The In Vitro Antibacterial Activity of Phytogenic and Acid-Based Eubiotics against Major Foodborne Zoonotic Poultry Pathogens
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Products under Examination
- Essential oil-based phytogenics: Phyto CSC Liquide B (Phytosynthese, Mozac, France), AEN 350 B Liquid (Phytosynthese, Mozac, France)
- Acid-based eubiotics: Salgard® liquid (Anpario plc, Manton Wood Enterprise Park, Worksop, Nottinghamshire, UK), Intesti-Flora (Kanters, Lieshout, The Netherlands)
- Blends of essential oils and organic acids: ProPhorceTM SA Exclusive (©Perstorp, Malmö, Sweden), Herbal acid (Pancosma, Rolle, Switzerland), Rigosol-N (Panaroma EPE, Kilkis, Greece), Eubisan 3000 (MIRAVIT®, Münster, Germany)
2.2. Tested Bacterial Strains
2.3. Preparation of the Tested Inoculum
2.4. MIC Assay
2.5. Statistical Analysis
3. Results
3.1. Gram-Negative Bacteria
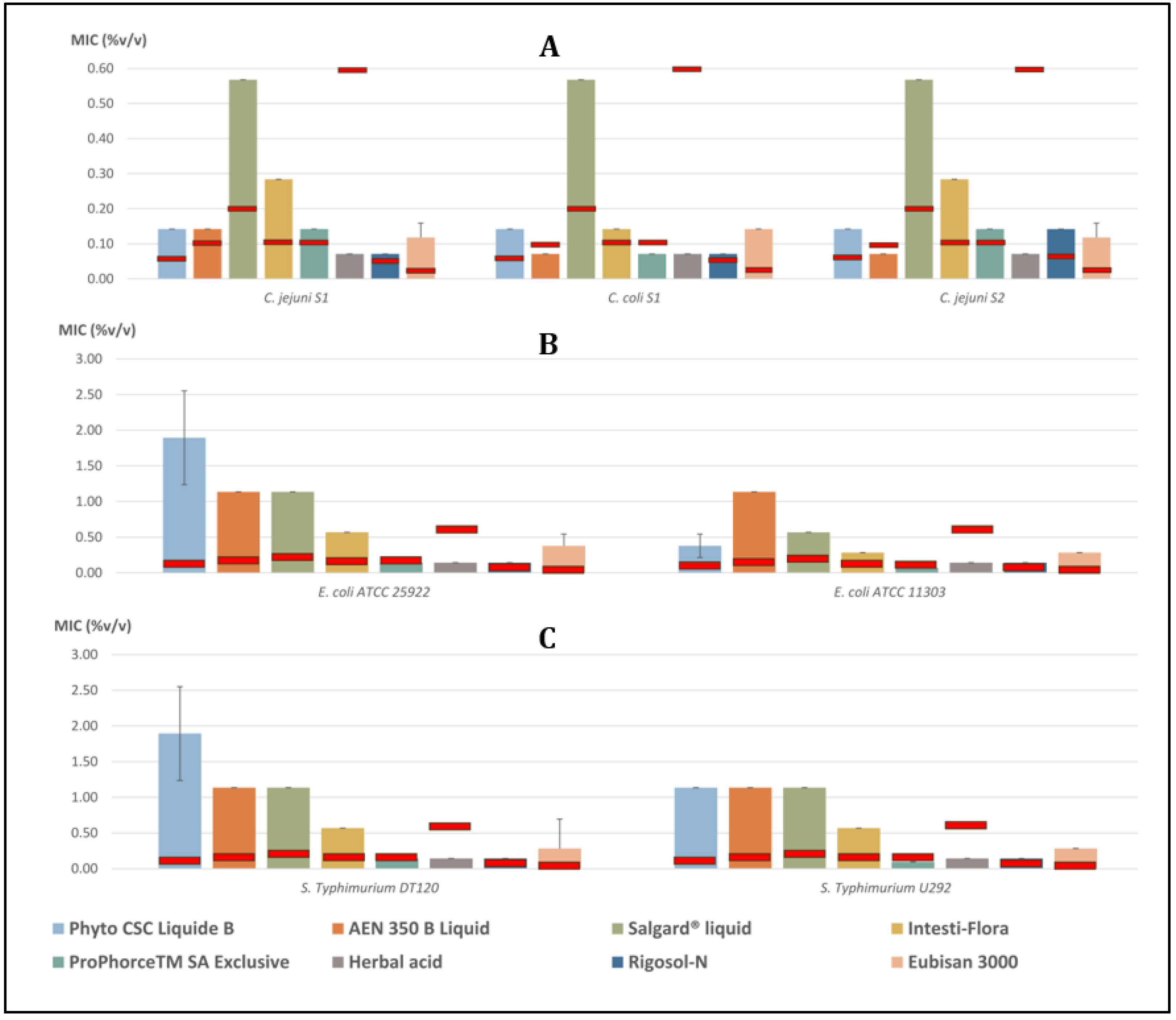
3.1.1. Campylobacter spp.
3.1.2. Escherichia coli
3.1.3. Salmonella Typhimurium
3.2. Gram-Positive Bacteria
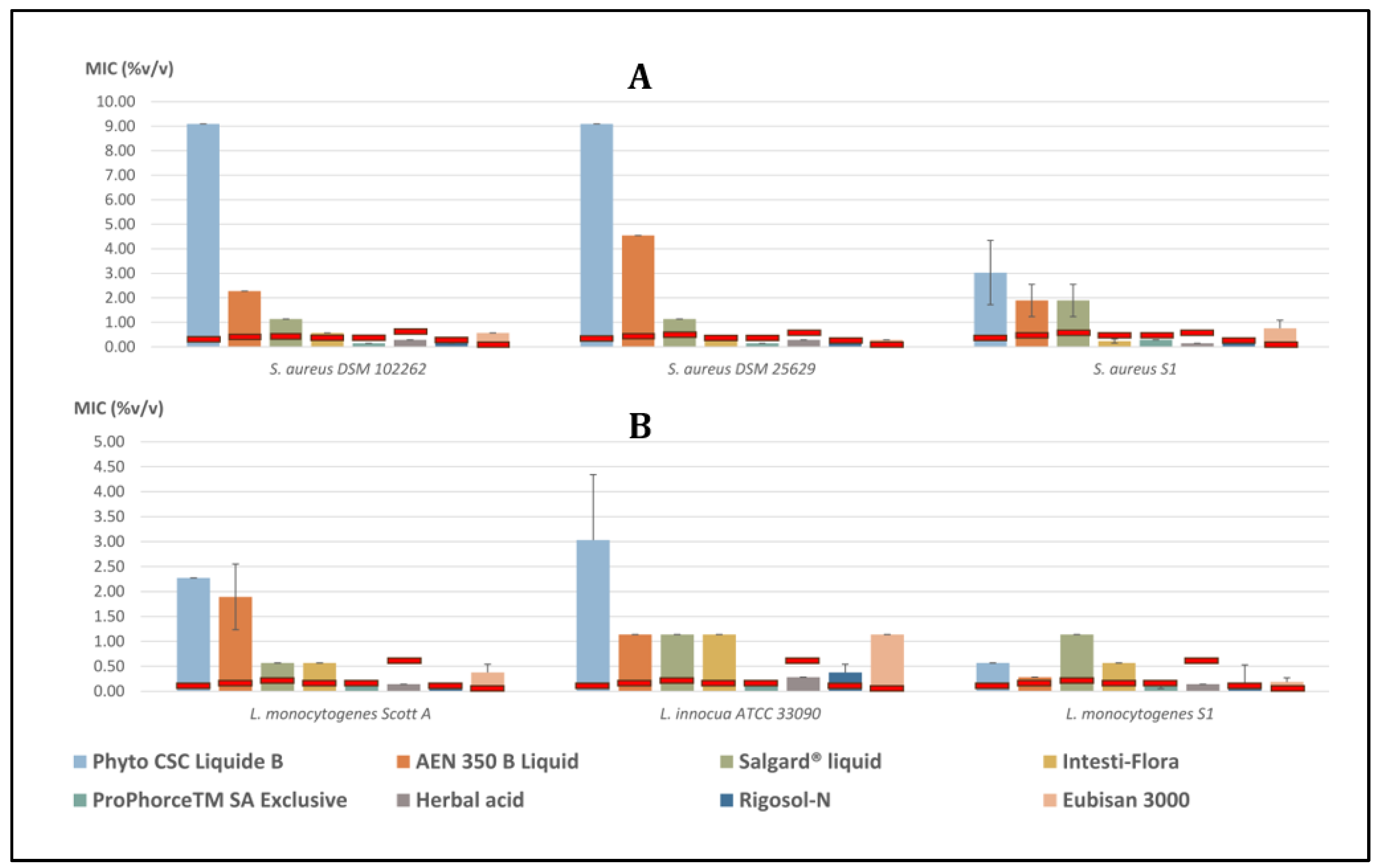
3.2.1. Staphylococcus aureus
3.2.2. Listeria spp.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- OECD (Economic Co-operation and Development); FAO (Food and Agriculture Organization). OECD-FAO Agricultural Outlook 2023–2032; Food and Agriculture Organization of the United Nations: Rome, Italy, 2023; pp. 1–90. [Google Scholar]
- EMA Committee for Medicinal Products for Veterinary Use (CVMP); EFSA Panel on Biological Hazards (BIOHAZ). EMA and EFSA Joint Scientific Opinion on measures to reduce the need to use antimicrobial agents in animal husbandry in the European Union, and the resulting impacts on food safety (RONAFA). EFSA J. 2017, 15, e04666. [Google Scholar]
- Gonçalves-Tenório, A.; Silva, B.; Rodrigues, V.; Cadavez, V.; Gonzales-Barron, U. Prevalence of Pathogens in Poultry Meat: A Meta-Analysis of European Published Surveys. Foods 2018, 7, 69. [Google Scholar] [CrossRef] [PubMed]
- Hafez, H.M.; Hauck, R. Zoonoses Transmitted by Poultry: Risks Related to Poultry Rearing and Eating Poultry Products Zoonoses. In Zoonoses: Infections Affecting Humans and Animals; Springer International Publishing: Cham, Switzerland, 2022; pp. 1–24. [Google Scholar]
- EFSA Panel on Biological Hazards (BIOHAZ); EFSA Panel on Contaminants in the Food Chain (CONTAM); EFSA Panel on Animal Health and Welfare (AHAW). Scientific Opinion on the public health hazards to be covered by inspection of meat (poultry). EFSA J. 2012, 10, 2741. [Google Scholar]
- Heredia, N.; García, S. Animals as sources of food-borne pathogens: A review. Anim. Nutr. 2018, 4, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Woyda, R.; Oladeinde, A.; Abdo, Z. Chicken Production and Human Clinical Escherichia coli Isolates Differ in Their Carriage of Antimicrobial Resistance and Virulence Factors. Appl. Environ. Microbiol. 2023, 89, e0116722. [Google Scholar] [CrossRef]
- European Food Safety Authority; European Centre for Disease Prevention and Control. The European Union One Health 2021 Zoonoses Report. EFSA J. 2022, 20, 7666. [Google Scholar]
- Arsenos, G.; Giannenas, I. Sustainable Use of Feed Additives in Livestock: Novel Ways for Animal Production; Springer International Publishing: Cham, Switzerland, 2023; pp. 1–969. [Google Scholar]
- Abreu, R.; Semedo-Lemsaddek, T.; Cunha, E.; Tavares, L.; Oliveira, M. Antimicrobial Drug Resistance in Poultry Production: Current Status and Innovative Strategies for Bacterial Control. Microorganisms 2023, 11, 953. [Google Scholar] [CrossRef]
