Effect of Olive Cake in Bísaro Pig Feed on Physicochemical Composition and Fatty Acid Profile of Three Different Muscles of Dry-Cured Shoulder
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Diets
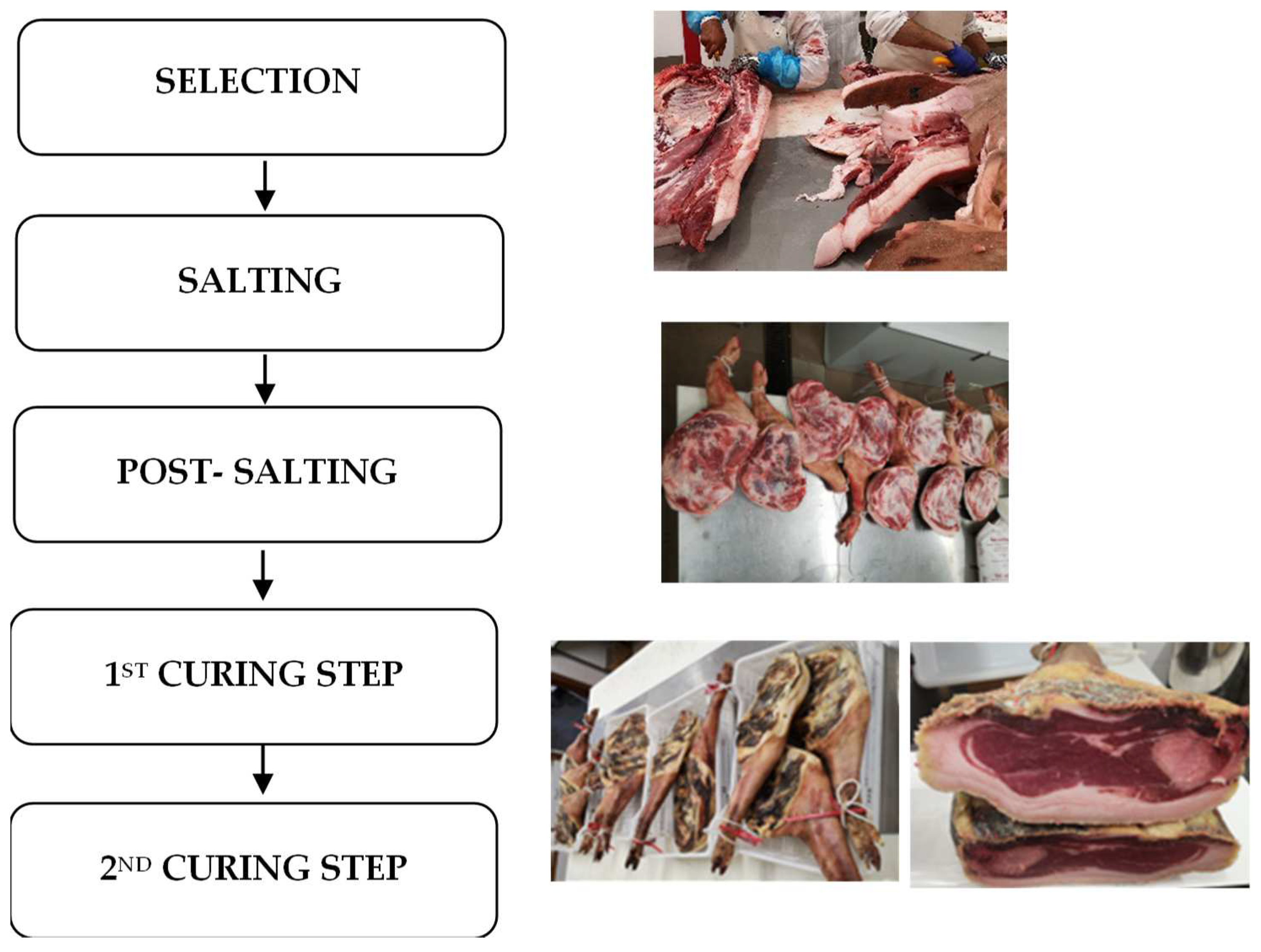
2.2. Dry-Cured Bísaro Shoulder
2.3. Chemical Composition and Physicochemical Analysis
2.4. Fatty Acid Analysis
2.5. Statistical Analysis
3. Results and Discussion
3.1. Influence of Olive Cake Treatments on Physicochemical Composition
3.2. Influence of Curing Time and Type of Muscle on Physicochemical Composition
3.3. Influence of Olive Cake Treatments on Fatty Acid Profile
3.4. Influence of Curing Time and Type of Muscle on Fatty Acid Profile
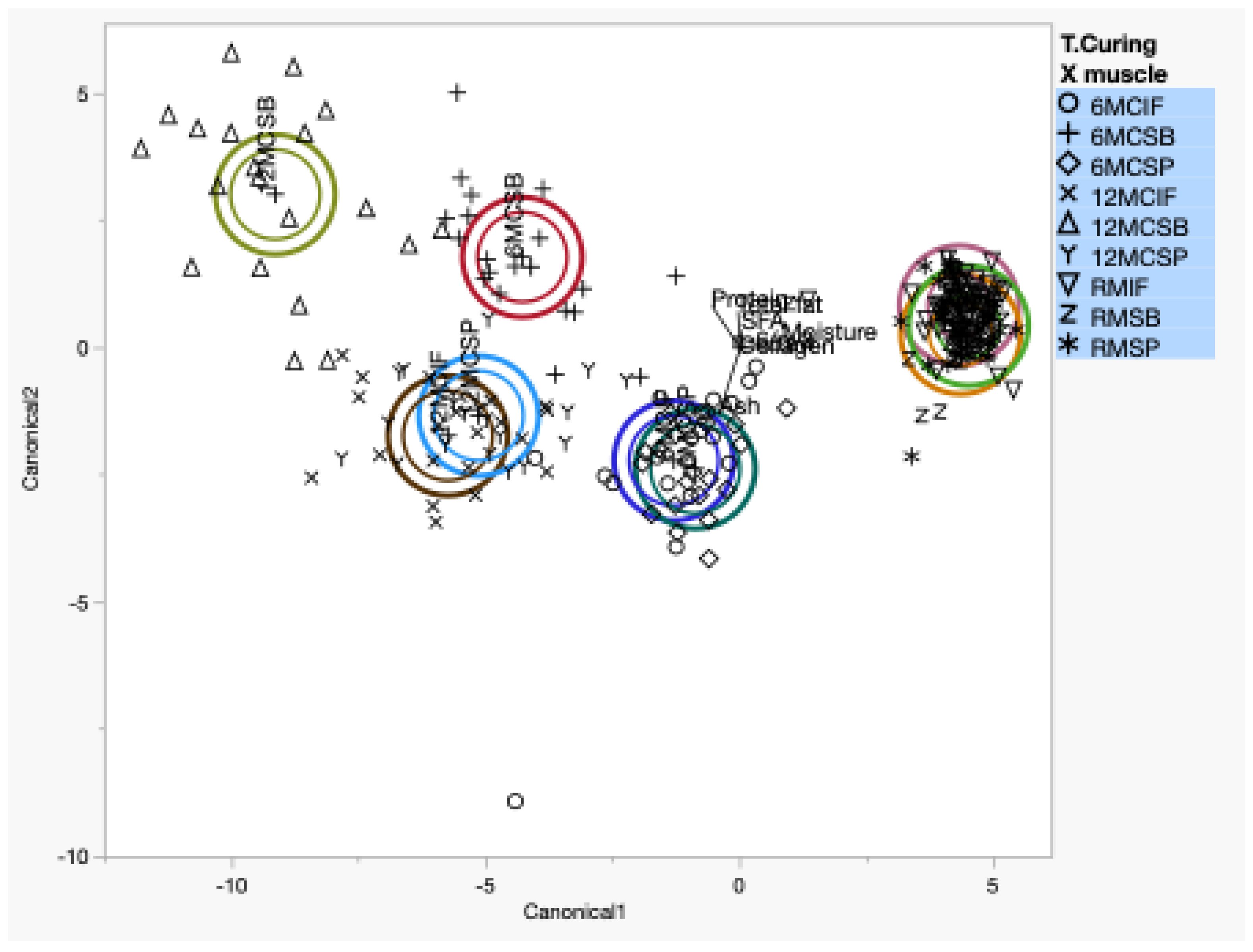
3.5. Discriminant Analysis
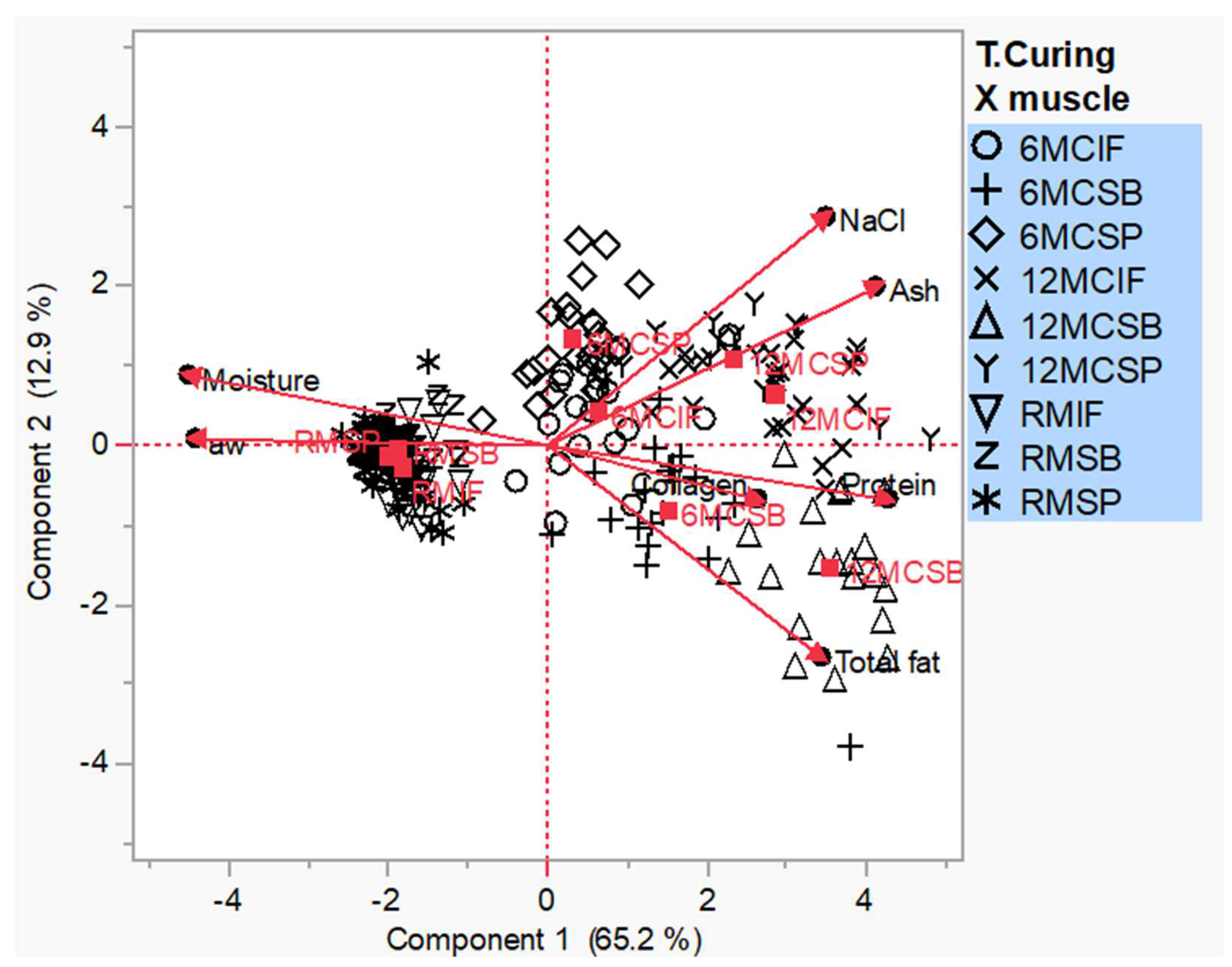
3.6. Principal Component Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Joven, M.; Pintos, E.; Latorre, M.A.; Suárez-Belloch, J.; Guada, J.A.; Fondevilla, M. Effect of replacing barley by increasing levels of olive cake in the diet of finishing pigs: Growth performances, digestibility, carcass, meat and fat quality. Anim. Feed Sci. Technol. 2014, 197, 185–193. [Google Scholar] [CrossRef]
- Teixeira, A.; Rodrigues, S. Pork meat quality of Preto Alentejano and Commercial Largewhite Landrace Cross. J. Integr. Agric. 2013, 12, 1961–1971. [Google Scholar] [CrossRef]
