Insight into Body Condition Variability in Small Mammals
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
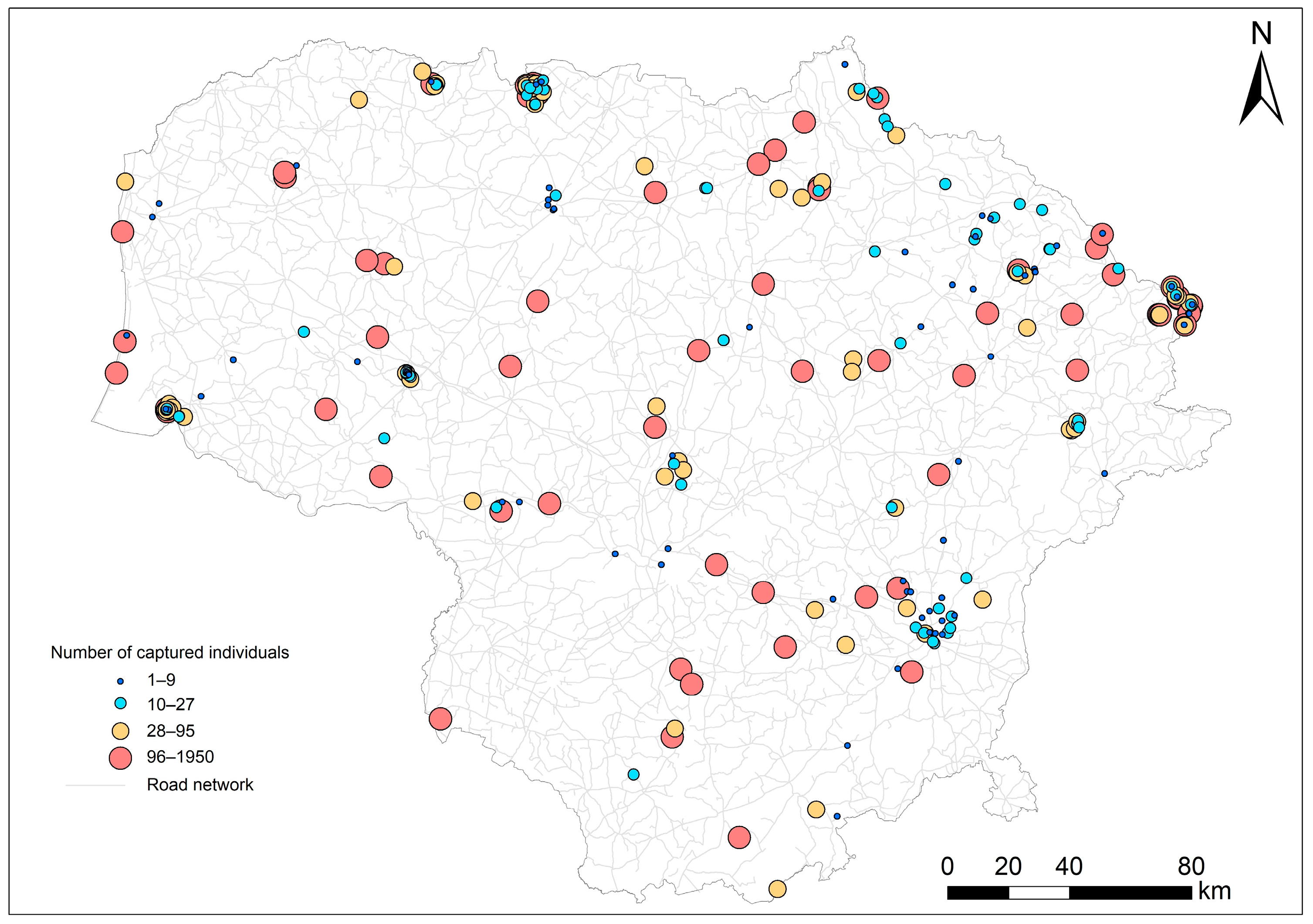
2.1. Small Mammal Collection Methods
2.2. Small Mammal Trapping and Sample Size
2.3. Body Size and Body Condition Index
2.4. Estimation of Small Mammal Age and Reproductive Status
2.5. Data Analysis
3. Results
3.1. BCI Differences in Relation to Small Mammal Species
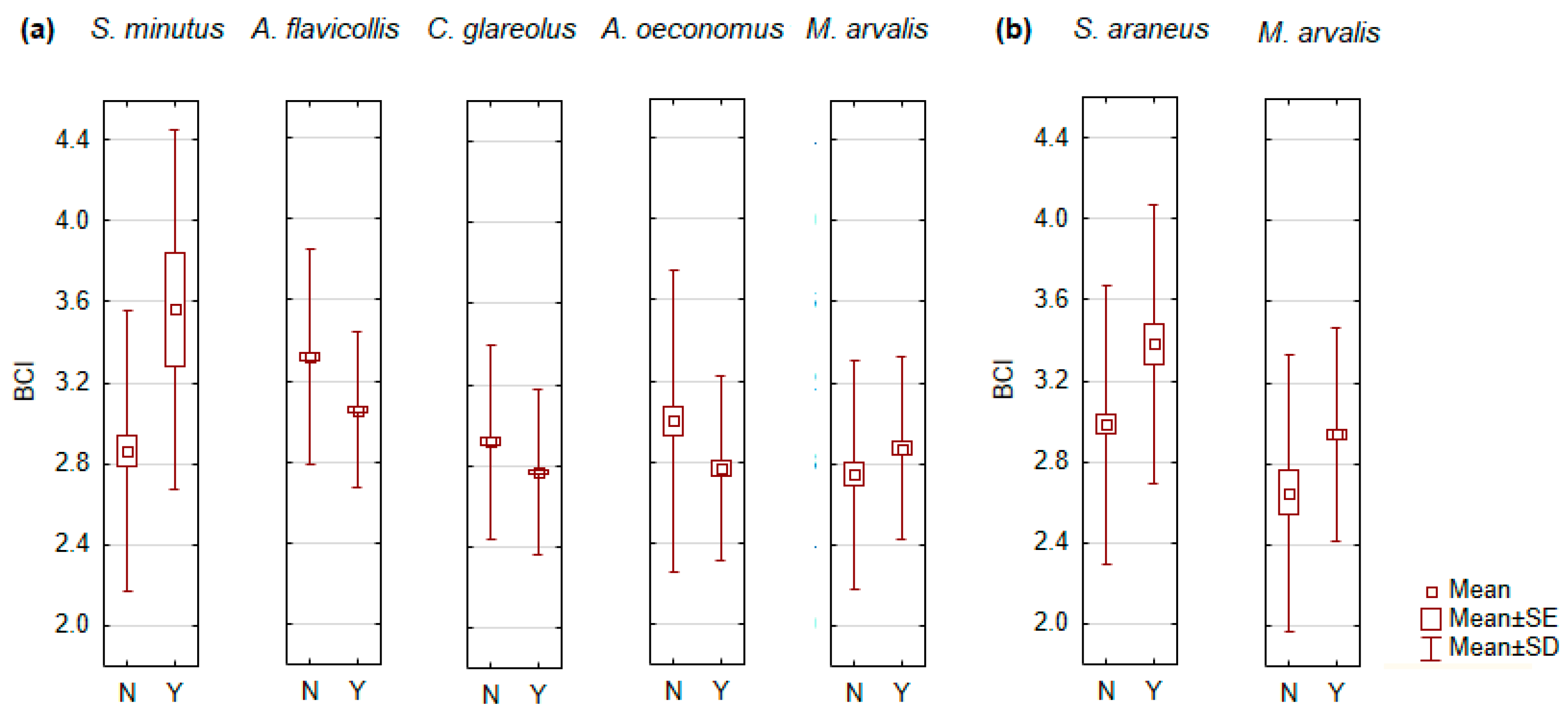
3.2. Age of Individual and Body Condition
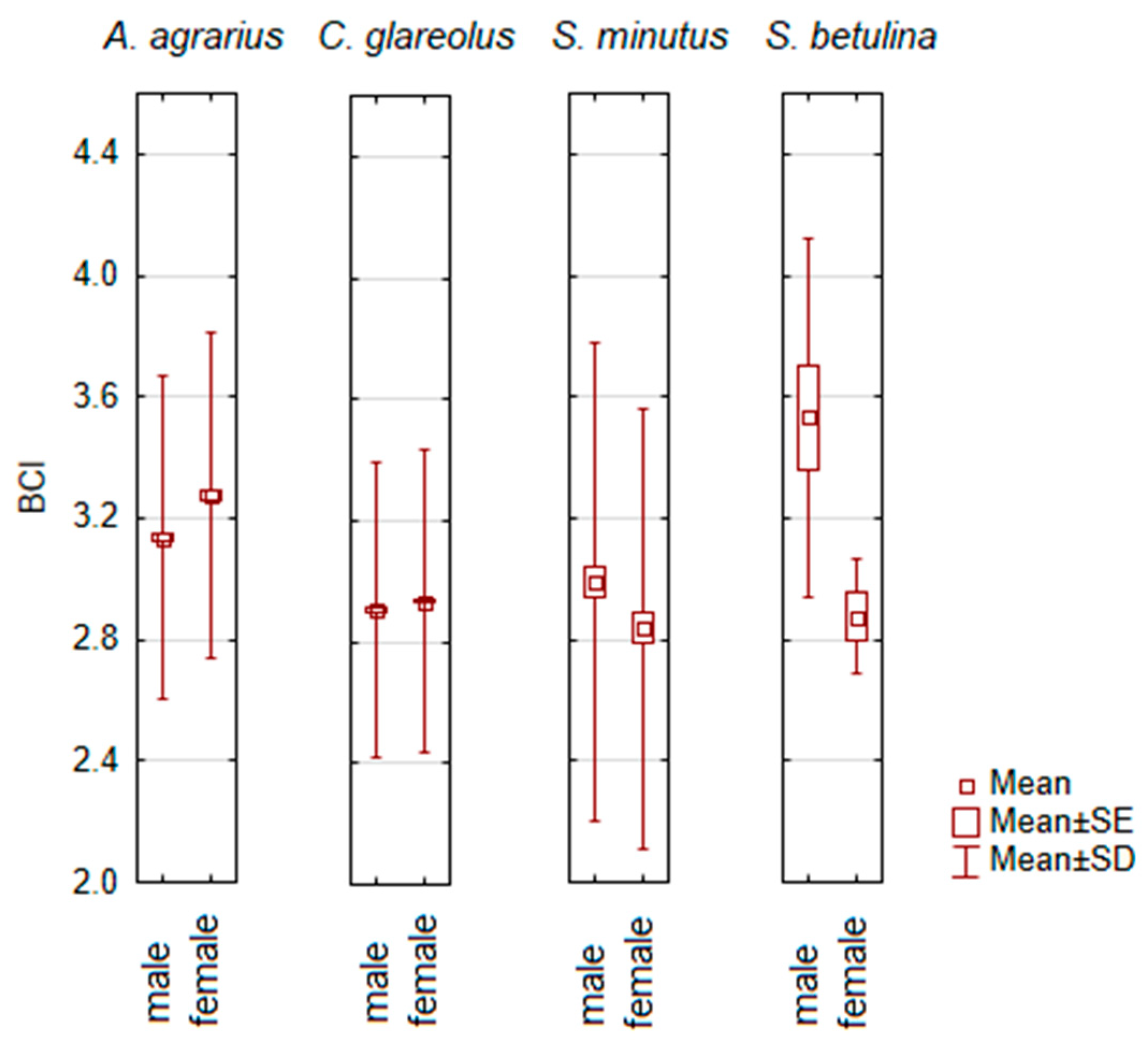
3.3. Gender of Individual and Body Condition
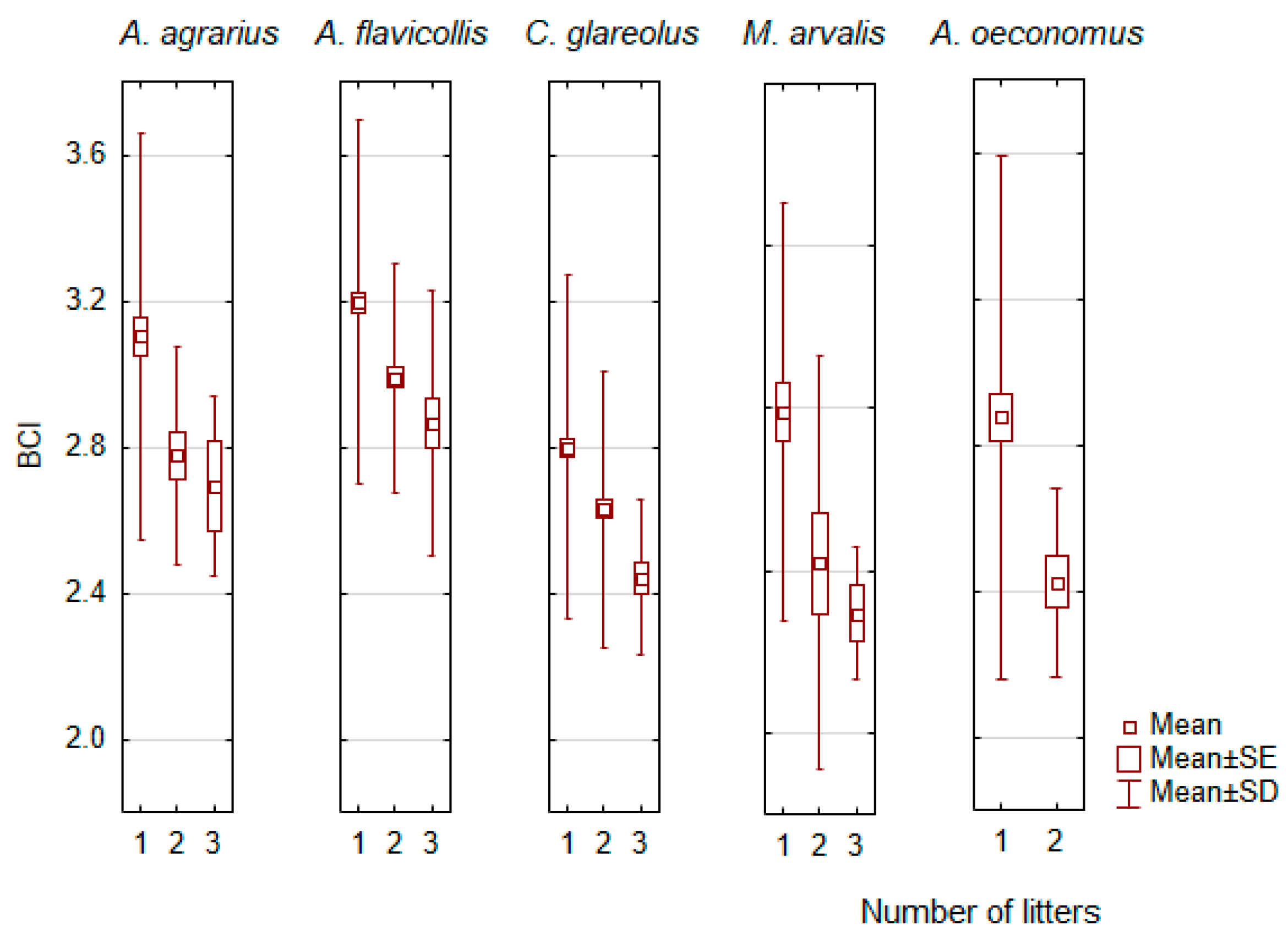
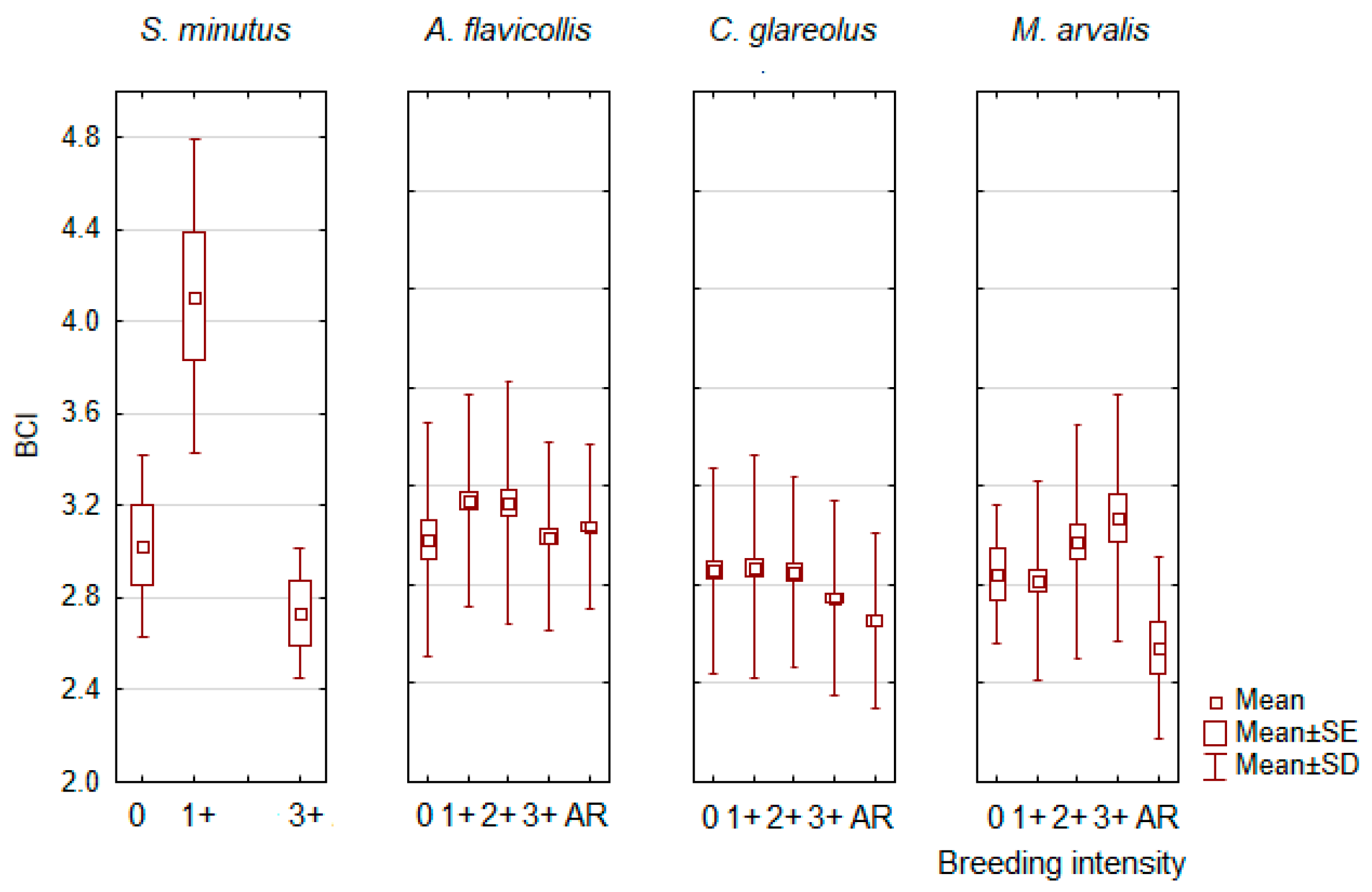
3.4. Influence of the Reproductive Status
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barnett, C.A.; Suzuki, T.N.; Sakaluk, S.K.; Thompson, C.F. Mass-based condition measures and their relationship with fitness: In what condition is condition? J. Zool. 2015, 296, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Wilder, S.M.; Raubenheimer, D.; Simpson, S.J. Moving beyond body condition indices as an estimate of fitness in ecological and evolutionary studies. Funct. Ecol. 2016, 30, 108–115. [Google Scholar] [CrossRef]
- Stevenson, R.D.; Woods, W.A., Jr. Condition indices for conservation: New uses for evolving tools. Integr. Comp. Biol. 2006, 46, 1169–1190. [Google Scholar] [CrossRef] [PubMed]
- Moya-Laraño, J.; Macías-Ordóñez, R.; Blanckenhorn, W.U.; Fernández-Montraveta, C. Analysing body condition: Mass, volume or density? J. Anim. Ecol. 2008, 77, 1099–1108. [Google Scholar] [CrossRef] [PubMed]
- Wishart, A.E.; Guerrero-Chacón, A.L.; Smith, R.; Hawkshaw, D.M.; McAdam, A.G.; Dantzer, B.; Boutin, S.; Lane, J.E. Inferring condition in wild mammals: Body condition indices confer no benefit over measuring body mass across ecological contexts. Oecologia 2024, 204, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Ergon, T.; Speakman, J.R.; Scantlebury, M.; Cavanagh, R.; Lambin, X. Optimal body size and energy expenditure during winter: Why are voles smaller in declining populations? Am. Nat. 2004, 163, 442–457. [Google Scholar] [CrossRef] [PubMed]