- Dos Santos, J.S.; Biduski, B.; Dos Santos, L.R. Listeria monocytogenes: Health risk and a challenge for food processing establishments. Arch. Microbiol. 2021, 203, 5907–5919. [Google Scholar] [CrossRef] [PubMed]
- Scharff, L.R. Food Attribution and Economic Cost Estimates for Meat- and Poultry-Related Illnesses. J. Food Prot. 2020, 83, 959–967. [Google Scholar] [CrossRef]
- Tariq, S.; Samad, A.; Hamza, M.; Ahmer, A.; Muazzam, A.; Ahmad, S.; Amhabj AM, A. Salmonella in Poultry; An Overview. Int. J. Multidiscip. Sci. Arts 2022, 1, 80–84. [Google Scholar] [CrossRef]
- Joseph, J.; Zhang, L.; Adhikari, P.; Evans, J.D.; Ramachandran, R. Avian Pathogenic Escherichia coli (APEC) in Broiler Breeders: An Overview. Pathogens 2023, 12, 1280. [Google Scholar] [CrossRef] [PubMed]
- Żbikowska, K.; Michalczuk, M.; Dolka, B. The Use of Bacteriophages in the Poultry Industry. Animals 2020, 10, 872. [Google Scholar] [CrossRef]
- De Mesquita Souza Saraiva, M.; Lim, K.; Do Monte DF, M.; Givisiez PE, N.; Alves LB, R.; De Freitas Neto, O.C.; Kariuki, S.; Júnior, A.B.; De Oliveira CJ, B.; Gebreyes, W.A. Antimicrobial resistance in the globalized food chain: A One Health perspective applied to the poultry industry. Braz. J. Microbiol. 2022, 53, 465–486. [Google Scholar] [CrossRef] [PubMed]
- Hlashwayo, D.F.; Sigaúque, B.; Bila, C.G. Epidemiology and antimicrobial resistance of Campylobacter spp. in animals in Sub-Saharan Africa: A systematic review. Heliyon 2020, 6, e03537. [Google Scholar] [CrossRef] [PubMed]
- Castro-Vargas, R.E.; Herrera-Sánchez, M.P.; Rodríguez-Hernández, R.; Rondón-Barragán, I.S. Antibiotic resistance in Salmonella spp. isolated from poultry: A global overview. Vet. World 2020, 13, 2070–2084. [Google Scholar]
- Kim, S.; Kim, H.; Kim, Y.; Kim, M.; Kwak, H.; Ryu, S. Antimicrobial Resistance of Escherichia coli from Retail Poultry Meats in Korea. J. Food Prot. 2020, 83, 1673–1678. [Google Scholar] [CrossRef]
- Panera-Martínez, S.; Rodríguez-Melcón, C.; Serrano-Galán, V.; Alonso-Calleja, C.; Capita, R. Prevalence, quantification and antibiotic resistance of Listeria monocytogenes in poultry preparations. Food Control 2022, 135, 108608. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA); European Centre for Disease Prevention and Control (ECDC). The European Union One Health 2018 Zoonoses Report. EFSA J. 2019, 17, e05926. [Google Scholar]
- Gerba, C.P. Environmentally Transmitted Pathogens. In Environmental Microbiology, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2009; Chapter 22; pp. 445–484. [Google Scholar]
- Ricke, S. Perspectives on the use of organic acids and short chain fatty acids as antimicrobials. Poult. Sci. 2003, 82, 632–639. [Google Scholar] [CrossRef]
- Al-Mnaser, A.; Dakheel, M.; Alkandari, F.; Woodward, M. Polyphenolic phytochemicals as natural feed additives to control bacterial pathogens in the chicken gut. Arch. Microbiol. 2022, 204, 253. [Google Scholar] [CrossRef]
- Abdelli, N.; Solà-Oriol, D.; Pérez, J.F. Phytogenic Feed Additives in Poultry: Achievements, Prospective and Challenges. Animals 2021, 11, 3471. [Google Scholar] [CrossRef]
- Yang, X.; Xin, H.; Yang, C.; Yang, X. Impact of essential oils and organic acids on the growth performance, digestive functions and immunity of broiler chickens. Anim. Nutr. 2018, 4, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.-Q.; Shi, H.-Q.; Xie, W.-Y.; Zhao, L.-H.; Zhang, J.-Y.; Ji, C.; Ma, Q.-G. Dietary supplementation with acidifiers improves the growth performance, meat quality and intestinal health of broiler chickens. Anim. Nutr. 2021, 7, 762–769. [Google Scholar] [CrossRef] [PubMed]
