Alleviating Heat Stress in Fattening Pigs: Low-Intensity Showers in Critical Hours Alter Body External Temperature, Feeding Pattern, Carcass Composition, and Meat Quality Characteristics
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Methods
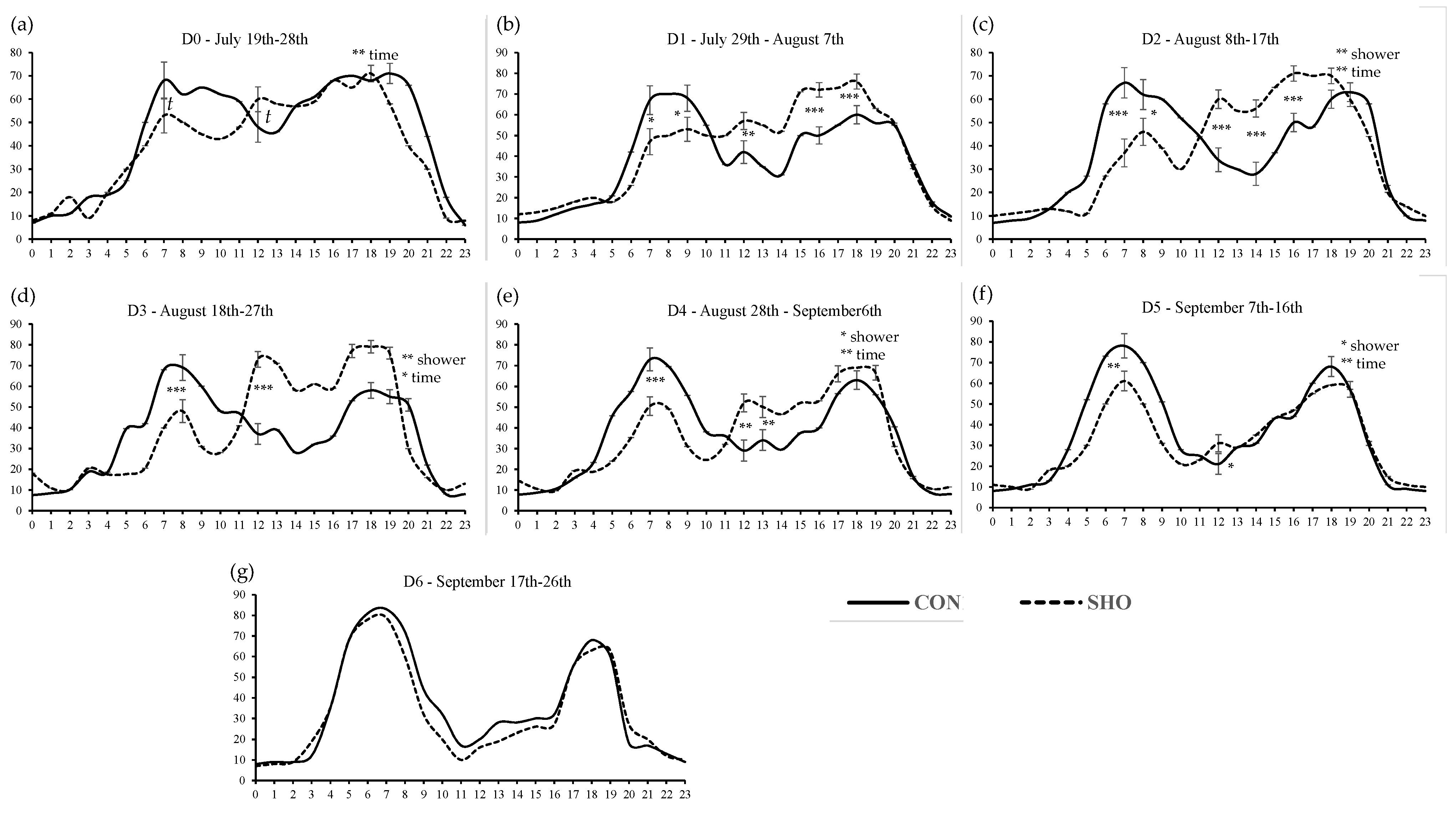
2.1.1. Feeder Occupancy Measurement
2.1.2. Thermographic Measures
2.1.3. Measurements at the Slaughterhouse: AutoFom III and pH
2.1.4. Drip Loss
2.1.5. Color
2.1.6. Intramuscular Fat (IMF) Content and Fatty Acid (FA) Profile
2.1.7. Thiobarbituric Acid Reactive Substances (TBARS)
2.2. Statistical Analysis
3. Results
3.1. Body Temperature, Feeding Pattern, and Carcass Characteristics
3.2. Meat Quality Parameters: pH, Water Holding Capacity, Color, Glycogen, Intramuscular Fat (IMF), Fatty Acid (FA) Profile, and Thiobarbituric Acid Reactive Substances (TBARS)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- González-Rivas, P.A.; Chauhan, S.S.; Ha, M.; Fegan, N.; Dunshea, F.R.; Warner, R.D. Effects of heat stress on animal physiology, metabolism, and meat quality: A review. Meat Sci. 2020, 162, 108025. [Google Scholar] [CrossRef]
- Mayorga, E.J.; Renaudeau, D.; Ramírez, B.C.; Ross, J.W.; Baumgard, L.H. Heat stress adaptations in pigs. Anim. Front. 2019, 9, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Gourdine, J.L.; Rauw, W.M.; Gilbert, H.; Poullet, N. The genetics of thermoregulation in pigs: A review. Front. Vet. Sci. 2021, 8, 770480. [Google Scholar] [CrossRef]
- dos Santos, L.S.; Pomar, C.; Campos, P.H.R.F.; Silva, W.C.; Gobi, J.P.; Veira, A.M.; Fraga, A.Z.; Hauschild, L. Precision feeding strategy for growing pigs under heat stress conditions. J. Anim. Sci. 2018, 96, 4789–4801. [Google Scholar] [CrossRef] [PubMed]
- Quiniou, N.; Dubois, S.; Noblet, J. Voluntary feed intake and feeding behaviour of group-housed growing pigs are affected by ambient temperature and body weight. Livest. Prod. Sci. 2000, 63, 245–253. [Google Scholar] [CrossRef]
