Possible Shifts in the Genetic Diversity of Red-crowned Cranes (Grus japonensis) in Hokkaido, Japan: Indications of Continental Gene Flow
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
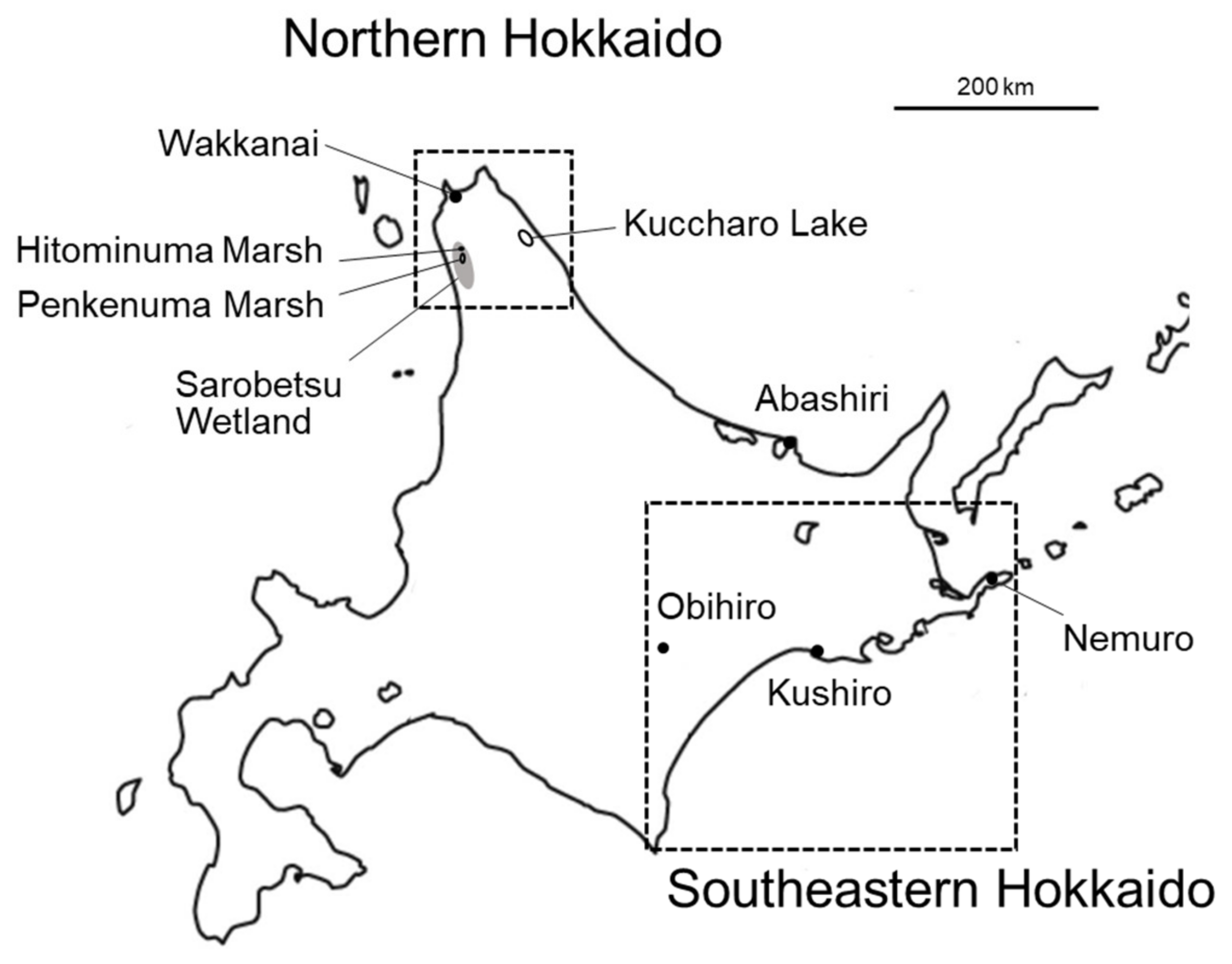
2.1. Samples and DNA Extraction
2.2. Genotyping of Major Histocompatibility Complex
2.3. Genotyping of Mitochondrial Haplotype
2.4. Genotyping InDels
2.5. Statistical Analyses
3. Results
3.1. Analysis of Insertion/Deletion in the Northern Hokkaido Population
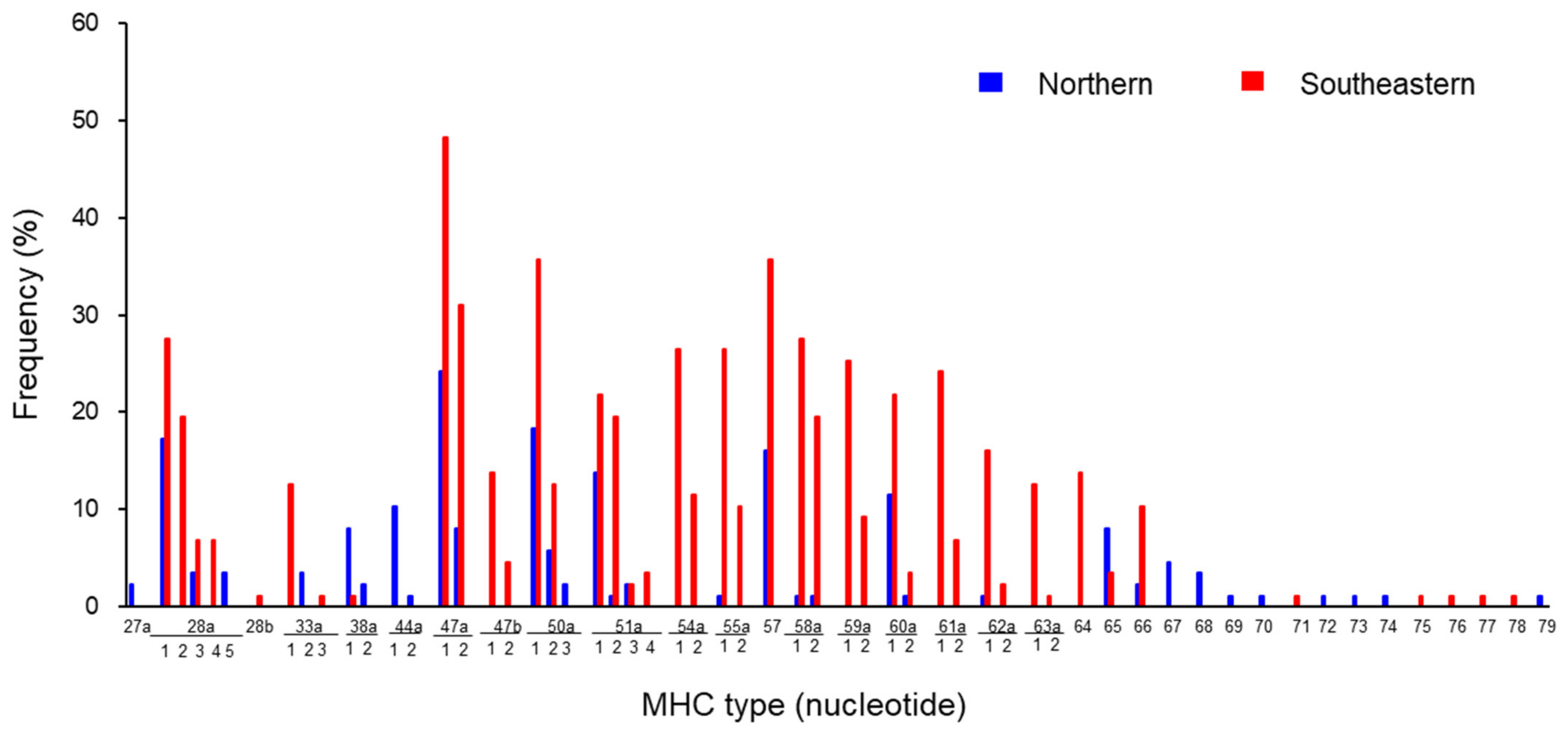
3.2. MHC Types Detected in Hokkaido Population
3.3. Distribution of MHC Types in Northern and Southeastern Hokkaido Populations
3.4. Comparison of MHC Types in Parents and Children
3.5. MHC Types of Hitominuma Swamp Cranes in Northern and Southeastern Hokkaido Populations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- BirdLife International. Species Factsheet: Grus japonensis. 2024. Available online: https://datazone.birdlife.org/species/factsheet/red-crowned-crane-grus-japonensis/text (accessed on 27 May 2024).