- Molina-Alcaide, E.; Yáñez-Ruiz, D.R. Potential use of olive by-products in ruminant feeding: A review. Anim. Feed Sci. Technol. 2008, 147, 247–264. [Google Scholar] [CrossRef]
- Liotta, L.; Chiofalo, V.; Lo Presti, V.; Chiofalo, B. In vivo, performances, carcass traits, and meat quality of pigs fed olive cake processing waste. Animals 2019, 9, 1155. [Google Scholar] [CrossRef] [PubMed]
- Gullón, P.; Gullón, B.; Astray, G.; Carpena, M.; Fraga-Corral, M.; Prieto, M.A.; Simal-Gandara, J. Valorization of by-products from the olive oil industry and added-value applications for innovative functional foods. Food Res. Int. 2020, 137, 109683. [Google Scholar] [CrossRef]
- Ferrer, P.; Calvet, S.; García-Rebollar, P.; Blas, C.; Jiménez-Belenguer, A.I.; Hernández, P.; Piquer, O.; Cerisuelo, A. Partially defatted olive cake in finishing pig diets: Implications on performance, fecal microbiota, carcass quality, slurry composition and gas emission. Animals 2020, 14, 426–434. [Google Scholar] [CrossRef]
- De la Casa, J.A.; Bueno, J.S.; Castro, E. Recycling of residues from the olive cultivation and olive oil production process for manufacturing of ceramic materials. A comprehensive review. J. Clean. Prod. 2021, 296, 126436. [Google Scholar] [CrossRef]
- Otero, P.; Garcia-Oliveira, P.; Carpena, M.; Barral-Martinez, M.; Chamorro, F.; Echave, J.; Garcia-Perez, P.; Cao, H.; Xiao, J.; Simal-Gandara, J.; et al. Applications of byproducts from the olive oil processing: Revalorization strategies based on target molecules and green extraction technologies. Trends Food Sci. Technol. 2021, 116, 1084–1104. [Google Scholar] [CrossRef]