- Andersson, M. The evolution of condition-dependent sex ornaments and mating preferences: Sexual selection based on viability differences. Evolution 1986, 40, 804–816. [Google Scholar] [CrossRef]
- Ross, J.G.B.; Newman, C.; Buesching, C.D.; Connolly, E.; Nakagawa, S.; Macdonald, D.W. A fat chance of survival: Body condition provides life-history dependent buffering of environmental change in a wild mammal population. Clim. Chang. Ecol. 2021, 2, 100022. [Google Scholar] [CrossRef]
- Dehnel, A. Studies on the genus Sorex L. Ann. Univ. M. Curie-Sklod. 1949, 4, 17–102. [Google Scholar]
- Taylor, J.R.; Rychlik, L.; Churchfield, S. Winter reduction in body mass in a very small, nonhibernating mammal: Consequences for heat loss and metabolic rates. Physiol. Biochem. Zool. 2013, 86, 9–18. [Google Scholar] [CrossRef]
- Wunder, B.A.; Dobkin, D.S.; Gettinger, R.D. Shifts of thermogenesis in the prairie vole (Microtus ochrogaster). Oecologia 1977, 29, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Jackson, D.M.; Trayhurn, P.; Speakman, J.R. Associations between energetics and over-winter survival in the short-tailed field vole Microtus agrestis. J. Anim. Ecol. 2001, 70, 633–640. [Google Scholar] [CrossRef]
- Aars, J.; Ims, R.A. Intrinsic and climatic determinants of population demography: The winter dynamics of tundra voles. Ecology 2002, 83, 3449–3456. [Google Scholar] [CrossRef]
- Wan, X.; Zhang, X.; Wang, G.; Chen, L. Optimal body weight of Brandt’s voles for winter survival. J. Arid Environ. 2014, 103, 31–35. [Google Scholar] [CrossRef]
- Balčiauskienė, L.; Balčiauskas, L.; Čepukienė, A. Winter growth depression of common vole (Microtus arvalis). Acta Zool. Litu. 2009, 19, 85–92. [Google Scholar] [CrossRef]
