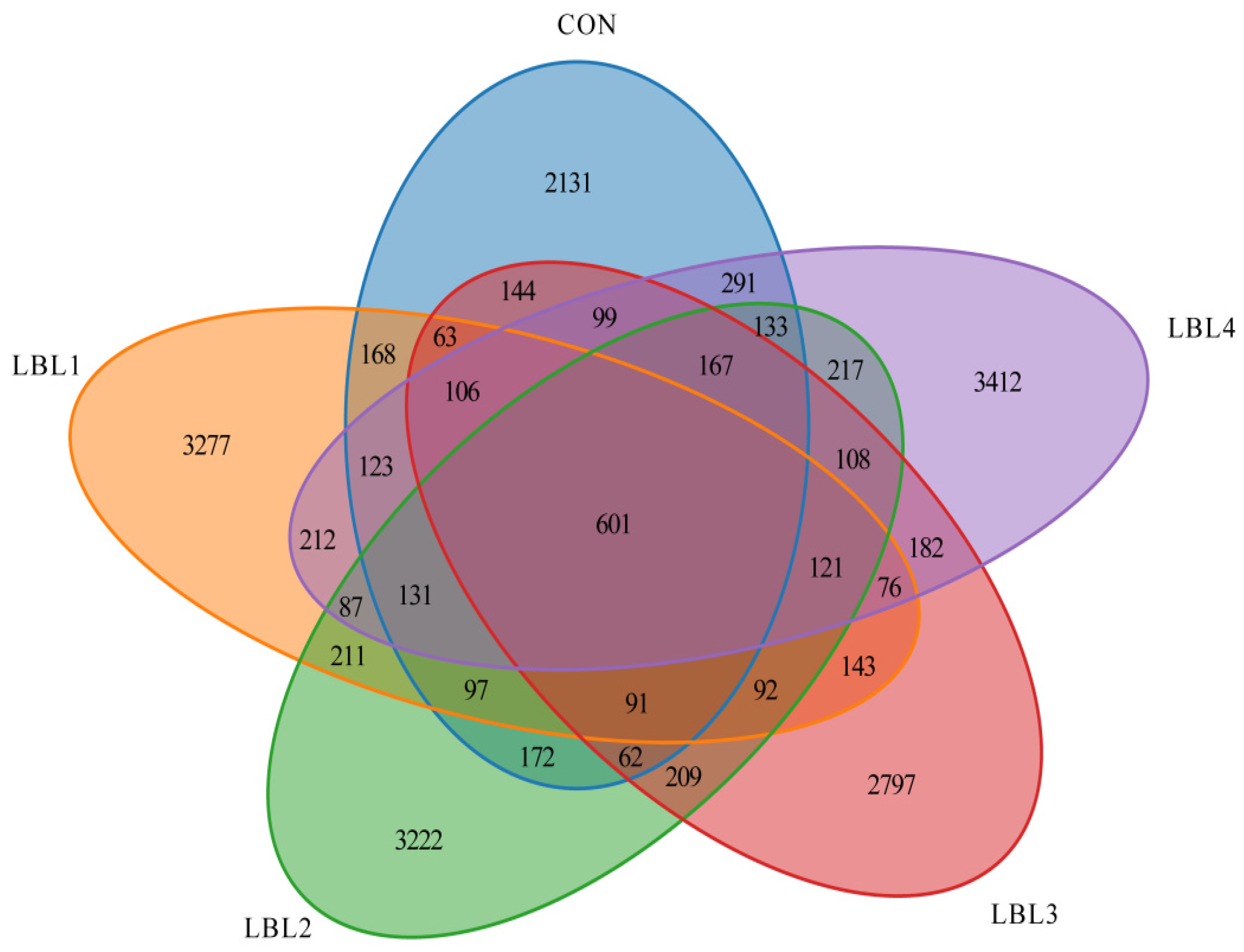


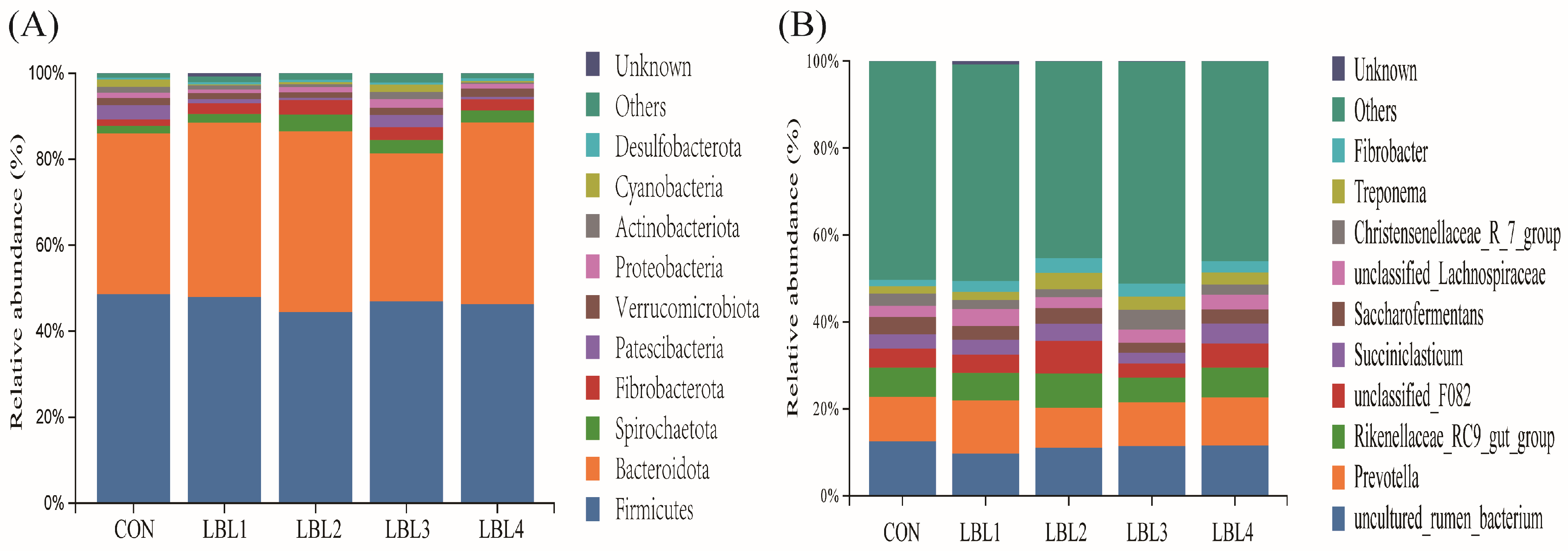

| Items | Group | ||||
|---|---|---|---|---|---|
| CON | LBL1 | LBL2 | LBL3 | LBL4 | |
| Ingredients (%) | |||||
| LBL | 0.00 | 3.00 | 6.00 | 9.00 | 12.00 |
| Alfalfa meal | 9.00 | 6.00 | 3.00 | 1.50 | 1.00 |
| Whole corn silage | 5.50 | 5.20 | 5.00 | 4.30 | 3.50 |
| Corn stalk | 45.50 | 45.80 | 46.00 | 45.20 | 43.50 |
| Corn | 12.00 | 12.00 | 12.00 | 12.00 | 12.00 |
| Wheat bran | 7.00 | 7.00 | 7.00 | 7.00 | 7.00 |
| Soybean meal | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 |
| cottonseed meal | 12.70 | 12.70 | 12.70 | 12.70 | 12.70 |
| CaHPO4 | 1.30 | 1.30 | 1.30 | 1.30 | 1.30 |
| NaCl | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Premix 1 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 |
| Nutrient components 2 | |||||
| ME (MJ/kg) | 9.75 | 9.88 | 9.73 | 9.53 | 9.42 |
| CP (%) | 13.72 | 13.69 | 13.68 | 13.74 | 13.87 |
| EE (%) | 9.70 | 10.07 | 9.32 | 9.69 | 9.86 |
| NDF (%) | 39.75 | 38.65 | 40.03 | 41.82 | 42.84 |
| ADF (%) | 24.14 | 23.81 | 24.19 | 24.17 | 24.02 |
| Ca (%) | 0.98 | 0.98 | 0.98 | 0.99 | 1.01 |
| P (%) | 0.66 | 0.66 | 0.66 | 0.66 | 0.66 |
| Items 1 | CON | LBL1 | LBL2 | LBL3 | LBL4 | SEM | p-Value |
|---|---|---|---|---|---|---|---|
| Growth performance | |||||||
| Initial BW, kg | 20.32 | 20.53 | 20.48 | 20.26 | 20.38 | 0.258 | 0.782 |
| Final weight, kg | 33.90 b | 35.46 a | 34.30 ab | 33.83 b | 32.40 c | 0.116 | 0.032 |
| ADG, g | 169.75 b | 186.56 a | 172.75 ab | 169.56 b | 150.25 c | 3.531 | 0.022 |
| ADFI, kg | 1.43 a | 1.43 ab | 1.45 a | 1.47 a | 1.35 b | 0.013 | 0.047 |
| F:G | 8.46 | 7.69 | 8.43 | 8.69 | 8.98 | 0.200 | 0.068 |
| Digestibility | |||||||
