Cardiac Disease Related to Primary Hyperthyroidism in a 20-Year-Old Mule
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Initial Presentation
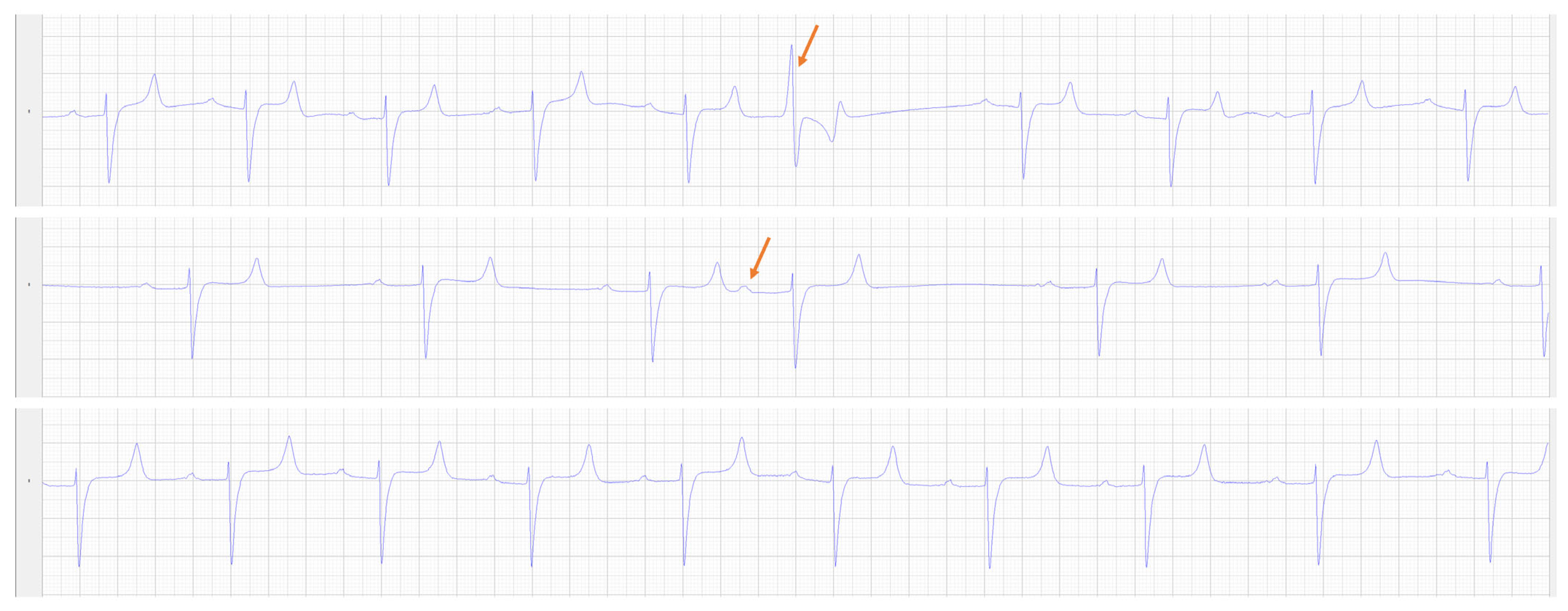
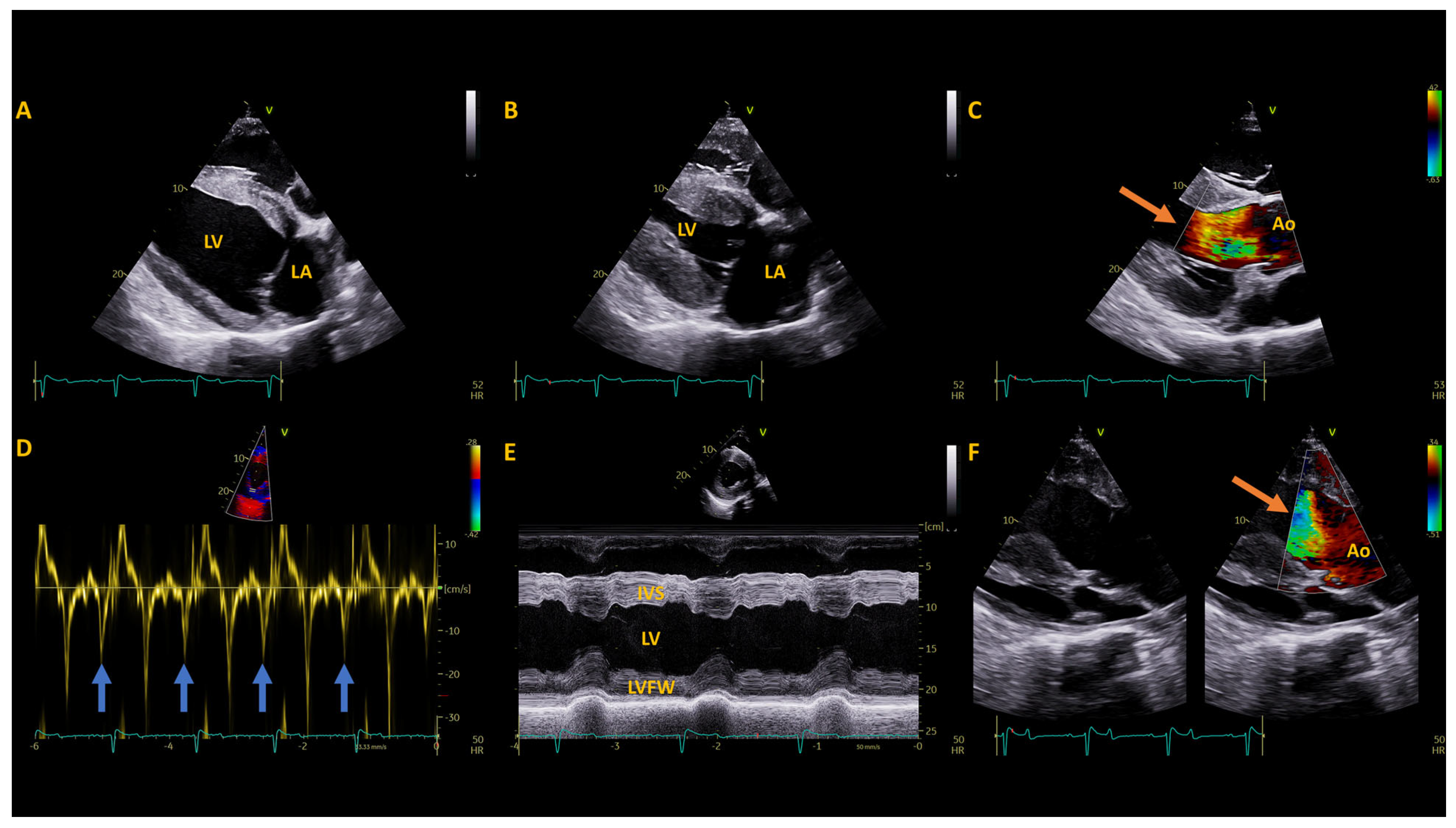
2.2. Pre-Operative Diagnostic Testing
2.3. Surgery and Treatments
3. Results
3.1. Post-Operative Diagnostics
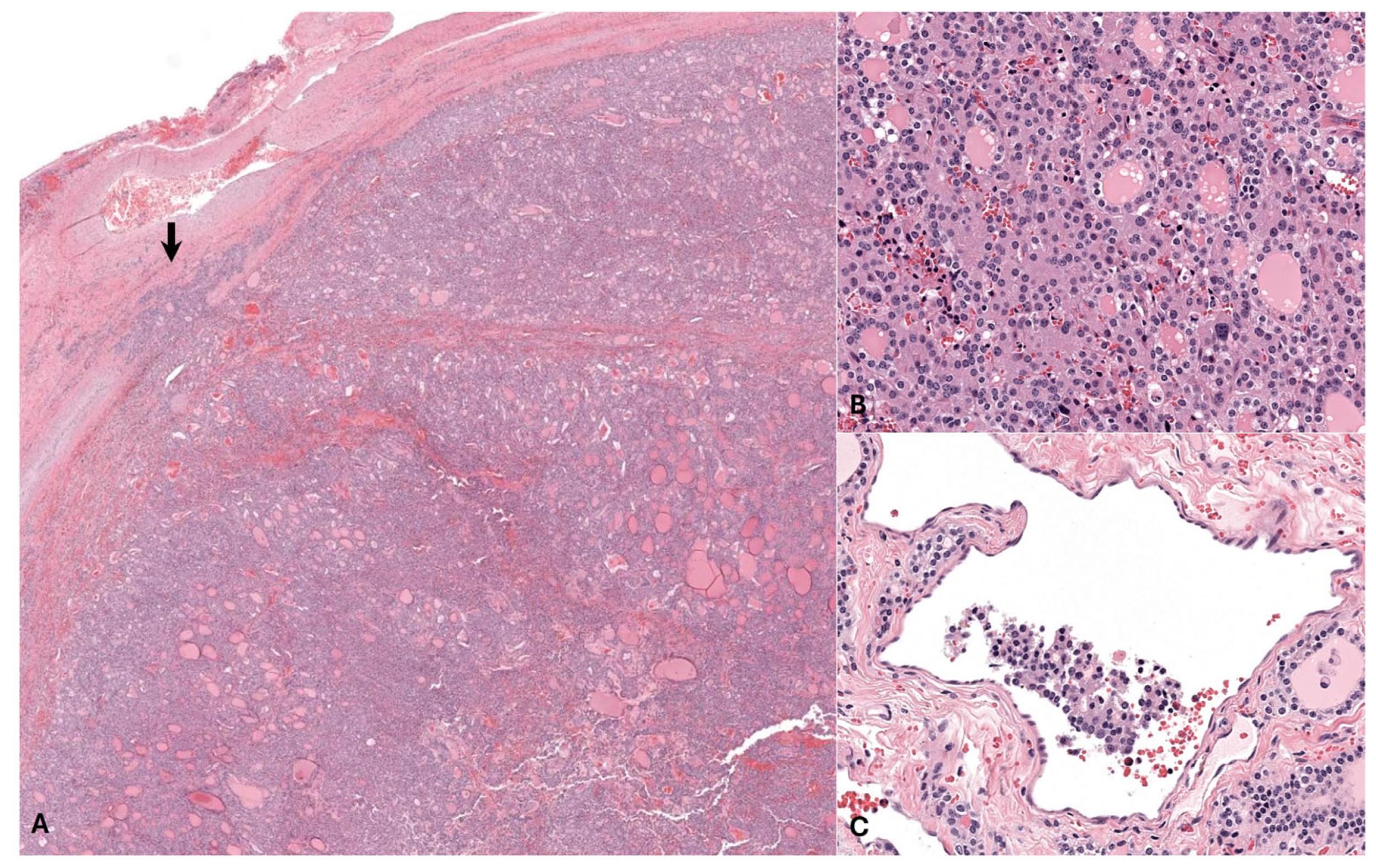
3.2. Histopathology
3.3. Follow Up
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Laboratory Equipment
- Calcium and phosphate were measured using the Cobas Roche chemistry analyzer
- Ionized calcium was measured using Zoetis iSTAT
- Parathyroid—Immunodiagnostic Systems
- ACTH, Total T3 and Total T4—Immulite 2000 CIA, Siemens Healthineers,
- Total T3—Antech RIA
- Alpha tocopherol—high performance liquid chromatography with fluorescence detector (HPLC-FLD) methodology
- Selenium—Graphite furnas atomic absorption spectrometry (AAS) methodology
References
- Ramirez, S.; McClure, J.J.; Moore, R.M.; Wolfsheimer, K.J.; Gaunt, S.D.; Mirza, M.H.; Taylor, W. Hyperthyroidism associated with a thyroid adenocarcinoma in a 21-year-old gelding. J. Vet. Intern. Med. 1998, 12, 475–477. [Google Scholar] [CrossRef] [PubMed]
- Alberts, M.K.; McCann, J.P.; Woods, P.R. Hemithyroidectomy in a horse with confirmed hyperthyroidism. J. Am. Vet. Med. 2000, 217, 1051–1054. [Google Scholar] [CrossRef]
- Tan, R.H.H.; Davies, S.E.; Crisman, M.V.; Coyle, L.; Daniel, G.B. The use of propylthiouracil for treatment of hyperthyroidism in a horse. J. Vet. Intern. Med. 2008, 22, 1253–1258. [Google Scholar] [CrossRef] [PubMed]