- Gandemer, G. Lipids in muscles and adipose tissues, changes during processing and sensory properties of meat products. Meat Sci. 2002, 62, 309–321. [Google Scholar] [CrossRef]
- Zhou, C.Y.; Wang, Y.; Pan, D.D.; Cao, J.X.; Chen, Y.J.; Liu, Y.; Sun, Y.Y.; Ou, R.C. The changes in the proteolysis activity and the accumulation of free amino acids during Chinese traditional dry-cured loins processing. Food Sci. Biotechnol. 2017, 26, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Salazar, E.; Cayuela, J.M.; Abellán, A.; Tejada, L. Effect of breed on proteolysis and free amino acid profiles of dry-cured loin during processing. Anim. Prod. Sci. 2018, 59, 1161–1167. [Google Scholar] [CrossRef]
- Toldrá, F. Dry-cured ham. In Handbook of Food Science, Technology and Engineering; Hui, Y.H., Culbertson, J.D., Duncan, S., Guerrero-Legarreta, I., Li-Chan, E.C.Y., Ma, C.Y., Manley, C.H., McMeekin, T.A., Nip, W.K., Nollet, L.M.L., et al., Eds.; MarcelDekker Inc./CRC Press: Boca Raton, FL, USA, 2005; Volume 1. [Google Scholar]