| DM, % | 70.45 | 72.31 | 72.16 | 71.17 | 71.38 | 0.813 | 0.553 |
| CP, % | 58.68 c | 65.62 a | 67.81 a | 60.16 b | 62.27 b | 0.617 | 0.036 |
| NDF, % | 49.37 | 51.08 | 51.57 | 49.68 | 52.49 | 1.671 | 0.621 |
| ADF, % | 38.24 | 42.69 | 42.07 | 39.83 | 38.24 | 1.441 | 0.416 |
| Items | CON | LBL1 | LBL2 | LBL3 | LBL4 | SEM | p-Value |
|---|---|---|---|---|---|---|---|
| pH | 7.05 a | 6.72 b | 6.99 ab | 7.02 a | 6.84 ab | 0.044 | 0.037 |
| NH3-N | 15.29 | 14.33 | 14.25 | 14.20 | 14.51 | 0.175 | 0.079 |
| TVFA | 87.87 | 94.85 | 94.45 | 90.13 | 91.62 | 1.168 | 0.095 |
| Acetate | 56.31 | 60.31 | 60.14 | 57.35 | 59.27 | 1.122 | 0.346 |
| Propionate | 19.64 c | 22.21 a | 21.40 ab | 20.16 bc | 19.85 c | 0.278 | 0.004 |
| Butyrate | 10.93 | 11.26 | 11.89 | 11.62 | 11.53 | 0.141 | 0.054 |
| Valerate | 0.99 bc | 1.07 a | 1.02 b | 1.00 bc | 0.97 c | 0.009 | 0.000 |
| A:P | 2.88 | 2.71 | 2.82 | 2.84 | 3.01 | 0.066 | 0.229 |
| Items | CON | LBL1 | LBL2 | LBL3 | LBL4 | SEM | p-Value |
|---|---|---|---|---|---|---|---|
| Slaughter performance | |||||||
| LWBS, kg | 35.84 | 36.98 | 36.55 | 36.18 | 33.87 | 0.452 | 0.053 |
| Carcass weight, kg | 15.62 | 16.46 | 16.34 | 16.24 | 15.04 | 0.220 | 0.093 |
| Dressing percentage, % | 43.70 | 44.55 | 44.73 | 44.98 | 44.46 | 0.584 | 0.571 |
| Eye muscle area, cm2 | 19.84 bc | 21.80 a | 20.46 b | 19.94 bc | 18.94 c | 0.237 | 0.000 |
| GR value, mm | 5.04 b | 5.93 a | 5.91 a | 4.66 c | 4.13 d | 0.120 | 0.002 |
| Backfat thickness, mm | 4.56 | 4.66 | 4.60 | 4.40 | 4.33 | 0.082 | 0.710 |
| Meat quality | |||||||
| Water loss rate, % | 19.97 bc | 21.12 a | 20.82 ab | 19.81 c | 19.67 c | 0.172 | 0.009 |
| pH 0 h | 6.25 | 6.30 | 6.35 | 6.31 | 6.24 | 0.038 | 0.438 |