- Vu Thuy Hong Loan, N.; Trung Thong, H.; Nu Anh Thu, L.; Viet Duc, H. Acidifiers as Alternatives for Antibiotics Reduction and Gut Health Improvement for Poultry and Swine. In Feed Additives—Recent Trends in Animal Nutrition; IntechOpen: London, UK, 2023. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, X.; Xin, H.; Chen, S.; Yang, C.; Duan, Y.; Yang, X. Effects of a protected inclusion of organic acids and essential oils as antibiotic growth promoter alternative on growth performance, intestinal morphology and gut microflora in broilers. Anim. Sci. J. 2017, 88, 1414–1424. [Google Scholar] [CrossRef] [PubMed]
- Stefanello, C.; Rosa, D.P.; Dalmoro, Y.K.; Segatto, A.L.; Vieira, M.S.; Moraes, M.L.; Santin, E. Protected Blend of Organic Acids and Essential Oils Improves Growth Performance, Nutrient Digestibility, and Intestinal Health of Broiler Chickens Undergoing an Intestinal Challenge. Front. Vet. Sci. 2020, 6, 491. [Google Scholar] [CrossRef] [PubMed]
- Kovanda, L.; Zhang, W.; Wei, X.; Luo, J.; Wu, X.; Atwill, E.R.; Vaessen, S.; Li, X.; Liu, Y. In Vitro Antimicrobial Activities of Organic Acids and Their Derivatives on Several Species of Gram-Negative and Gram-Positive Bacteria. Molecules 2019, 24, 3770. [Google Scholar] [CrossRef]
- Milagres De Almeida, J.; Crippa, B.L.; Martins Alencar De Souza, V.V.; Perez Alonso, V.P.; Da Motta Santos Júnior, E.; Siqueira Franco Picone, C.; Prata, A.S.; Cirone Silva, N.C. Antimicrobial action of Oregano, Thyme, Clove, Cinnamon and Black pepper essential oils free and encapsulated against foodborne pathogens. Food Control 2023, 144, 109356. [Google Scholar] [CrossRef]
- Mantzios, T.; Tsiouris, V.; Kiskinis, K.; Economou, V.; Petridou, E.; Tsitsos, A.; Patsias, A.; Apostolou, I.; Papadopoulos, G.A.; Giannenas, I.; et al. In Vitro Investigation of the Antibacterial Activity of Nine Commercial Water Disinfectants, Acidifiers, and Glyceride Blends against the Most Important Poultry Zoonotic Bacteria. Pathogens 2023, 12, 381. [Google Scholar] [CrossRef] [PubMed]
- Merino, N.; Berdejo, D.; Pagán, E.; Girard, C.; Kerros, S.; Spinozzi, E.; Pagán, R.; García-Gonzalo, D. Phenotypic and Genotypic Comparison of Antimicrobial-Resistant Variants of Escherichia coli and Salmonella Typhimurium Isolated from Evolution Assays with Antibiotics or Commercial Products Based on Essential Oils. Pharmaceuticals 2023, 16, 1443. [Google Scholar] [CrossRef] [PubMed]
- Standard ATCC 25922. Available online: https://www.atcc.org/products/25922 (accessed on 25 May 2024).
- Standard ATCC 11303. Available online: https://www.atcc.org/products/11303?matchtype=&network=x&device=c&adposition=&keyword=&gad_source=1&gclid=EAIaIQobChMI9777zJ2whgMVdZKDBx02azKTEAAYAiAAEgLRofD_BwE (accessed on 25 May 2024).
- Standard ATCC 33090. Available online: https://www.atcc.org/products/33090 (accessed on 25 May 2024).
- M07-A10; Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically. Approved Standard, 10th ed. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2015.
- M100-S28; Performance Standards for Antimicrobial Susceptibility Testing. Twenty-Eighth Informational Supplement. CLSI Document; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018.
- Abd El-Hack, M.E.; El-Saadony, M.T.; Salem, H.M.; El-Tahan, A.M.; Soliman, M.M.; Youssef, G.B.A.; Taha, A.E.; Soliman, S.M.; Ahmed, A.E.; El-Kott, A.F.; et al. Alternatives to antibiotics for organic poultry production: Types, modes of action and impacts on bird’s health and production. Poult. Sci. 2022, 101, 101696. [Google Scholar] [CrossRef]