- Keane, B.; Bryant, L.; Goyal, U.; Williams, S.; Kortering, S.L.; Lucia, K.E.; Richmond, A.R.; Solomon, N.G. No effect of body condition at weaning on survival and reproduction in prairie voles. Can. J. Zool. 2007, 85, 718–727. [Google Scholar] [CrossRef]
- Sibly, R.M.; Brown, J.H. Effects of body size and lifestyle on evolution of mammal life histories. Proc. Natl. Acad. Sci. USA 2007, 104, 17707–17712. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.M.; Lajeunesse, M.J.; Rohr, J.R. A global synthesis of animal phenological responses to climate change. Nat. Clim. Chang. 2018, 8, 224–228. [Google Scholar] [CrossRef]
- Moors, P.J. Norway rats (Rattus norvegicus) on the Noises and Motukawao islands, Hauraki Gulf, New Zealand. N. Z. J. Ecol. 1985, 8, 37–54. [Google Scholar]
- Krebs, C.J.; Singleton, G.R. Indexes of condition for small mammals. Aust. J. Zool. 1993, 41, 317–323. [Google Scholar] [CrossRef]
- Blackwell, G.L. A potential multivariate index of condition for small mammals. N. Z. J. Zool. 2002, 29, 195–203. [Google Scholar] [CrossRef]
- Peig, J.; Green, A.J. The paradigm of body condition: A critical reappraisal of current methods based on mass and length. Funct. Ecol. 2010, 24, 1323–1332. [Google Scholar] [CrossRef]
- Wauters, A.L.; Vermeulen, M.; Van Dongen, S.; Bertolino, S.; Molinari, A.; Tosi, G.; Matthysen, E. Effects of spatio-temporal variation in food supply on red squirrel Sciurus vulgaris body size and body mass and its consequences for some fitness components. Ecography 2007, 30, 51–65. [Google Scholar] [CrossRef]
- Adjapong, A.O.; Oppong, S.K.; Danquah, E.; Nsor, C.A.; Kumi, S. Habitat effects on morphometrics and body condition of Tullberg’s soft-furred mouse (Praomys tullbergi) in two tropical forests. Glob. Ecol. Conserv. 2023, 48, e02741. [Google Scholar] [CrossRef]
- Delciellos, A.C.; Barros, C.D.S.D.; Prevedello, J.A.; Ferreira, M.S.; Cerqueira, R.; Vieira, M.V. Habitat fragmentation affects individual condition: Evidence from small mammals of the Brazilian Atlantic Forest. J. Mammal. 2018, 99, 936–945. [Google Scholar] [CrossRef]