- Costello, J.; Firshman, A.M.; Brown, J.C.; Maher, M.; Tadros, E.M. Response to thyrotropin-releasing hormone (TRH) in a horse with hyperthyroidism associated with a functional thyroid adenoma. Can. Vet. J. 2019, 60, 1189–1193. [Google Scholar] [PubMed]
- Schlotthauer, C.F. The incidence and types of disease of the thyroid gland of adult horses. J. Am. Vet. Med. 1931, 78, 211–218. [Google Scholar]
- Dalefield, R.R.; Palmer, D.N. The frequent occurrence of thyroid tumours in aged horses. J. Comp. Pathol. 1994, 110, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Marcatili, M.; Voss, S.J.; Pollock, P.J. Standing thyroidectomy in 10 horses. Vet. Surg. 2018, 47, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Osuna, P.M.; Udovcic, M.; Sharma, M.D. Hyperthyroidism and the heart. Methodist DeBakey Cardiovasc. J. 2017, 13, 60–63. [Google Scholar] [CrossRef] [PubMed]
- Syme, H.M. Cardiovascular and renal manifestations of hyperthyroidism. Vet. Clin. N. Am. Small Anim. 2007, 37, 723–743. [Google Scholar] [CrossRef]
- Deaton, K.E.; Bishop, C.M.; Butler, P.J. The effect of thyroid hormones on the aerobic development of locomotor and cardiac muscles in the barnacle goose. J. Comp. Physiol. B 1997, 167, 319–327. [Google Scholar] [CrossRef]
- Hammond, H.K.; White, F.C.; Buxton, I.L.; Saltzstein, P.; Brunton, L.L.; Longhurst, J.C. Increased myocardial β-receptors and adrenergic responses in hyperthyroid pigs. Am. J. Physiol.-Heart Circ. Physiol. 1987, 252, H283–H290. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Xu, A.; Tokmakejian, S.; Narayanan, N. Thyroid hormone-induced overexpression of functional ryanodine receptors in the rabbit heart. Am. J. Physiol.-Heart Circ. Physiol. 2000, 278, H1429–H1438. [Google Scholar] [CrossRef] [PubMed]