- Bus, J.D.; Boumans, I.J.M.M.; Webb, L.E.; Bokkers, E.A.M. The potential of feeding patterns to assess generic welfare in growing-finishing pigs. Appl. Anim. Behav. Sci. 2021, 241, 105383. [Google Scholar] [CrossRef]
- Toft, N.; Madsen, T.N.; Dina, K. Modeling Eating Patterns of Growing Pigs Using Dynamic Linear Models; Royal Veterinary and Agricultural University: Copenhagen, Denmark, 1998. [Google Scholar]
- Weiler, U.; Götz, M.; Schmidt, A.; Otto, M.; Müller, S. Influence of sex and immunocastration on feed intake behavior, skatole and indole concentrations in adipose tissue of pigs. Animal 2013, 7, 300–308. [Google Scholar] [CrossRef]
- Hyun, Y.; Ellis, M. Effect of group size and feeder type on growth performance and feeding patterns in finishing pigs. J. Anim. Sci. 2002, 80, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, R.F.D.; Soares, R.D.T.R.N.; Moreira, R.H.R.; Andrade, R.P.D.; Rosenfield, D.A.; Pizzutto, C.S. Effects of the environmental enrichment on pigs’ behavior and performance. Rev. Bras. Zootec. 2023, 52, e20210123. [Google Scholar] [CrossRef]
- Lee, N.; Choi, J.W.; Ko, H.S.; Ohh, S.J.; Kim, Y.H.; Jang, A.R.; Kim, J.S. Comparison of linear functions to estimate growth performance and feed intake variations pattern in growing and finishing pigs in high ambient temperature. J. Indones. Trop. Anim. Agric. 2019, 44, 177–186. [Google Scholar] [CrossRef]
- Shi, Z.B.; Ma, X.Y.; Zheng, C.T.; Hu, Y.J.; Yang, X.F.; Gao, K.G.; Wang, L.; Jiang, Z.Y. Effects of high ambient temperature on meat quality, serum hormone concentrations, and gene expression in the Longissimus dorsi muscle of finishing pigs. Anim. Prod. Sci. 2017, 57, 1031–1039. [Google Scholar] [CrossRef]
- Rauw, W.M.; de Mercado de la Peña, E.; Gómez-Raya, L.; García Cortés, L.A.; Ciruelos, J.J.; Gómez Izquierdo, E. Impact of environmental temperature on production traits in pigs. Sci. Rep. 2020, 10, 2106. [Google Scholar] [CrossRef]