- Masatomi, H.; Masatomi, Y. Ecology of the Red-crowned Crane and Conservation Activities in Japan. In Biodiversity Conservation Using Umbrella Species: Blakiston’s Fish Owl and the Red-crowned Crane; Ecological Research Monographs; Nakamura, F., Ed.; Springer: Singapore, 2018; Chapter 6. [Google Scholar]
- Hisai, A.; Akasaka, T. Historical Relationship between Japanese Crane and Society -the Use and Product of Japanese Crane in Hokkaido. J. Rakuno Gakuen Univ. 2009, 34, 31–50. (In Japanese) [Google Scholar]
- Masatomi, H.; Masatomi, Y. Promoting the Coexistence of Humans and Tancho in Japan. Jpn. J. Conserv. Ecol. 2009, 14, 223–242, (in Japanese with English abstract). [Google Scholar]
- The Red-crowned Crane Conservation Research Group. Available online: https://www6.marimo.or.jp/tancho1213/sosutyosa.html (accessed on 25 April 2024).
- Hasegawa, O.; Takada, S.; Yoshida, M.C.; Abe, S. Variation of Mitochondrial Control Region Sequences in Three Crane Species, the Red-crowned Crane Grus japonensis, the Common Crane G. grus and the Hooded Crane G. monacha. Zool. Sci. 1999, 16, 685–692. [Google Scholar] [CrossRef]
- Miura, Y.; Shiomi, A.; Shiraishi, J.; Makita, K.; Asakawa, M.; Kitazawa, T.; Hiraga, T.; Momose, Y.; Momose, K.; Masatomi, H.; et al. Large-Scale Survey of Mitochondrial D-Loop of the Red-crowned Crane Grus Japonensis in Hokkaido, Japan by Convenient Genotyping Method. J. Vet. Med. Sci. 2013, 75, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, T.; Momose, K.; Onuma, M.; Matsumoto, F.; Masuda, R. Low Genetic Variation of Red-crowned Cranes on Hokkaido Island, Japan, over the Hundred Years. Zool. Sci. 2017, 34, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, E.; Hasebe, M.; Hwang, J.H.; Kim, E.Y.; Lee, K.; Momose, K.; Teraoka, H. Origin of a Pair of Red-crowned Cranes (Grus Japonensis) Found in Sarobetsu Wetland, Northwestern Hokkaido, Japan: A Possible Crossbreeding between the Island and the Mainland Population. J. Vet. Med. Sci. 2022, 84, 233–237. [Google Scholar] [CrossRef]
- Masatomi, H.; Masatomi, Y.; Fujimoto, T.; Masuzawa, T.; Konishi, K.; Fujimura, A. Increased Breeding among Grus Japonensis Observed via Aircraft Surveys over Northern Hokkaido, Japan. Jpn. J. Conserv. Ecol. 2020, 25, 87–98, (In Japanese with English abstract). [Google Scholar]
- Sugimoto, T.; Hasegawa, O.; Azuma, N.; Masatomi, H.; Sato, F.; Matsumoto, F.; Masatomi, Y.; Izumi, H.; Abe, S. Genetic Structure of the Endangered Red-crowned Cranes in Hokkaido, Japan and Conservation Implications. Conserv. Genet. 2015, 16, 1395–1401. [Google Scholar] [CrossRef]
- Kawasaki, E.; Wenjing, D.; Sawada, A.; Nakajima, M.; Momose, K.; Yoshino, T.; Amano, T.; Endoh, D.; Nakajima, N.; Teraoka, H. Conventional Gel Electrophoresis-Resolvable Insertion/Deletion Markers for Individual Identification and Analysis of Population Genetics in Red-crowned Cranes in Eastern Hokkaido, Japan. Animals 2022, 12, 2293. [Google Scholar] [CrossRef] [PubMed]
- Miura, Y.; Shiraishi, J.; Shiomi, A.; Kitazawa, T.; Hiraga, T.; Matsumoto, F.; Teraoka, H.; Masatomi, H. Origin of Three Red-crowned Cranes Grus Japonensis Found in Northeast Honshu and West Hokkaido, Japan, from 2008 to 2012. J. Vet. Med. Sci. 2013, 75, 1241–1244. [Google Scholar] [CrossRef] [PubMed]