- Klein, I.; Hong, C. Effects of thyroid hormone on cardiac size and myosin content of the heterotropically transplanted rat heart. J. Clin. Investig. 1986, 77, 1694–1698. [Google Scholar] [CrossRef] [PubMed]
- Carney, H.C.; Ward, C.R.; Bailey, S.J.; Bruyette, D.; Dennis, S.; Ferguson, D.; Hinc, A.; Rucinsky, A.R. 2016 AAFP guidelines for the management of feline hyperthyroidism. J. Fel. Med. Surg. 2016, 18, 400–416. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.; Sikanderkhel, S.; Gui, J.; Adeniyi, A.; O’ Dell, K.; Erickson, M.; Malpartida, J.; Mufti, Z.; Khat, T.; Mufti, H.; et al. Thyroid and cardiovascular disease: A focused review on the impact of hyperthyroidism in heart failure. Cardiol. Res. 2020, 11, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Spalla, I.; Locatelli, C.; Riscazzi, G.; Santagostino, S.; Cremaschi, E.; Brambilla, P. Survival in cats with primary and secondary cardiomyopathies. J. Fel. Med. Surg. 2016, 18, 501–509. [Google Scholar] [CrossRef]
- Hart, K.; Durham, A.; Frank, N.; McGowan, C.; Schott, H.; Stewart, A. Recommendations for the Diagnosis and Management of Pituitary Pars Intermedia Dysfunction (PPID); Equine Endocrinology Group: Columbia, MO, USA, 2023. [Google Scholar]
- Heliczer, N.; Lorello, O.; Casoni, D.; Navas de Solís, C. Accuracy and Precision of Noninvasive Blood Pressure in Normo-, Hyper-, and Hypotensive Standing and Anesthetized Adult Horses. J. Vet. Intern. Med. 2016, 30, 866–872. [Google Scholar] [CrossRef]