- Afonso, B.C.; Matias, G.; Teixeira, D.; Pereira, R.; Rosalino, L.M. Determinants of Small Mammals’ Body Condition in Eucalyptus Dominated Landscapes. Sustainability 2023, 16, 128. [Google Scholar] [CrossRef]
- Schweiger, B.R.; Frey, J.K.; Cain, J.W., III. A case for multiscale habitat selection studies of small mammals. J. Mammal. 2021, 102, 1249–1265. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Chardi, A.; García-Pando, M.; López-Fuster, M.J. Chronic exposure to environmental stressors induces fluctuating asymmetry in shrews inhabiting protected Mediterranean sites. Chemosphere 2013, 93, 916–923. [Google Scholar] [CrossRef] [PubMed]
- Tête, N.; Fritsch, C.; Afonso, E.; Coeurdassier, M.; Lambert, J.C.; Giraudoux, P.; Scheifler, R. Can body condition and somatic indices be used to evaluate metal-induced stress in wild small mammals? PLoS ONE 2013, 8, e66399. [Google Scholar] [CrossRef]
- Balciauskas, L.; Balciauskiene, L.; Jasiulionis, M. Mammals under a colony of great cormorants: Population structure and body condition of yellow-necked mice. Turk. J. Zool. 2015, 39, 941–948. [Google Scholar] [CrossRef]
- Brochu, L.; Caron, L.; Bergeron, J.M. Diet quality and body condition of dispersing and resident voles (Microtus pennsylvanicus). J. Mammal. 1988, 69, 704–710. [Google Scholar] [CrossRef]
- Hansson, L. Condition and diet in relation to habitat in bank voles Clethrionomys glareolus: Population or community approach? Oikos 1979, 33, 55–63. [Google Scholar] [CrossRef]
- Gliwicz, J. Dispersal in Bank voles: Benefits to emigrants or to residents? Acta Theriol. 1993, 38, 31–38. [Google Scholar] [CrossRef]
- Lidicker, W.Z., Jr.; Ostfeld, R.S. Extra-large body size in California voles: Causes and fitness consequences. Oikos 1991, 61, 108–121. [Google Scholar] [CrossRef]