- Beier, R.; Byrd, J.; Caldwell, D.; Andrews, K.; Crippen, T.; Anderson, R.; Nisbet, D. Inhibition and Interactions of Campylobacter jejuni from Broiler Chicken Houses with Organic Acids. Microorganisms 2019, 7, 223. [Google Scholar] [CrossRef]
- Mutlu-Ingok, A.; Tasir, S.; Seven, A.; Akgun, N.; Karbancioglu-Guler, F. Evaluation of the single and combined antibacterial efficiency of essential oils for controlling Campylobacter coli, Campylobacter jejuni, Escherichia coli, Staphylococcus aureus, and mixed cultures. Flavour. Fragr. J. 2019, 34, 280–287. [Google Scholar] [CrossRef]
- Peh, E.; Kittler, S.; Reich, F.; Kehrenberg, C. Antimicrobial activity of organic acids against Campylobacter spp. and development of combinations—A synergistic effect? PLoS ONE 2020, 15, e0239312. [Google Scholar] [CrossRef]
- Ammar, A.M.; El-Naenaeey, E.-S.Y.; Abd El-Hamid, M.I.; El-Gedawy, A.A.; Elmalt, R.M.S. Campylobacter as a major foodborne pathogen: A review of its characteristics, pathogenesis, antimicrobial resistance and control. J. Microbiol. Biotechnol. Food Sci. 2021, 10, 609–619. [Google Scholar] [CrossRef]
- EFSA Panel on Biological Hazards (BIOHAZ); Koutsoumanis, K.; Allende, A.; Alvarez-Ordóñez, A.; Bolton, D.; Bover-Cid, S.; Davies, R.; De Cesare, A.; Herman, L.; Hilbert, F.; et al. Update and review of control options for Campylobacter in broilers at primary production. EFSA J. 2020, 18, e06090. [Google Scholar]
- Navarro, M.; Stanley, R.; Cusack, A.; Sultanbawa, Y. Combinations of plant-derived compounds against Campylobacter in vitro. J. Appl. Poult. Res. 2015, 24, 352–363. [Google Scholar] [CrossRef]
- De Souza, G.T.; De Carvalho, R.J.; De Sousa, J.P.; Tavares, J.F.; Schaffner, D.; De Souza, E.L.; Magnani, M. Effects of the Essential Oil from Origanum vulgare L. on Survival of Pathogenic Bacteria and Starter Lactic Acid Bacteria in Semihard Cheese Broth and Slurry. J. Food Prot. 2016, 79, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Albano, M.; Crulhas, B.P.; Alves, F.C.B.; Pereira, A.F.M.; Andrade, B.F.M.T.; Barbosa, L.N.; Furlanetto, A.; Lyra, L.P.D.S.; Rall, V.L.M.; Júnior, A.F. Antibacterial and anti-biofilm activities of cinnamaldehyde against S. epidermidis. Microb. Pathog. 2019, 126, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Ashrafudoulla, M.; Mizan, M.F.R.; Ha, A.J.; Park, S.H.; Ha, S.-D. Antibacterial and antibiofilm mechanism of eugenol against antibiotic resistance Vibrio parahaemolyticus. Food Microbiol. 2020, 91, 103500. [Google Scholar] [CrossRef]
- Rathod, N.B.; Kulawik, P.; Ozogul, F.; Regenstein, J.M.; Ozogul, Y. Biological activity of plant-based carvacrol and thymol and their impact on human health and food quality. Trends Food Sci. Technol. 2021, 116, 733–748. [Google Scholar] [CrossRef]
- Elmi, A.; Nasher, F.; Dorrell, N.; Wren, B.; Gundogdu, O. Revisiting Campylobacter jejuni Virulence and Fitness Factors: Role in Sensing, Adapting, and Competing Front. Cell. Infect. Microbiol. 2021, 10, 607704. [Google Scholar] [CrossRef]
- Mantzios, T.; Tsiouris, V.; Papadopoulos, G.A.; Economou, V.; Petridou, E.; Brellou, G.D.; Giannenas, I.; Biliaderis, C.G.; Kiskinis, K.; Fortomaris, P. Investigation of the Effect of Three Commercial Water Acidifiers on the Performance, Gut Health, and Campylobacter jejuni Colonization in Experimentally Challenged Broiler Chicks. Animals 2023, 13, 2037. [Google Scholar] [CrossRef]