- Pardo, Z.; Fernández-Fígares, I.; Lachica, M.; Lara, L.; Nieto, R.; Seiquer, I. Impact of heat stress on meat quality and antioxidant markers in iberian pigs. Antioxidants 2021, 10, 1911. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.S. Heat stress: Impact on livestock well-being and productivity and mitigation strategies to alleviate the negative effects. Anim. Prod. Sci. 2018, 58, 1404–1413. [Google Scholar] [CrossRef]
- Fraga, A.Z.; Reis Furtado Campos, P.H.; da Silva, W.C.; Caetano, R.P.; Veira, A.M.; dos Santos, L.S.; Hauschild, L. Sequential feeding with high-fat/low-crude protein diets for two lines of growing-finishing pigs under daily cyclic high ambient temperature conditions. J. Anim. Sci. 2019, 97, 2493–2504. [Google Scholar] [CrossRef]
- Bin-Jumah, M.; Abd El-Hack, M.E.; Abdelnour, S.A.; Hendy, Y.A.; Ghanem, H.A.; Alsafy, S.A.; Khafaga, A.F.; Noreldin, A.E.; Shaheen, H.; Samak, D.; et al. Potential use of chromium to combat thermal stress in animals: A review. Sci. Total Environ. 2020, 707, 135996. [Google Scholar] [CrossRef] [PubMed]
- Barbari, M.; Conti, L. Use of different cooling systems by pregnant sows in experimental pen. Biosyst. Eng. 2009, 103, 239–244. [Google Scholar] [CrossRef]
- Godyn, D.; Herbut, P.; Angrecka, S. Impact of fogging system on thermal comfort of lactating sows. Trans. ASABE 2018, 61, 1933–1938. [Google Scholar] [CrossRef]
- Haeussermann, A.; Hartung, E.; Jungbluth, T.; Vranken, E.; Aerts, J.-M.; Berckmans, D. Cooling effects and evaporation characteristics of fogging systems in an experimental piggery. Biosyst. Eng. 2007, 97, 395–405. [Google Scholar] [CrossRef]
- Huynh, T.T.T.; Aarnink, A.J.A.; Truong, C.T.; Kemp, B.; Verstegen, M.W.A. Effects of tropical climate and water cooling methods on growing pigs’ responses. Livest. Sci. 2006, 104, 278–291. [Google Scholar] [CrossRef]
- BOE. RD 53/2013, de 21 de Octubre por la que se Establecen las Normas Básicas Aplicables para la Protección de los Animales Utilizados en Experimentación y otros Fines Científicos, Incluyendo la Docencia; Boletín Oficial del Estado: Madrid, Spain, 2013; Volume 252, pp. 34367–34391. Available online: https://www.boe.es/buscar/pdf/2013/BOE-A-2013-1337-consolidado.pdf (accessed on 1 May 2024).