- Suchomel, J.; Heroldova, M. Population of Apodemus flavicollis in three large isolated forests under various environmental conditions in southern Moravia (the Czech Republic). Ekol. Bratisl. 2006, 25, 377–386. [Google Scholar]
- Zduniak, M.; Serafini, S.; Wróbel, A.; Zwolak, R. Host body mass, not sex, affects ectoparasite loads in yellow-necked mouse Apodemus flavicollis. Parasitol. Res. 2023, 122, 2599–2607. [Google Scholar] [CrossRef]
- Velickovic, M. Measures of the developmental stability, body size and body condition in the black-striped mouse (Apodemus agrarius) as indicators of a disturbed environment in northern Serbia. Belg. J. Zool. 2007, 137, 147. [Google Scholar]
- Alcántara, M.; Díaz, M. Patterns of body weight, body size, and body condition in the wood mouse Apodemus sylvaticus L.: Effects of sex and habitat quality. In Proceedings of the I European Congress of Mammalogy, Lisboa, Portugal, 18–23 March 1991. [Google Scholar]
- Fragoso, R.; Santos-Reis, M.; Rosalino, L.M. Cattle grazing in agroforest landscape. Eur. J. Wildl. Res. 2020, 66, 13. [Google Scholar] [CrossRef]
- Ylönen, H.; Eccard, J.A. Does quality of winter food affect spring condition and breeding in female bank voles (Clethrionomys glareolus)? Écoscience 2004, 11, 1–5. [Google Scholar] [CrossRef]
- Karlsson, A.F. Over-winter survival in a boreal population of the bank vole, Clethrionomys glareolus. Can. J. Zool. 1988, 66, 1835–1840. [Google Scholar] [CrossRef]
- Zub, K.; Borowski, Z.; Szafrańska, P.A.; Wieczorek, M.; Konarzewski, M. Lower body mass and higher metabolic rate enhance winter survival in root voles, Microtus oeconomus. Biol. J. Linn. Soc. 2014, 113, 297–309. [Google Scholar] [CrossRef]
- Rémy, A.; Le Galliard, J.F.; Gundersen, G.; Steen, H.; Andreassen, H.P. Effects of individual condition and habitat quality on natal dispersal behaviour in a small rodent. J. Anim. Ecol. 2011, 80, 929–937. [Google Scholar] [CrossRef] [PubMed]