- Wieczorek, M.; Abualrous, E.T.; Sticht, J.; Álvaro-Benito, M.; Stolzenberg, S.; Noé, F.; Freund, C. Major Histocompatibility Complex (MHC) Class I and MHC Class II Proteins: Conformational Plasticity in Antigen Presentation. Front. Immunol. 2017, 8, 248429. [Google Scholar] [CrossRef] [PubMed]
- Fleming-Canepa, X.; Jensen, S.M.; Mesa, C.M.; Diaz-Satizabal, L.; Roth, A.J.; Parks-Dely, J.A.; Moon, D.A.; Wong, J.P.; Evseev, D.; Gossen, D.A.; et al. Extensive Allelic Diversity of MHC Class I in Wild Mallard Ducks. J. Immunol. 2016, 197, 783–794. [Google Scholar] [CrossRef] [PubMed]
- Maibach, V.; Hans, J.B.; Hvilsom, C.; Marques-Bonet, T.; Vigilant, L. MHC Class I Diversity in Chimpanzees and Bonobos. Immunogenetics 2017, 69, 661–676. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Ye, W.; Sun, C.; He, K.; Zhu, Y.; Lan, H.; Lu, C.; Liu, H. Genetic Diversity and Differentiation of MHC Class I Genes in Red-crowned Crane Populations. Front. Ecol. Evol. 2022, 10, 898581. [Google Scholar] [CrossRef]
- Akiyama, T.; Kohyama, T.I.; Nishida, C.; Onuma, M.; Momose, K.; Masuda, R. Genetic Variation of Major Histocompatibility Complex Genes in the Endangered Red-crowned Crane. Immunogenetics 2017, 69, 451–462. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, J.C.; Huber, C.D. The Inflated Significance of Neutral Genetic Diversity in Conservation Genetics. Proc. Natl. Acad. Sci. USA 2021, 118, e2015096118. [Google Scholar] [CrossRef] [PubMed]
- Winternitz, J.; Chakarov, N.; Rinaud, T.; Ottensmann, M.; Krüger, O. High Functional Allelic Diversity and Copy Number in Both MHC Classes in the Common Buzzard. BMC Ecol. Evol. 2023, 23, 24. [Google Scholar] [CrossRef] [PubMed]
- Guenay-Greunke, Y.; Bohan, D.A.; Traugott, M.; Wallinger, C. Handling of Targeted Amplicon Sequencing Data Focusing on Index Hopping and Demultiplexing Using a Nested Metabarcoding Approach in Ecology. Sci. Rep. 2021, 11, 19510. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, A.; Migalska, M.; Biedrzycka, A. AmpliSAS and AmpliHLA: Web Server Tools for MHC Typing of Non-Model Species and Human Using NGS Data. In Methods in Molecular Biology; Humana Press: New York, NY, USA, 2018; Volume 1802. [Google Scholar]
- Kalinowski, S.T.; Taper, M.L.; Marshall, T.C. Revising How the Computer Program CERVUS Accommodates Genotyping Error Increases Success in Paternity Assignment. Mol. Ecol. 2007, 16, 1099–1106. [Google Scholar] [CrossRef]
- Hardy, O.J.; Vekemans, X. SPAGeDI: A Versatile Computer Program to Analyse Spatial Genetic Structure at the Individual or Population Levels. Mol. Ecol. Notes 2002, 2, 618–620. [Google Scholar] [CrossRef]
- Weir, B.S.; Cockerham, C.C. Estimating F-Statistics for the Analysis of Population Structure. Evolution 1984, 38, 1358–1370. [Google Scholar] [CrossRef] [PubMed]
- Frankham, R.; Ballou, J.D.; Briscoe, D.A. Chapter 14. Population fragmentation. In Introduction to Conservation Genetics, 2nd ed.; Kindle Ver.; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
- Choo, S.Y. The HLA System: Genetics, Immunology, Clinical Testing, and Clinical Implications. Yonsei Med. J. 2007, 48, 11. [Google Scholar] [CrossRef] [PubMed]