- Choi, J.S.; Kwon, K.M.; Lee, Y.K.; Joeng, J.U.; Lee, K.O.; Jin, S.K.; Choi, Y.I.; Lee, J.J. Application of AutoFom III equipment for prediction of primal and commercial cut weight of Korean pig carcasses. Asian-Australas. J. Anim. Sci. 2018, 31, 1670–1676. [Google Scholar] [CrossRef] [PubMed]
- Honikel, K.O. Reference methods for the assessment of physical characteristics of meat. Meat Sci. 1998, 49, 447–457. [Google Scholar] [CrossRef] [PubMed]
- CIE. Recommendations on uniform color spaces, color-difference equations, and metric color terms. Color Res. Appl. 1977, 2, 5–6. [Google Scholar] [CrossRef]
- Segura, J.; Calvo, L.; Óvilo, C.; González-Bulnes, A.; Olivares, Á.; Cambero, M.I.; López-Bote, C.J. Alternative method for intramuscular fat analysis using common laboratory equipment. Meat Sci. 2015, 103, 24–27. [Google Scholar] [CrossRef]
- Calvo, L.; Segura, J.; Toldrá, F.; Flores, M.; Rodríguez, A.I.; López-Bote, C.J.; Rey, A.I. Meat quality, free fatty acid concentration, and oxidative stability of pork from animals fed diets containing different sources of selenium. Food Sci. Technol. Int. 2017, 23, 716–728. [Google Scholar] [CrossRef]
- Salih, A.M.; Smith, D.M.; Price, J.F.; Dawson, L.E. Modified extraction 2-thiobarbituric acid method for measuring lipid oxidation in poultry. Poult. Sci. 1987, 66, 1483–1488. [Google Scholar] [CrossRef]
- SAS Institute Inc. SAS 9.4 for Windows; SAS Institute Inc.: Cary, NC, USA, 2014. [Google Scholar]
- Poullet, N.; Rauw, W.M.; Renaudeau, D.; Riquet, J.; Giorgi, M.; Billon, Y.; Gilbert, H.; Gourdine, J.L. Plasticity of feeding behaviour traits in response to production environment (temperate vs. tropical) in group-housed growing pigs. Sci. Rep. 2022, 12, 847. [Google Scholar] [CrossRef] [PubMed]
- Bus, J.D.; Boumans, I.J.M.M.; Engel, J.; te Beest, D.E.; Webb, L.E.; Bokkers, E.A.M. Circadian rhythms and diurnal patterns in the feed intake behaviour of growing-finishing pigs. Sci. Rep. 2023, 13, 16021. [Google Scholar] [CrossRef]
- Cross, A.J.; Brown-Brandl, T.M.; Keel, B.N.; Cassady, J.P.; Rohrer, G.A. Feeding behavior of grow-finish swine and the impacts of heat stress. Transl. Anim. Sci. 2020, 4, 986–992. [Google Scholar] [CrossRef]
- Fornós, M.; Sanz-Fernández, S.; Jiménez-Moreno, E.; Carrión, D.; Gasa, J.; Rodríguez-Estévez, V. The feeding behaviour habits of growing-finishing pigs and its effects on growth performance and carcass quality: A review. Animals 2022, 12, 1128. [Google Scholar] [CrossRef]
- Cervantes, M.; Antoine, D.; Valle, J.A.; Vásquez, N.; Camacho, R.L.; Bernal, H.; Morales, A. Effect of feed intake level on the body temperature of pigs exposed to heat stress conditions. J. Thermal. Biol. 2018, 76, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Traulsen, I.; Naunin, K.; Muller, K.; Krieter, J. Untersuchungen zum einsatz der infrarotthermographie zur messung der körpertemperatur bei sauen. Zuchtungskunde 2010, 82, 437–446. [Google Scholar]