- Tucker, R.L.; Wickler, S.J.; London, C.; Wyle, A.; Gabbard, M. Selected echocardiographic parameters and right sided pressures of the mule. Equine Vet. J. 1995, 27, 117–119. [Google Scholar] [CrossRef]
- Tucker, R.L.; Wickler, S.J.; London, C.; Wyle, A.; Greene, H.N. Echocardiographic and right-sided cardiac pressure comparison of the mule and horse. J. Equine Vet. Sci. 1995, 15, 404–408. [Google Scholar] [CrossRef]
- Huesler, I.M.; Mitchell, K.J.; Schwarzwald, C.C. Echocardiographic assessment of left atrial size and function in warmblood horses: Reference intervals, allometric scaling and agreement of different echocardiographic variables. J. Vet. Intern. Med. 2016, 30, 1241–1252. [Google Scholar] [CrossRef]
- Berthoud, D.; Schwarzwald, C.C. Echocardiographic assessment of left ventricular size and systolic function in Warmblood horses using linear measurements, area-based indices, and volume estimates: A retrospective database analysis. J. Vet. Intern. Med. 2021, 35, 504–520. [Google Scholar] [CrossRef] [PubMed]
- Fukui, S.; Endo, Y.; Hirayama, K.; Taniyama, H.; Kadosawa, T. Identification and preservation of the parathyroid gland during total thyroidectomy in dogs with bilateral thyroid carcinoma: A report of six cases. J. Vet. Med. Sci. 2015, 77, 747–751. [Google Scholar] [CrossRef] [PubMed]
- Nelson, R.W. Disorders of the thyroid gland. In Small Animal Internal Medicine, 5th ed.; Nelson, R.W., Couto, C.G., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2014; Volume 2, pp. 740–766. [Google Scholar]
- Hyperthyroidism. Available online: https://www.ncbi.nlm.nih.gov/books/NBK537053/ (accessed on 25 January 2022).