- Agrell, J.; Erlinge, S.; Nelson, J.; Sandell, M. Body weight and population dynamics: Cyclic demography in a noncyclic population of the field vole (Microtus agrestis). Can. J. Zool. 1992, 70, 494–501. [Google Scholar] [CrossRef]
- Norrdahl, K.; Korpimäki, E. Changes in individual quality during a 3-year population cycle of voles. Oecologia 2002, 130, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Frafjord, K. Can environmental factors explain size variation in the common shrew (Sorex araneus)? Mamm. Biol. 2008, 73, 415–422. [Google Scholar] [CrossRef]
- White, T.A.; Searle, J.B. Factors explaining increased body size in common shrews (Sorex araneus) on Scottish islands. J. Biogeogr. 2007, 34, 356–363. [Google Scholar] [CrossRef]
- Balčiauskas, L.; Stirkė, V.; Balčiauskienė, L. Rodent fertility in commercial orchards in relation to body mass and body condition. Agric. Ecosyst. Environ. 2022, 329, 107886. [Google Scholar] [CrossRef]
- Balčiauskas, L.; Balčiauskienė, L. Small Mammal Diversity Changes in a Baltic Country, 1975–2021: A Review. Life 2022, 12, 1887. [Google Scholar] [CrossRef]
- Prūsaitė, J. Fauna of Lithuania. Mammals; Mokslas: Vilnius, Lithuania, 1988; pp. 28–43. [Google Scholar]
- Butet, A.; Delettre, Y.R. Diet differentiation between European arvicoline and murine rodents. Acta Theriol. 2011, 56, 297–304. [Google Scholar] [CrossRef]
- Myllymaki, A. Demographic mechanisms in the fluctuating populations of the field vole Microtus agrestis. Oikos 1977, 29, 468–493. [Google Scholar] [CrossRef]
- Crespin, L.; Verhagen, R.; Stenseth, N.C.; Yoccoz, N.G.; Prevot-Julliard, A.C.; Lebreton, J.D. Survival in fluctuating bank vole populations: Seasonal and yearly variations. Oikos 2002, 98, 467–479. [Google Scholar] [CrossRef]
- O’Rourke, D. Sexing or Gender Determination in Small Mammals. 3 June 2010. LafeberVet Web Site. Available online: https://lafeber.com/vet/sexing-or-gender-determination-in-small-mammals/ (accessed on 15 April 2024).