- Weschenfelder, A.V.; Saucier, L.; Maldague, X.; Rocha, L.M.; Schaefer, A.L.; Faucitano, L. Use of infrared ocular thermography to assess physiological conditions of pigs prior to slaughter and predict pork quality variation. Meat Sci. 2013, 95, 616–620. [Google Scholar] [CrossRef] [PubMed]
- Soerensen, D.D.; Clausen, S.; Mercer, J.B.; Pedersen, L.J. Determining the emissivity of pig skin for accurate infrared thermography. Comput. Electron. Agric. 2014, 109, 52–58. [Google Scholar] [CrossRef]
- Soerensen, D.D.; Pedersen, L.J. Infrared skin temperature measurements for monitoring health in pigs: A review. Acta Vet. Scand. 2015, 57, 5. [Google Scholar] [CrossRef] [PubMed]
- Baumgard, L.H.; Rhoads, R.P. Effects of heat stress on postabsorptive metabolism and energetics. Annu. Rev. Anim. Biosci. 2013, 1, 311–337. [Google Scholar] [CrossRef]
- Renaudeau, D.; Collin, A.; Yahav, S.; de Basilio, V.; Gourdine, J.L.; Collier, R.J. Adaptation to hot climate and strategies to alleviate heat stress in livestock production. Animal 2012, 6, 707–728. [Google Scholar] [CrossRef]
- Srikanth, K.; Park, J.-E.; Ji, S.Y.; Kim, K.H.; Lee, Y.K.; Kumar, H.; Kim, M.; Baek, Y.C.; Kim, H.; Jang, G.W.; et al. Genome-Wide Transcriptome and Metabolome analyses provide novel insights and suggest a sex-specific response to heat stress in pigs. Genes 2020, 11, 540. [Google Scholar] [CrossRef] [PubMed]
- Lebret, B.; Čandek-Potokar, M. Review: Pork quality attributes from farm to fork. Part I. Carcass and fresh meat. Animal 2022, 16, 100402. [Google Scholar] [CrossRef]
- Yang, P.; Feng, Y.; Hao, Y.; Gu, X.; Yang, C.; Cao, Z. Effects of constant heat stress on performance, carcass traits, nutrition content and myofiber characteristics of Longissimus dorsi in finishing pigs. Chin. Anim. Nutr. 2014, 26, 2503–2512. [Google Scholar]
- Gao, J.; Yang, P.; Cui, Y.; Meng, Q.; Feng, Y.; Hao, Y.; Liu, J.; Piao, X.; Gu, X. Identification of metabonomics changes in Longissimus dorsi muscle of finishing pigs following heat stress through LC-MS/MS-Based Metabonomics method. Animals 2020, 10, 129. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Feng, Y.; Yang, P.; Feng, J.; Lin, H.; Gu, X. Nutritional and physiological responses of finishing pigs exposed to a permanent heat exposure during three weeks. Arch. Anim. Nutr. 2014, 68, 296–308. [Google Scholar] [CrossRef] [PubMed]
- Segura, J.; Escudero, R.; Romero de Ávila, M.D.; Cambero, M.I.; López-Bote, C.J. Effect of fatty acid composition and positional distribution within the triglyceride on selected physical properties of dry-cured ham subcutaneous fat. Meat Sci. 2015, 103, 90–95. [Google Scholar] [CrossRef]
- Dinh, T.T.; To, K.V.; Schilling, M.W. Fatty acid composition of meat animals as flavor precursors. Meat Muscle Biol. 2021, 5, 1–16. [Google Scholar] [CrossRef]