- Pearlin, B.V.; Muthuvel, S.; Govidasamy, P.; Villavan, M.; Alagawany, M.; Ragab Farag, M.; Dhama, K.; Gopi, M. Role of acidifiers in livestock nutrition and health: A review. Anim. Physiol. Nutr. 2020, 104, 558–569. [Google Scholar] [CrossRef] [PubMed]
- Hamed, D.M.; Hassan, A.M.A. Acids Supplementation to Drinking Water and Their Effects on Japanese Quails Experimentally Challenged with Salmonella Enteritidis. Res. Zool. 2013, 3, 15–22. [Google Scholar]
- Hao, Y.; Kang, J.; Yang, R.; Li, H.; Cui, H.; Bai, H.; Tsitsilin, A.; Li, J.; Shi, L. Multidimensional exploration of essential oils generated via eight oregano cultivars: Compositions, chemodiversities, and antibacterial capacities. Food Chem. 2022, 374, 131629. [Google Scholar] [CrossRef] [PubMed]
- Mith, H.; Clinquart, A.; Zhiri, A.; Daube, G.; Delcenserie, V. The impact of oregano (Origanum heracleoticum) essential oil and carvacrol on virulence gene transcription by Escherichia coli O157:H7. FEMS Microbiol. Lett. 2015, 362, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kachur, K.; Suntres, Z. The antibacterial properties of phenolic isomers, carvacrol and thymol. Crit. Rev. Food Sci. Nutr. 2020, 60, 3042–3053. [Google Scholar] [CrossRef] [PubMed]
- Dan, S.D.; Mihaiu, M.; Reget, O.; Oltean, D.; Tăbăran, A. Pathogens Contamination Level Reduction on Beef Using Organic Acids Decontamination Methods. Bull. Univ. Agric. Sci. Veter-Med. Cluj-Napoca. Veter-Med. 2017, 74, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Huang, T.-Y.; Ye, C.; Chen, L.; Liang, Y.; Wang, K.; Liu, J. Formation and Control of the Viable but Non-Culturable State of Foodborne Pathogen Escherichia coli O157:H7. Front. Microbiol. 2020, 11, 1202. [Google Scholar] [CrossRef] [PubMed]
- McDermott, A.; Whyte, P.; Brunton, N.; Lyng, J.; Fagan, J.; Bolton, D.J. The effect of organic acid and sodium chloride dips on the shelf-life of refrigerated Irish brown crab (Cancer pagurus) meat. LWT 2018, 98, 141–147. [Google Scholar] [CrossRef]
- Wang, J.; Lei, Y.; Yu, Y.; Yin, L.; Zhang, Y. Use of Acetic Acid to Partially Replace Lactic Acid for Decontamination against Escherichia coli O157:H7 in Fresh Produce and Mechanism of Action. Foods 2021, 10, 2406. [Google Scholar] [CrossRef]
- Ijabadeniyi, O.; Mbedla, A.; Ajayeoba, T. Microbiological quality and antimicrobial efficacy of combined oregano essential oil and acetic acid on fresh lettuce. Ital. J. Food Sci. 2020, 32, 399–409. [Google Scholar]
- Galán-Relaño, Á.; Valero Díaz, A.; Huerta Lorenzo, B.; Gómez-Gascón, L.; Mena Rodríguez, M.Á.; Carrasco Jiménez, E.; Pérez Rodríguez, F.; Astorga Márquez, R.J. Salmonella and Salmonellosis: An Update on Public Health Implications and Control Strategies. Animals 2023, 13, 3666. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Ji, B.; Zhang, H.; Jiang, H.; Yang, Z.; Li, J.; Li, J.; Ren, Y.; Yan, W. Synergistic Effect of Thymol and Carvacrol Combined with Chelators and Organic Acids against Salmonella Typhimurium. J. Food Prot. 2007, 70, 1704–1709. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Bae, Y.-M.; Jung, K.-S.; Heu, S.; Lee, S.-Y. Antimicrobial activity of natural antimicrobial substances against spoilage bacteria isolated from fresh produce. Food Control 2013, 32, 665–672. [Google Scholar] [CrossRef]
- Walker, K. Antimicrobial Effectiveness of Citrus Essential Oils and Organic Acids against Salmonella and Listeria monocytogenes; West Virginia University Libraries: Morgantown, WV, USA, 2015; pp. 1–67. [Google Scholar]