| pH 24 h | 5.65 ab | 5.58 b | 5.66 ab | 5.69 ab | 5.79 a | 0.023 | 0.044 |
| Cooking loss, % | 26.08 | 25.97 | 27.51 | 27.30 | 27.42 | 0.390 | 0.287 |
| Shear force, N | 60.21 | 65.49 | 64.22 | 53.31 | 54.73 | 2.047 | 0.091 |
| Shear force, kgf | 6.16 | 6.70 | 6.54 | 5.45 | 5.60 | 0.209 | 0.088 |
| L* | 32.60 a | 28.53 c | 30.27 b | 31.13 ab | 32.87 a | 0.401 | 0.000 |
| a* | 14.13 | 16.00 | 15.67 | 16.47 | 14.53 | 0.412 | 0.117 |
| b* | 19.33 | 20.40 | 20.20 | 19.60 | 19.47 | 0.160 | 0.052 |
| Items | CON | LBL1 | LBL2 | LBL3 | LBL4 | SEM | p-Value |
|---|---|---|---|---|---|---|---|
| Organ weight, g | |||||||
| Heart | 136.62 | 147.918 | 143.536 | 140.302 | 140.61 | 1.342 | 0.080 |
| Liver | 549.756 | 562.076 | 570.672 | 547.426 | 543.014 | 14.391 | 0.979 |
| Spleen | 42.744 | 48.108 | 45.636 | 45.434 | 45.058 | 1.021 | 0.628 |
| Lung | 525.87 | 564.324 | 558.034 | 528.176 | 536.776 | 9.665 | 0.655 |
| Kidney | 105.758 | 114.798 | 107.99 | 101.65 | 105.87 | 3.340 | 0.825 |
| Pancreas | 34.264 | 27.718 | 25.344 | 25.928 | 26.672 | 1.209 | 0.114 |
| Rumen | 664.524 | 665.408 | 675.166 | 669.442 | 658.42 | 10.426 | 0.993 |
| Reticulum | 96.664 | 103.298 | 106.848 | 94.834 | 100.618 | 2.178 | 0.430 |
| Omasum | 111.174 | 102.772 | 113.452 | 105.114 | 102.242 | 3.960 | 0.881 |
| Abomasum | 128.75 | 134.268 | 130.07 | 133.846 | 133.592 | 4.242 | 0.993 |
| Small intestine | 566.112 | 591.612 | 585.248 | 579.598 | 522.388 | 14.957 | 0.637 |
| Organ index, % | |||||||
| Heart | 0.388 | 0.416 | 0.394 | 0.39 | 0.414 | 0.005 | 0.144 |
| Liver | 1.554 | 1.576 | 1.564 | 1.518 | 1.604 | 0.036 | 0.971 |
| Spleen | 0.12 | 0.134 | 0.126 | 0.124 | 0.132 | 0.003 | 0.644 |
| Lung | 1.49 | 1.582 | 1.538 | 1.466 | 1.58 | 0.028 | 0.625 |