- Kouba, M.; Hermier, D.; Le Dividich, J. Influence of a high ambient temperature on stearoyl-CoA-desaturase activity in the growing pig. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 1999, 124, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Kloareg, M.; Le Bellego, L.; Mourot, J.; Noblet, J.; van Milgen, J. Deposition of dietary fatty acids and of de novo synthesised fatty acids in growing pigs: Effects of high ambient temperature and feeding restriction. Br. J. Nutr. 2005, 93, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Yi, W.; Huang, Q.; Wang, Y.; Shan, T. Lipo-nutritional quality of pork: The lipid composition, regulation, and molecular mechanisms of fatty acid deposition. Anim. Nutr. 2023, 13, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Qu, H.; Ajuwon, K.M. Adipose tissue-specific responses reveal an important role of lipogenesis during heat stress adaptation in pigs. J. Anim. Sci. 2018, 96, 975–989. [Google Scholar] [CrossRef] [PubMed]
- Qu, H.; Ajuwon, K.M. Metabolomics of heat stress response in pig adipose tissue reveals alteration of phospholipid and fatty acid composition during heat stress. J. Anim. Sci. 2018, 96, 3184–3195. [Google Scholar] [CrossRef]
- Zappaterra, M.; Catillo, G.; Lo Fiego, D.P.; Belmonte, A.M.; Padalino, B.; Davoli, R. Describing backfat and Semimembranosus muscle fatty acid variability in heavy pigs: Analysis of non–genetic factors. Meat Sci. 2022, 183, 108645. [Google Scholar] [CrossRef]
- Heng, J.; Tian, M.; Zhang, W.; Chen, F.; Guan, W.; Zhang, S. Maternal heat stress regulates the early fat deposition partly through modification of m6A RNA methylation in neonatal piglets. Cell Stress Chaperon. 2019, 24, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Paton, C.M.; Ntambi, J.M. Biochemical and physiological function of stearoyl-CoA desaturase. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E28–E37. [Google Scholar] [CrossRef] [PubMed]
- Vessby, B.; Gustafsson, I.B.; Tengblad, S.; Boberg, M.; Andersson, A. Desaturation and elongation of fatty acids and insulin action. Ann. N. Y. Acad. Sci. 2002, 967, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, S.; Summers, C.M.; Pearce, S.C.; Gabler, N.K.; Valentine, R.J.; Baumgard, L.H.; Rhoads, R.P.; Selsby, J.T. Short-term heat stress altered metabolism and insulin signaling in skeletal muscle. J. Anim. Sci. 2018, 96, 154–167. [Google Scholar] [CrossRef]
- Seibert, J.T.; Abuajamieh, M.; Sanz Fernández, M.V.; Johnson, J.S.; Kvidera, S.K.; Horst, E.A.; Mayorga, E.J.; Lei, S.; Patience, J.F.; Ross, J.W.; et al. Effects of heat stress and insulin sensitizers on pig adipose tissue. J. Anim. Sci. 2018, 96, 510–520. [Google Scholar] [CrossRef]

| Anatomical Location | Gilts (n = 60) | Barrows (n = 60) | RMSE 1 | p-Value 2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CON | SHO | CON | SHO | S | T | S × T | ||||||
| Eye | 36.74 | a | 35.86 | b | 36.27 | ab | 36.09 | b | 0.1974 | NS | ** | * |