- Machado, P.C.; Beirão, B.C.B.; Filho, T.F.; Lourenço, M.C.; Joineau, M.L.; Santin, E.; Caron, L.F. Use of blends of organic acids and oregano extracts in feed and water of broiler chickens to control Salmonella Enteritidis persistence in the crop and ceca of experimentally infected birds. J. Appl. Poult. Res. 2014, 23, 671–682. [Google Scholar] [CrossRef]
- Hu, Z.; Liu, L.; Guo, F.; Huang, J.; Qiao, J.; Bi, R.; Huang, J.; Zhang, K.; Guo, Y.; Wang, Z. Dietary supplemental coated essential oils and organic acids mixture improves growth performance and gut health along with reduces Salmonella load of broiler chickens infected with Salmonella Enteritidis. J. Anim. Sci. Biotechnol. 2023, 14, 95. [Google Scholar] [CrossRef] [PubMed]
- Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of Essential Oils on Pathogenic Bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Sánchez, D.; Cabo, M.L.; Ibusquiza, P.S.; Rodríguez-Herrera, J.J. Biofilm-forming ability and resistance to industrial disinfectants of Staphylococcus aureus isolated from fishery products. Food Control 2014, 39, 8–16. [Google Scholar] [CrossRef]
- Mejía, L.; Espinosa-Mata, E.; Freire, A.L.; Zapata, S.; González-Candelas, F. Listeria monocytogenes, a silent foodborne pathogen in Ecuador. Front. Microbiol. 2023, 14, 1278860. [Google Scholar] [CrossRef] [PubMed]
- Engstrom, S.K.; Anderson, K.M.; Glass, K.A. Effect of Commercial Protective Cultures and Bacterial Fermentates on Listeria monocytogenes Growth in a Refrigerated High-Moisture Model Cheese. J. Food Prot. 2021, 84, 772–780. [Google Scholar] [CrossRef]
- Upadhyay, A.; Johny, A.K.; Amalaradjou, M.A.R.; Ananda Baskaran, S.; Kim, K.S.; Venkitanarayanan, K. Plant-derived antimicrobials reduce Listeria monocytogenes virulence factors in vitro, and down-regulate expression of virulence genes. Int. J. Food Microbiol. 2012, 157, 88–94. [Google Scholar] [CrossRef]
- Corrêa, J.A.F.; Santos, J.V.G.D.; Evangelista, A.G.; Pinto, A.C.S.M.; Macedo, R.E.F.D.; Luciano, F.B. Combined application of phenolic acids and essential oil components against Salmonella Enteritidis and Listeria monocytogenes in vitro and in ready-to-eat cooked ham. LWT 2021, 149, 111881. [Google Scholar] [CrossRef]
- Rogiers, G.; Kebede, B.T.; Van Loey, A.; Michiels, C.W. Membrane fatty acid composition as a determinant of Listeria monocytogenes sensitivity to trans-cinnamaldehyde. Res. Microbiol. 2017, 168, 536–546. [Google Scholar] [CrossRef] [PubMed]
- Borges, A.; Simões, L.C.; Saavedra, M.J.; Simões, M. The action of selected isothiocyanates on bacterial biofilm prevention and control. Int. Biodeterior. Biodegrad. 2014, 86, 25–33. [Google Scholar] [CrossRef]
- Lopez-Romero, J.C.; González-Ríos, H.; Borges, A.; Simões, M. Antibacterial Effects and Mode of Action of Selected Essential Oils Components against Escherichia coli and Staphylococcus aureus. Evid. -Based Complement. Altern. Med. 2015, 2015, 795435. [Google Scholar] [CrossRef] [PubMed]
- Oulkheir, S.; Aghrouch, M.; Mourabit, F.E.; Dalha, F.; Graich, H.; Amouch, F.; Ouzaid, K.; Moukale, A.; Chadli, S. Antibacterial Activity of Essential Oils Extracts from Cinnamon, Thyme, Clove and Geranium against a Gram Negative and Gram Positive Pathogenic Bacteria. J. Dis. Med. Plants 2017, 3, 1–5. [Google Scholar]
- Muchaamba, F.; Eshwar, A.K.; Stevens, M.J.A.; Stephan, R.; Tasara, T. Different Shades of Listeria monocytogenes: Strain, Serotype, and Lineage-Based Variability in Virulence and Stress Tolerance Profiles. Front. Microbiol. 2022, 12, 792162. [Google Scholar] [CrossRef] [PubMed]