- Daniel, G.B.; Neelis, D.A. Thyroid scintigraphy in veterinary medicine. Semin. Nucl. Med. 2014, 44, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Olson, J.A., Jr.; DeBenedetti, M.K.; Baumann, D.S.; Wells, S.A., Jr. Parathyroid autotransplantation during thyroidectomy. Results of long-term follow-up. Ann. Surg. 1996, 223, 472. [Google Scholar] [CrossRef] [PubMed]
- Nabbout, L.A.; Robbings, R.J. The cardiovascular effects of hyperthyroidism. Methodist DeBakey Cardiovasc. J. 2010, 6, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Berta, E.; Lengyel, I.; Halmi, S.; Zrínyi, M.; Erdei, A.; Harangi, M.; Páll, D.; Nagy, E.V.; Bodor, M. Hypertension in thyroid disorders. Front. Endocrinol. 2019, 10, 482. [Google Scholar] [CrossRef]
- Lorell, B.H.; Carabello, B.A. Left ventricular hypertrophy: Pathogenesis, detection and prognosis. Circulation 2000, 102, 470–479. [Google Scholar] [CrossRef]

| Electrolytes (Reference) | Prior to Referral | Pre-op | 12 h Post-op | 24 h Post-op | 2 Days Post-op | 3 Days Post-op | 6 Days Post-op | 17 Days Post-op | 3 Months Post-op | 8 Months Post-op | 10 Months Post-op | 23 Months Post-op | 25 Months Post-op |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Calcium (10.9–12.9 mg/dL) | 12.5 | 11.9 | 13.3 | 11.9 | |||||||||
| Phosphate (2.1–4.7 mg/dL) | 3.0 | 1.7 | 1.9 | 2.6 | |||||||||
| Ionized Calcium (1.4–1.72 mmol/L) | 1.78 | 1.65 | 1.68 | 1.8 | 1.81 | 1.68 | |||||||
| Hormones (reference) | |||||||||||||
| Total T4 (1–3 µg/dL) | 5.78 | 6.7 | 3.4 | 0.45 | <0.05 | 0.58 | 4.4 | 3.5 | 2.32 | ||||
| Free T4 (1.2–1.8 ng/dL) | 8.05 | 7.22 | 4.3 | 0.62 | <0.15 | 0.38 | 2.05 | ||||||
| T3 (30–80 ng/dL) | 204 | 289 | 43.8 | 43.5 | <10 | 74.0 | 90.8 | ||||||
| Parathyroid hormone (1.3–15.0 mg/dL) | 7.8 | ||||||||||||
| Adrenocorticotropic Hormone (Fall: 30–90 pg/mL) | 113 | 217 | 25 | ||||||||||
| Vitamins/minerals (reference) | |||||||||||||
| Alpha-tocopherol (200–1000 µg/dL) | 264 | ||||||||||||
| Selenium (14–24 µg/dL) | 25.34 | 20.08 |
| Measurement (Units) | Available References (mean ± SD) | Pre-Operative | 1 Year Post-Operative | 2 Years Post-Operative |
|---|---|---|---|---|
| Left Atrium | ||||
| LAAmax (cm2) | 92.8 ± 5.0 | 71.4 | 71.8 | 70.9 |
| LADmax (R) (cm) | 11.9 ± 0.7 | 10 | 10.4 | 9.8 |
| LA FAC active (%) | 20 ± 7.0 | 41 | 34 | 30 |
| LADmax (L) (cm) | 12.9 ± 0.5 | 13.3 | 13.1 | 11.2 |
| LAAmax sx (cm2) | 108.8 ± 12.2 | 102.9 | 87.4 | 74.6 |