| Ear INT | 36.83 | a | 34.67 | b | 36.42 | a | 34.41 | b | 0.3430 | NS | *** | NS |
| Ear EXT | 37.29 | a | 33.42 | c | 36.97 | a | 35.81 | b | 0.6084 | * | *** | ** |
| Nose | 33.76 | a | 31.67 | b | 33.52 | a | 32.57 | ab | 0.2894 | NS | ** | 0.0524 |
| Shoulder | 37.68 | a | 34.95 | c | 37.33 | a | 35.83 | b | 0.5289 | NS | *** | * |
| Loin | 37.40 | a | 35.89 | c | 37.41 | a | 36.71 | b | 0.4003 | * | *** | * |
| Belly | 37.85 | a | 35.88 | b | 37.79 | a | 37.01 | a | 0.3953 | 0.0685 | *** | * |
| Ham | 37.39 | a | 35.96 | c | 37.43 | a | 36.61 | b | 0.4198 | 0.0894 | *** | 0.0828 |
| CON (n = 192) | SHO (n = 190) | RMSE | p-Value | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ♀ | ♂ | ♀ | ♂ | S | T | S × T | |||||||
| HCW (kg) | 92.98 | b | 88.95 | c | 97.95 | a | 93.00 | b | 1.099 | *** | *** | NS | |
| LMY (%) | 58.99 | b | 57.39 | c | 61.17 | a | 59.90 | ab | 1.548 | 0.0885 | * | NS | |
| Fat width (mm) | LD | 16.51 | b | 17.91 | ab | 19.05 | a | 19.20 | a | 1.298 | NS | ** | * |
| GL | 11.88 | b | 12.88 | ab | 14.01 | a | 14.32 | a | 1.377 | NS | * | * | |
| CON (n = 20) | SHO (n = 20) | RMSE | p-Value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ♀ | ♂ | ♀ | ♂ | S | T | S × T | ||||||
| Drip loss (%) | 12.08 | a | 12.56 | a | 10.48 | b | 10.49 | b | 1.033 | NS | ** | NS |
| TBARS (mg MDA/kg) | 0.382 | a | 0.421 | a | 0.321 | b | 0.330 | b | 0.0301 | NS | ** | NS |
| IMF (%) | 2.041 | b | 2.557 | ab | 2.822 | a | 3.131 | a | 0.517 | 0.0744 | ** | NS |
| Glycogen (µmol/g) | 1.000 | a | 0.800 | b | 1.095 | a | 0.865 | b | 0.2000 | 0.0712 | NS | NS |
| L* | 56.19 | b | 57.67 | a | 55.38 | c | 56.06 | b | 1.281 | * | * | NS |
| a* | 3.712 | b | 3.892 | b | 4.297 | a | 4.738 | a | 0.4193 | NS | * | NS |
| b* | 6.584 | b | 6.842 | ab | 7.060 | ab | 7.689 | a | 0.7403 | 0.0550 | * | NS |
| CON (n = 20) | SHO (n = 20) | RMSE | p-Value | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ♀ | ♂ | ♀ | ♂ | S | T | S × T | |||||||
| INNER | |||||||||||||
| C16:0 | 22.61 | 22.74 | 22.17 | 22.09 | 1.013 | NS | NS | NS | |||||
| C16:1n-7 | 1.506 | d | 1.840 | c | 2.034 | b | 2.464 | a | 0.2693 | ** | ** | NS | |
| C18:0 | 14.70 | a | 14.53 | a | 13.10 | b | 13.05 | b | 0.5885 | NS | * | NS | |
| C18:1n-9 | 38.52 | b | 37.60 | c | 40.71 | a | 40.37 | a | 0.8237 | 0.1311 | * | 0.0999 | |
| C18:2n-6 | 15.81 | a | 15.91 | a | 13.60 | b | 13.25 | b | 0.8206 | NS | ** | NS | |
| ΣSFA | 39.16 | a | 39.21 | a | 37.10 | b | 37.02 | b | 0.9290 | NS | * | NS | |
| ΣMUFA | 43.71 | b | 43.69 | b | 47.75 | a | 48.31 | a | 1.685 | NS | ** | NS | |
| ΣPUFA | 17.13 | a | 17.09 | a | 15.15 | b | 14.67 | b | 1.555 | NS | ** | NS | |
| Δ9 | 0.5090 | b | 0.5051 | b | 0.5388 | a | 0.5400 | a | 0.0202 | NS | * | NS | |
| Δ5 | 0.7042 | b | 0.7812 | a | 0.6968 | b | 0.7885 | a | 0.0302 | * | NS | NS | |
| OUTER | |||||||||||||