- Lianou, A.; Nychas, G.-J.E.; Koutsoumanis, K.P. Strain variability in biofilm formation: A food safety and quality perspective. Food Res. Int. 2020, 137, 109424. [Google Scholar] [CrossRef] [PubMed]
- Meléndez-Carmona, M.Á.; Mancheño-Losa, M.; Ruiz-Sorribas, A.; Muñoz-Gallego, I.; Viedma, E.; Chaves, F.; Van Bambeke, F.; Lora-Tamayo, J. Strain-to-strain variability among Staphylococcus aureus causing prosthetic joint infection drives heterogeneity in response to levofloxacin and rifampicin. J. Antimicrob. Chemother. 2022, 77, 3265–3269. [Google Scholar] [CrossRef]
- Ferreira, M.A.; Bernardo, L.G.; Neves, L.S.; Campos, M.R.H.; Lamaro-Cardoso, J.; André, M.C.P. Virulence profile and genetic variability of Staphylococcus aureus isolated from artisanal cheese. J. Dairy Sci. 2016, 99, 8589–8597. [Google Scholar] [CrossRef]

| Category | Tested Product | Active Ingredients | r. Dosage Range 1 |
|---|---|---|---|
| Essential oil-based phytogenics | Phyto CSC Liquide B | Trans-cinnamaldehyde (43.93%), Thymol (29.83%), Carvacrol (10.56%) | 0.030–0.060% |
| AEN 350 B Liquid | Trans-cinnamaldehyde (87.12%), Eugenol (10.83%), (E)-caryophyllene (1.40%) | 0.030–0.100% | |
| Acid-Based Eubiotics | Salgard® liquid | Ammonium formate (20%), Propionic acid (5.2%), Ammonium Propionate (1%) and Carrier | 0.100–0.200% |
| Intesti-Flora | Lactic acid, Propionic acid, Sorbic acid Copper-chelates of glycine, Oligofructose syrup | 0.020–0.100% | |
| Blends of essential oils and organic acids | ProPhorceTM SA Exclusive | Formic acid (50–60%), Sodium formate (20–30%), L-(+)-lactic acid (5–10%), Cinnamaldehyde (1–5%) | 0.080–0.100% |
| Herbal acid | Lactic acid 30%, Phosphoric acid 20%, Formic acid 15%, Acetic acid 5%, Citric acid 1%, Malic acid 1%, Xtract anabasis (Thyme oil, Oregano oil) 0.5% | 0.050–0.600% | |
| Rigosol-N | Lactic acid 60–80%, Acetic acid 20–10%, Propionic acid 5–10%, Benzoic acid 1–2%, Oregano oil 5% | 0.040–0.060% | |
| Eubisan 3000 | Oregano oil (80,000 mg), Cinnamon oil (3000 mg), Aniseed oil (3000 mg), Citric acid | 0.020–0.025% |
| Phytogenics | Acid-Based Eubiotics | Blends of Essential Oils and Organic Acids | ||||||
|---|---|---|---|---|---|---|---|---|
| Tested Strains | Phyto CSC Liquide B | AEN 350 B Liquid | Salgard® Liquid | Intesti-Flora | ProPhorceTM SA Exclusive | Herbal Acid | Rigosol-N | Eubisan 3000 |
| Gram-negative bacteria | ||||||||
| C. jejuni S1 | 0.142 ± 0.000 | 0.142 ± 0.000 | 0.568 ± 0.000 | 0.284 ± 0.000 | 0.142 ± 0.000 | 0.071 ± 0.000 | 0.071 ± 0.000 | 0.118 ± 0.041 |
| C. coli S1 | 0.142 ± 0.000 | 0.071 ± 0.000 | 0.568 ± 0.000 | 0.142 ± 0.000 | 0.071 ± 0.000 | 0.071 ± 0.000 | 0.071 ± 0.000 | 0.142 ± 0.000 |
| C. jejuni S2 | 0.142 ± 0.000 | 0.071 ± 0.000 | 0.568 ± 0.000 | 0.284 ± 0.000 | 0.142 ± 0.000 | 0.071 ± 0.000 | 0.142 ± 0.000 | 0.118 ± 0.041 |
| E. coli ATCC 25922 | 1.894 ± 0.657 | 1.136 ± 0.000 | 1.136 ± 0.000 | 0.568 ± 0.000 | 0.142 ± 0.000 | 0.142 ± 0.000 | 0.142 ± 0.000 | 0.379 ± 0.164 |
| E. coli ATCC 11303 | 0.379 ± 0.164 | 1.136 ± 0.000 | 0.568 ± 0.000 | 0.284 ± 0.000 | 0.071 ± 0.000 | 0.142 ± 0.000 | 0.142 ± 0.000 | 0.284 ± 0.000 |
| S. Typhimurium DT120 | 1.894 ± 0.657 | 1.136 ± 0.000 | 1.136 ± 0.000 | 0.568 ± 0.000 | 0.142 ± 0.000 | 0.142 ± 0.000 | 0.142 ± 0.000 | 0.284 ± 0.000 |
| S. Typhimurium U292 | 1.136 ± 0.000 | 1.136 ± 0.000 | 1.136 ± 0.000 | 0.568 ± 0.000 | 0.095 ± 0.041 | 0.142 ± 0.000 | 0.142 ± 0.000 | 0.284 ± 0.000 |
| Gram-positive bacteria | ||||||||