- Terman, R.C. Weights of selected organs of deer mice (Peromyscus maniculatus bairdii) from asymptotic laboratory populations. J. Mammal. 1969, 50, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Massányi, P.; Jancova, A.; Uhrín, V. Morphometric study of male reproductive organs in the rodent species Apodemus sylvaticus and Apodemus flavicollis. Bull.-Vet. Inst. Pulawy 2003, 47, 133–138. [Google Scholar]
- Cook, M.J. The Anatomy of the Laboratory Mouse. Available online: https://www.informatics.jax.org/cookbook/figures/figure65.shtml (accessed on 30 April 2024).
- Accialini, P.; Hernandez, S.F.; Abramovich, D.; Tesone, M. The rodent corpus luteum. In The Life Cycle of the Corpus Luteum; Meidan, R., Ed.; Springer: Cham, Switzerland, 2017; pp. 117–131. [Google Scholar]
- Wydoski, R.S.; Davis, D.E. The occurrence of placental scars in mammals. Proc. Pa. Acad. Sci. 1961, 35, 197–204. [Google Scholar]
- Martin, K.H.; Stehn, R.A.; Richmond, M.E. Reliability of placental scar counts in the prairie vole. J. Wildl. Manag. 1976, 40, 264–271. [Google Scholar] [CrossRef]
- Past 4—The Past of the Future. Available online: https://www.nhm.uio.no/english/research/resources/past/ (accessed on 1 January 2024).
- GLM in R: Generalized Linear Model. Available online: https://www.datacamp.com/tutorial/generalized-linear-models (accessed on 30 October 2023).
- TIBCO Software Inc. Data Science Textbook. Available online: https://docs.tibco.com/data-science/textbook (accessed on 15 January 2024).
- Juškaitis, R.; Baltrūnaitė, L. Feeding on the edge: The diet of the hazel dormouse Muscardinus avellanarius (Linnaeus 1758) on the northern periphery of its distributional range. Mammalia 2013, 77, 149–155. [Google Scholar] [CrossRef]
- Goodwin, C.E.; Swan, G.J.; Hodgson, D.J.; Bailey, S.; Chanin, P.; McDonald, R.A. Effects of food availability on the trophic niche of the hazel dormouse Muscardinus avellanarius. For. Ecol. Manag. 2020, 470, 118215. [Google Scholar] [CrossRef]
- Trout, R.C. A review of studies on captive Harvest mice (Micromys minutus (Pallas)). Mammal Rev. 1978, 8, 159–175. [Google Scholar] [CrossRef]
- Yamao, K.; Ishiwaka, R.; Murakami, M.; Takatsuki, S. Seasonal variation in the food habits of the Eurasian harvest mouse (Micromys minutus) from western Tokyo, Japan. Zool. Sci. 2016, 33, 611–615. [Google Scholar] [CrossRef]
- Lawal, R.A.; Arora, U.P.; Dumont, B.L. Selection shapes the landscape of functional variation in wild house mice. BMC Biol. 2021, 19, 239. [Google Scholar] [CrossRef]
- Ivanter, E.V.; Korosov, A.V.; Makarov, A.M. Study of the trophic relations of small insectivorous mammals. Biol. Bull. Russ. Acad. Sci. 2015, 42, 845–856. [Google Scholar] [CrossRef]
- Gębczyńska, Z.; Sołtys, H.; Sienkiewicz, M. Food composition in striped field mice living at localities of various degrees of urban development. Acta Theriol. 1987, 32, 325–330. [Google Scholar] [CrossRef]
- Abt, K.F.; Bock, W.F. Seasonal variations of diet composition in farmland field mice Apodemus spp. and bank voles Clethrionomys glareolus. Acta Theriol. 1998, 43, 379–389. [Google Scholar] [CrossRef]
- Watts, C.H. The foods eaten by wood mice (Apodemus sylvaticus) and bank voles (Clethrionomys glareolus) in Wytham Woods, Berkshire. J. Anim. Ecol. 1968, 37, 25–41. [Google Scholar] [CrossRef]
- Holisova, V.; Obrtel, R. Food resource partitioning among four myomorph rodent populations coexisting in a spruce forest. Folia Zool. 1980, 3, 193–207. [Google Scholar]
- Ivanter, E.V. Study of the Water Vole (Arvicola amphibius) at the Northwestern Boundary of Its Range. Biol. Bull. Russ. Acad. Sci. 2023, 50, 162–174. [Google Scholar] [CrossRef]
- White, T.C.R. The Inadequate Environment: Nitrogen and the Abundance of Animals; Springer: Berlin, Germany, 1993; pp. 1–425. [Google Scholar]
- Iskjaer, C.; Slade, N.A.; Childs, J.E.; Glass, G.E.; Korch, G.W. Body mass as a measure of body size in small mammals. J. Mammal. 1989, 70, 662–667. [Google Scholar] [CrossRef]
- Lee, J.K.; Eom, T.K.; Lee, D.H.; Ko, H.; Rhim, S.J. Effects of multiple ecological factors on the body mass of small rodents in a forest ecosystem. Wildl. Biol. 2023, 2023, e01078. [Google Scholar] [CrossRef]