| LA/Ao (Sx) Ratio | 2.5 ± 0.3 | 2.7 | 2.2 | 2.1 |
| Left Ventricle | ||||
| IVSd (cm) | 2.8 ± 0.3 * | 3.2 | 3.3 | 2.7 |
| LVIDd (cm) | 11.1 ± 1.5 * | 9 | 8.9 | 9.0 |
| LVFWd (cm) | 3.3 ± 0.3 * | 2.6 | 2.6 | 2.8 |
| IVSs (cm) | 4.4 ± 0.4 | 5 | 4.7 | 4.4 |
| LVIDs (cm) | 6.7 ± 1.3 * | 4.2 | 4.6 | 5.0 |
| LVFWs (cm) | 4.4 ± 0.4 | 4.3 | 3.9 | 3.9 |
| FS (%) | 40.4 ± 5.7 * | 54 | 49 | 44 |
| LVIVd (ml) | 1475 ± 200.7 | 694 | 672 | 757 |
| LVIVs (ml) | 412 ± 81.6 | 146 | 148 | 189 |
| SV (mL) | 1065 ± 139.2 | 548 | 524 | 568 |
| CO (L) | 37.1 ± 6.02 | 30.6 | 30.4 | 21.2 |
| EF (%) | 71 ± 4.9 | 79 | 78 | 75 |
| MWT (cm) | 3.05 ± 0.2 * | 2.9 | 2.9 | 2.8 |
| RWT | 0.52 ± 0.2 * | 0.65 | 0.66 | 0.61 |
| LAD/LVID ratio | 1.1 ± 0.1 | 1.11 | 1.17 | 1.09 |
| HR (bpm) | 38 ± 4.4 * | 50 | 57 | 37 |
| Great Vessels | ||||
| PADed (cm) | 6.5 ± 0.47 | 5.8 | 5.8 | 5.9 |
| AoDed (cm) | 7.0 ± 0.9 * | 6.6 | 6.7 | 6.5 |
| PAed-sx (cm) | 5.0 ± 0.31 | 4.1 | 4.1 | 4.7 |
| AoAsx (cm2) | 45 ± 5.5 | 38 | 39.5 | 36 |
| Ao/PA (sx) Ratio | 1.4 ± 0.1 | 1.6 | 1.6 | 1.4 |
| Tissue Doppler Imaging | ||||
| IMP | 0.38 | 0.29 | 0.44 | |
| Em/Am | 3.1 ± 0.8 | 1.7 | 1.2 | 1.9 |
| Velocity | ||||
| AoVmax (m/s) | 1.1 | 1.2 | 0.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brown, K.; Williams Louie, E.; Pinn-Woodcock, T.; Pearson, E.; Pearson, G.B.; Marr, J.; Hackett, E.S.; Rath Brown, L.; Mitchell, K.J. Cardiac Disease Related to Primary Hyperthyroidism in a 20-Year-Old Mule. Animals 2024, 14, 1660. https://doi.org/10.3390/ani14111660
Brown K, Williams Louie E, Pinn-Woodcock T, Pearson E, Pearson GB, Marr J, Hackett ES, Rath Brown L, Mitchell KJ. Cardiac Disease Related to Primary Hyperthyroidism in a 20-Year-Old Mule. Animals. 2024; 14(11):1660. https://doi.org/10.3390/ani14111660
Chicago/Turabian StyleBrown, Kaitlin, Elizabeth Williams Louie, Toby Pinn-Woodcock, Erin Pearson, Garett B. Pearson, Jacqueline Marr, Eileen S. Hackett, Laura Rath Brown, and Katharyn J. Mitchell. 2024. "Cardiac Disease Related to Primary Hyperthyroidism in a 20-Year-Old Mule" Animals 14, no. 11: 1660. https://doi.org/10.3390/ani14111660
APA StyleBrown, K., Williams Louie, E., Pinn-Woodcock, T., Pearson, E., Pearson, G. B., Marr, J., Hackett, E. S., Rath Brown, L., & Mitchell, K. J. (2024). Cardiac Disease Related to Primary Hyperthyroidism in a 20-Year-Old Mule. Animals, 14(11), 1660. https://doi.org/10.3390/ani14111660