| S. aureus DSM 102262 | 9.090 ± 0.000 | 2.273 ± 0.000 | 1.136 ± 0.000 | 0.568 ± 0.000 | 0.142 ± 0.000 | 0.284 ± 0.000 | 0.142 ± 0.000 | 0.568 ± 0.000 |
| S. aureus DSM 25629 | 9.090 ± 0.000 | 4.545 ± 0.000 | 1.136 ± 0.000 | 0.284 ± 0.000 | 0.142 ± 0.000 | 0.284 ± 0.000 | 0.142 ± 0.000 | 0.284 ± 0.000 |
| S. aureus S1 | 3.030 ± 1.312 | 1.894 ± 0.657 | 1.894 ± 0.657 | 0.237 ± 0.082 | 0.284 ± 0.000 | 0.142 ± 0.000 | 0.142 ± 0.000 | 0.758 ± 0.328 |
| L. monocytogenes Scott A | 2.273 ± 0.000 | 1.894 ± 0.657 | 0.568 ± 0.000 | 0.568 ± 0.000 | 0.142 ± 0.000 | 0.142 ± 0.000 | 0.142 ± 0.000 | 0.379 ± 0.164 |
| L. innocua ATCC 33090 | 3.030 ± 1.312 | 1.136 ± 0.000 | 1.136 ± 0.000 | 1.136 ± 0.000 | 0.142 ± 0.000 | 0.284 ± 0.000 | 0.379 ± 0.164 | 1.136 ± 0.000 |
| L. monocytogenes S1 | 0.568 ± 0.000 | 0.284 ± 0.000 | 1.136 ± 0.000 | 0.568 ± 0.000 | 0.095 ± 0.041 | 0.142 ± 0.000 | 0.118 ± 0.411 | 0.189 ± 0.082 |
| Two-Fold Serial Dilutions | 1 | 1/2 | 1/4 | 1/8 | 1/16 | 1/32 | 1/64 | 1/128 | 1/256 | 1/512 | 1/1024 | 1/2048 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phyto CSC Liquide B | 7.25 | 7.30 | 7.35 | 7.39 | 7.45 | 7.46 | 7.47 | 7.47 | 7.47 | 7.47 | 7.48 | 7.48 |
| AEN 350 B Liquid | 7.10 | 7.19 | 7.28 | 7.37 | 7.42 | 7.46 | 7.47 | 7.47 | 7.47 | 7.47 | 7.47 | 7.47 |
| Salgard® liquid | 4.58 | 4.63 | 4.74 | 5.02 | 5.30 | 6.20 | 7.00 | 7.21 | 7.31 | 7.37 | 7.41 | 7.43 |
| Intesti-Flora | 2.92 | 3.27 | 3.82 | 4.43 | 5.01 | 5.52 | 6.79 | 7.14 | 7.28 | 7.36 | 7.40 | 7.41 |
| ProPhorceTM SA Exclusive | 3.12 | 3.23 | 3.39 | 3.58 | 3.81 | 3.97 | 4.00 | 4.35 | 5.10 | 5.90 | 6.60 | 7.40 |
| Herbal acid | 2.07 | 2.41 | 2.83 | 3.28 | 3.75 | 4.27 | 4.91 | 5.93 | 6.76 | 7.12 | 7.29 | 7.39 |
| Rigosol-N | 2.55 | 2.83 | 3.13 | 3.49 | 3.87 | 4.34 | 4.91 | 5.73 | 6.69 | 7.14 | 7.31 | 7.39 |
| Eubisan 3000 | 3.33 | 3.78 | 4.27 | 4.77 | 5.39 | 6.35 | 6.95 | 7.14 | 7.31 | 7.37 | 7.42 | 7.42 |
| Negative control 1 | 7.48 | 7.48 | 7.48 | 7.48 | 7.48 | 7.48 | 7.48 | 7.48 | 7.48 | 7.48 | 7.48 | 7.48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiskinis, K.; Mantzios, T.; Economou, V.; Petridou, E.; Tsitsos, A.; Patsias, A.; Apostolou, I.; Papadopoulos, G.A.; Giannenas, I.; Fortomaris, P.; et al. The In Vitro Antibacterial Activity of Phytogenic and Acid-Based Eubiotics against Major Foodborne Zoonotic Poultry Pathogens. Animals 2024, 14, 1611. https://doi.org/10.3390/ani14111611
Kiskinis K, Mantzios T, Economou V, Petridou E, Tsitsos A, Patsias A, Apostolou I, Papadopoulos GA, Giannenas I, Fortomaris P, et al. The In Vitro Antibacterial Activity of Phytogenic and Acid-Based Eubiotics against Major Foodborne Zoonotic Poultry Pathogens. Animals. 2024; 14(11):1611. https://doi.org/10.3390/ani14111611
Chicago/Turabian StyleKiskinis, Konstantinos, Tilemachos Mantzios, Vangelis Economou, Evanthia Petridou, Anestis Tsitsos, Apostolos Patsias, Ioanna Apostolou, Georgios A. Papadopoulos, Ilias Giannenas, Paschalis Fortomaris, and et al. 2024. "The In Vitro Antibacterial Activity of Phytogenic and Acid-Based Eubiotics against Major Foodborne Zoonotic Poultry Pathogens" Animals 14, no. 11: 1611. https://doi.org/10.3390/ani14111611
APA StyleKiskinis, K., Mantzios, T., Economou, V., Petridou, E., Tsitsos, A., Patsias, A., Apostolou, I., Papadopoulos, G. A., Giannenas, I., Fortomaris, P., & Tsiouris, V. (2024). The In Vitro Antibacterial Activity of Phytogenic and Acid-Based Eubiotics against Major Foodborne Zoonotic Poultry Pathogens. Animals, 14(11), 1611. https://doi.org/10.3390/ani14111611