- Hasebe, M. Breeding status and migratory survey of red-crowned cranes around Sarobetsu. In Proceedings of the North Hokkaido Crane Report Meeting 2023, Online, 23 February 2022; p. 4. (In Japanese). [Google Scholar]
- Inoue, M. Insights Gleaned from the Data Organization of Tagged Red-crowned Cranes. Part 1: A Giant Falls! TANCHO 2021, 43, 5–7. (In Japanese) [Google Scholar]
- Stoeckel, S.; Grange, J.; Fernández-Manjarres, J.F.; Bilger, I.; Frascaria-Lacoste, N.; Mariette, S. Heterozygote Excess in a Self-Incompatible and Partially Clonal Forest Tree Species—Prunus avium L. Mol. Ecol. 2006, 15, 2109–2118. [Google Scholar] [CrossRef] [PubMed]
- Nishioka, H. Detection and Wintering of Hooded Cranes in the Kushiro Area. TANCHO 2014, 21, 4. (In Japanese) [Google Scholar]
| N | AR | He | Ho | Fis | P | ||
|---|---|---|---|---|---|---|---|
| InDel | Total | 51 | 1.90 | 0.3352 | 0.315 | 0.062 | 0.1868 |
| Northern | 20 | 1.71 | 0.2737 | 0.381 | −0.412 | 0.0000 | |
| Southeastern | 31 | 1.90 | 0.3088 | 0.282 | 0.090 | 0.1139 | |
| MHC | Total | 51 | 34.09 | 0.9513 | 1.00 | −0.044 | 0.0000 |
| Northern | 20 | 33.00 | 0.9221 | 1.00 | −0.075 | 0.0000 | |
| Southeastern | 31 | 30.55 | 0.9606 | 1.00 | −0.041 | 0.0000 |
| Fst | P | |
|---|---|---|
| InDel | 0.214387 | 0.0000 |
| MHC | 0.012881 | 0.0000 |
| Grja-UA* | 28a1 | 28a3 | 28a5 | 33a2 | 38a1 | 38a2 | 44a1 | 44a2 | 47a1 | 47a2 | 50a1 | 50a2 | 50a3 | 51a1 | 57 | 59a1 | 60a1 | 60a2 | 65 | 67 | 68 | 69 | 74 | 76 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hitominuma male | |||||||||||||||||||||||||
| Hitominuma female | |||||||||||||||||||||||||
| % northern | 48.6 | 8.6 | 8.6 | 20 | 5.7 | 51.4 | 14.3 | 28.6 | 2.9 | 20 | 11.4 | 8.6 | |||||||||||||
| % southeastern | 41.4 | 10 | 0 | 1.7 | 0 | 53.4 | 19 | 32.8 | 5.2 | 5.2 | 0 | 0 | |||||||||||||
| % of total | 44.1 | 9.7 | 3.2 | 8.6 | 2.2 | 52.7 | 17.2 | 31.2 | 4.3 | 10.8 | 4.3 | 3.2 | |||||||||||||
| Lake Kuccharo 1 | |||||||||||||||||||||||||
| Lake Kuccharo 4 | |||||||||||||||||||||||||
| Lake Kuccharo 6 | |||||||||||||||||||||||||
| Penkenuma 9 | |||||||||||||||||||||||||
| Kabutonuma 3 | |||||||||||||||||||||||||
| Kabutonuma 5 | |||||||||||||||||||||||||
| Kabutonuma 6 | |||||||||||||||||||||||||
| 429 | |||||||||||||||||||||||||
| 315 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, W.; Tomita, K.; Sawada, A.; Hasebe, M.; Inoue, M.; Momose, K.; Nakamura, T.; Teraoka, H. Possible Shifts in the Genetic Diversity of Red-crowned Cranes (Grus japonensis) in Hokkaido, Japan: Indications of Continental Gene Flow. Animals 2024, 14, 1633. https://doi.org/10.3390/ani14111633
Dong W, Tomita K, Sawada A, Hasebe M, Inoue M, Momose K, Nakamura T, Teraoka H. Possible Shifts in the Genetic Diversity of Red-crowned Cranes (Grus japonensis) in Hokkaido, Japan: Indications of Continental Gene Flow. Animals. 2024; 14(11):1633. https://doi.org/10.3390/ani14111633
Chicago/Turabian StyleDong, Wenjing, Kai Tomita, Akira Sawada, Makoto Hasebe, Masako Inoue, Kunikazu Momose, Tatsuro Nakamura, and Hiroki Teraoka. 2024. "Possible Shifts in the Genetic Diversity of Red-crowned Cranes (Grus japonensis) in Hokkaido, Japan: Indications of Continental Gene Flow" Animals 14, no. 11: 1633. https://doi.org/10.3390/ani14111633