| C16:0 | 22.77 | a | 22.67 | a | 21.01 | b | 20.93 | b | 0.9656 | NS | * | NS | |
| C16:1n-7 | 2.292 | b | 2.671 | ab | 2.444 | ab | 2.958 | a | 0.2582 | * | NS | NS | |
| C18:0 | 11.54 | 11.56 | 10.90 | 10.73 | 0.9881 | NS | NS | NS | |||||
| C18:1n-9 | 39.91 | b | 38.57 | b | 41.90 | a | 41.33 | a | 1.184 | NS | * | NS | |
| C18:2n-6 | 15.88 | 15.93 | 15.45 | 15.41 | 1.270 | NS | NS | NS | |||||
| ΣSFA | 36.35 | a | 36.24 | a | 33.84 | b | 33.55 | b | 1.783 | NS | ** | NS | |
| ΣMUFA | 46.09 | b | 46.20 | b | 48.90 | a | 49.36 | a | 1.401 | NS | ** | NS | |
| ΣPUFA | 17.57 | 17.57 | 17.25 | 17.09 | 1.393 | NS | NS | NS | |||||
| Δ9 | 0.5414 | b | 0.5365 | b | 0.5713 | a | 0.5731 | a | 0.0182 | NS | * | NS | |
| Δ5 | 0.6985 | c | 0.7712 | b | 0.7382 | c | 0.8029 | a | 0.0272 | * | * | NS | |
| CON (n = 20) | SHO (n = 20) | RMSE | p-Value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ♀ | ♂ | ♀ | ♂ | S | T | S × T | ||||||
| C16:0 | 23.44 | a | 22.36 | ab | 23.14 | a | 21.87 | b | 1.217 | *** | 0.1213 | NS |
| C16:1n-7 | 3.203 | a | 3.064 | ab | 3.422 | a | 2.858 | b | 0.4340 | * | NS | 0.1451 |
| C18:0 | 12.80 | a | 12.07 | ab | 11.83 | b | 12.25 | ab | 0.8646 | NS | NS | 0.0782 |
| C18:1n-9 | 38.44 | b | 37.40 | c | 40.58 | a | 38.74 | b | 2.004 | * | * | NS |
| C18:2n-6 | 11.47 | ab | 13.12 | a | 9.921 | b | 11.63 | ab | 1.858 | * | * | NS |
| ΣSFA | 38.07 | a | 36.24 | b | 36.82 | ab | 35.91 | b | 1.728 | * | 0.1650 | 0.1898 |
| ΣMUFA | 45.98 | bc | 45.21 | c | 48.69 | a | 46.31 | bc | 2.351 | * | * | 0.0551 |
| ΣPUFA | 15.95 | ab | 18.56 | a | 14.49 | b | 17.78 | a | 3.235 | ** | 0.1405 | 0.0662 |
| Δ9 | 0.5256 | c | 0.5314 | bc | 0.5476 | a | 0.5409 | ab | 0.0147 | NS | * | NS |
| Δ5 | 0.8700 | 0.8753 | 0.8758 | 0.8775 | 0.0157 | NS | NS | NS | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Segura, J.; Calvo, L.; Escudero, R.; Rodríguez, A.I.; Olivares, Á.; Jiménez-Gómez, B.; López-Bote, C.J. Alleviating Heat Stress in Fattening Pigs: Low-Intensity Showers in Critical Hours Alter Body External Temperature, Feeding Pattern, Carcass Composition, and Meat Quality Characteristics. Animals 2024, 14, 1661. https://doi.org/10.3390/ani14111661
Segura J, Calvo L, Escudero R, Rodríguez AI, Olivares Á, Jiménez-Gómez B, López-Bote CJ. Alleviating Heat Stress in Fattening Pigs: Low-Intensity Showers in Critical Hours Alter Body External Temperature, Feeding Pattern, Carcass Composition, and Meat Quality Characteristics. Animals. 2024; 14(11):1661. https://doi.org/10.3390/ani14111661
Chicago/Turabian StyleSegura, José, Luis Calvo, Rosa Escudero, Ana Isabel Rodríguez, Álvaro Olivares, Beatriz Jiménez-Gómez, and Clemente José López-Bote. 2024. "Alleviating Heat Stress in Fattening Pigs: Low-Intensity Showers in Critical Hours Alter Body External Temperature, Feeding Pattern, Carcass Composition, and Meat Quality Characteristics" Animals 14, no. 11: 1661. https://doi.org/10.3390/ani14111661