| Kidney | 0.298 | 0.322 | 0.296 | 0.28 | 0.31 | 0.009 | 0.656 |
| Pancreas | 0.096 | 0.078 | 0.07 | 0.07 | 0.078 | 0.004 | 0.113 |
| Rumen | 1.882 | 1.878 | 1.856 | 1.856 | 1.934 | 0.030 | 0.988 |
| Reticulum | 0.272 | 0.292 | 0.292 | 0.262 | 0.294 | 0.007 | 0.434 |
| Omasum | 0.318 | 0.288 | 0.314 | 0.29 | 0.3 | 0.011 | 0.878 |
| Abomasum | 0.362 | 0.376 | 0.356 | 0.374 | 0.392 | 0.010 | 0.845 |
| Small intestine | 1.596 | 1.654 | 1.608 | 1.606 | 1.538 | 0.034 | 0.900 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, P.; Rehemujiang, H.; Zhang, L.; Lu, M.; Li, C.; Hu, L.; Wang, Y.; Diao, Q.; Xu, G. Lycium barbarum (Wolfberry) Branches and Leaves Enhance the Growth Performance and Improve the Rumen Microbiota in Hu Sheep. Animals 2024, 14, 1610. https://doi.org/10.3390/ani14111610
Duan P, Rehemujiang H, Zhang L, Lu M, Li C, Hu L, Wang Y, Diao Q, Xu G. Lycium barbarum (Wolfberry) Branches and Leaves Enhance the Growth Performance and Improve the Rumen Microbiota in Hu Sheep. Animals. 2024; 14(11):1610. https://doi.org/10.3390/ani14111610
Chicago/Turabian StyleDuan, Pingping, Halidai Rehemujiang, Lidong Zhang, Mulong Lu, Changchang Li, Lihong Hu, Youli Wang, Qiyu Diao, and Guishan Xu. 2024. "Lycium barbarum (Wolfberry) Branches and Leaves Enhance the Growth Performance and Improve the Rumen Microbiota in Hu Sheep" Animals 14, no. 11: 1610. https://doi.org/10.3390/ani14111610
APA StyleDuan, P., Rehemujiang, H., Zhang, L., Lu, M., Li, C., Hu, L., Wang, Y., Diao, Q., & Xu, G. (2024). Lycium barbarum (Wolfberry) Branches and Leaves Enhance the Growth Performance and Improve the Rumen Microbiota in Hu Sheep. Animals, 14(11), 1610. https://doi.org/10.3390/ani14111610