- Speakman, J.R. The physiological costs of reproduction in small mammals. Philos. Trans. R. Soc. 2008, 363, 375–398. [Google Scholar] [CrossRef]
- Schaeffer, P.J.; O’Mara, M.T.; Breiholz, J.; Keicher, L.; Lázaro, J.; Muturi, M.; Dechmann, D.K. Metabolic rate in common shrews is unaffected by temperature, leading to lower energetic costs through seasonal size reduction. R. Soc. Open Sci. 2020, 7, 191989. [Google Scholar] [CrossRef]
- Sundell, J.; Norrdahl, K. Body size-dependent refuges in voles: An alternative explanation of the Chitty effect. Ann. Zool. Fenn. 2002, 39, 325–333. [Google Scholar]
- Ochocińska, D.; Taylor, J.R. Bergmann’s rule in shrews: Geographical variation of body size in Palearctic Sorex species. Biol. J. Linn. Soc. 2003, 78, 365–381. [Google Scholar] [CrossRef]
- Promislow, D.E.; Harvey, P.H. Living fast and dying young: A comparative analysis of life-history variation among mammals. J. Zool. 1990, 220, 417–437. [Google Scholar] [CrossRef]
- Wells, C.P.; Barbier, R.; Nelson, S.; Kanaziz, R.; Aubry, L.M. Life history consequences of climate change in hibernating mammals: A review. Ecography 2022, 2022, e06056. [Google Scholar] [CrossRef]
- Bujalska, G.; Grüm, L. Winter survival of Apodemus flavicollis in Crabapple island (NE Poland). Hystrix 2006, 17, 173–177. [Google Scholar]
- Ren, S.; Li, Y.; Peichl, M. Diverse effects of climate at different times on grassland phenology in mid-latitude of the Northern Hemisphere. Ecol. Indic. 2020, 113, 106260. [Google Scholar] [CrossRef]
- Buermann, W.; Forkel, M.; O’sullivan, M.; Sitch, S.; Friedlingstein, P.; Haverd, V.; Jain, A.K.; Kato, E.; Kautz, M.; Lienert, S.; et al. Widespread seasonal compensation effects of spring warming on northern plant productivity. Nature 2018, 562, 110–114. [Google Scholar] [CrossRef]


| Species | TG | N * | Male ** | Female | Ad | Sub | Juv |
|---|---|---|---|---|---|---|---|
| Common shrew (Sorex araneus) | I | 2536 | 1079 | 739 | 658 | 1315 | 270 |
| Pygmy shrew (S. minutus) | I | 805 | 260 | 202 | 159 | 128 | 35 |
| Water shrew (Neomys fodiens) | I | 100 | 44 | 33 | 48 | 38 | 7 |
| Mediterranean water shrew (N. milleri) | I | 3 | 1 | 2 | 1 | 1 | |
| Hazel dormouse (Muscardinus avellanarius) | O | 1 | 1 | 1 | |||
| Northern birch mouse (Sicista betulina) | G | 18 | 12 | 6 | 11 | 3 | 4 |
| House mouse (Mus musculus) | O | 432 | 271 | 161 | 183 | 117 | 132 |
| Striped field mouse (Apodemus agrarius) | G | 3781 | 2152 | 1580 | 774 | 1067 | 1898 |
| Yellow-necked mouse (A. flavicollis) | G | 5561 | 2912 | 2566 | 2583 | 1621 | 1284 |
| Wood mouse (A. sylvaticus) | G | 5 | 4 | 1 | 2 | 1 | 2 |
| Pygmy field mouse (A. uralensis) | G | 74 | 27 | 46 | 20 | 23 | 31 |
| Harvest mouse (Micromys minutus) | G | 347 | 186 | 149 | 58 | 95 | 190 |
| Bank vole (Clethrionomys glareolus) | O | 10,316 | 5373 | 4860 | 3482 | 3505 | 3270 |
| Water vole (Arvicola amphibius) | H | 10 | 7 | 3 | 1 | 4 | 5 |
| Root vole (Alexandromys oeconomus) | H | 1337 | 622 | 706 | 688 | 238 | 405 |
| Common vole (Microtus arvalis) *** | H | 2537 | 1257 | 1257 | 758 | 427 | 1333 |
| Short-tailed vole (M. agrestis) | H | 674 | 346 | 326 | 308 | 188 | 177 |
| Sibling vole (M. rossiaemeridionalis) | H | 30 | 13 | 17 | 19 | 6 | 5 |
| Stage | Abbreviation | Description |
|---|---|---|
| No spermatogenesis | (0) | testes scrotal, but no other signs, thymus absent |
| Beginning of spermatogenesis | (1+) | testes enlarged, epididymis empty |
| Spermatogenesis of medium intensity | (2+) | testes and epididymis enlarged, seminal vesicles empty or slightly enlarged |
| Intensive spermatogenesis | (3+) | testes enlarged, epididymis and seminal vesicles full |
| Individuals after reproduction | (AR) | seminal vesicles empty, testes and epididymis slumped, slate colored |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balčiauskas, L.; Balčiauskienė, L. Insight into Body Condition Variability in Small Mammals. Animals 2024, 14, 1686. https://doi.org/10.3390/ani14111686
Balčiauskas L, Balčiauskienė L. Insight into Body Condition Variability in Small Mammals. Animals. 2024; 14(11):1686. https://doi.org/10.3390/ani14111686
Chicago/Turabian StyleBalčiauskas, Linas, and Laima Balčiauskienė. 2024. "Insight into Body Condition Variability in Small Mammals" Animals 14, no. 11: 1686. https://doi.org/10.3390/ani14111686