- European Union. COMMISSION REGULATION (EC) No 676/2008 of 16 July 2008 registering certain names in the Register of protected designations of origin and protected geographical indications (Ail de la Drôme (PGI), Všestarská cibule (PDO), Slovenská bryndza (PGI), Ajo Morado de Las Pedroñeras (PGI), Gamoneu or Gamonedo (PDO), Alheira de Vinhais (PGI), Presunto de Vinhais or Presunto Bísaro de Vinhais (PGI)) L189/19—17 July 2008. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32008R0676 (accessed on 6 May 2024).
- European Union. COMMISSION REGULATION (EC) No 723/2008 of 25 July 2008 registering certain names in the Register of protected designations of origin and protected geographical indications (Afuega’l Pitu (PDO), Mazapán de Toledo (PGI), Agneau de Lozère (PGI), Oignon doux des Cévennes (PDO), Butelo de Vinhais or Bucho de Vinhais or Chouriço de Ossos de Vinhais (PGI), Chouriça Doce de Vinhais (PGI)) L198/28—26 July 2008. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32008R0723 (accessed on 6 May 2024).
- European Union. COMMISSION REGULATION (EC) No 944/2008 of 25 September 2008 entering certain names in the Register of protected designations of origin and protected geographical indications (Salame S. Angelo (PGI), Chouriço Azedo de Vinhais or Azedo de Vinhais or Chouriço de Pão de Vinhais (PGI), Presunto do Alentejo or Paleta do Alentejo (PDO). Available online: https://eur-lex.europa.eu/eli/reg/2008/944/oj (accessed on 6 May 2024).
- Álvarez-Rodríguez, J.; Teixeira, A. Slaughter weight rather than sex affects carcass cuts and tissue composition of Bísaro pigs. Meat Sci. 2019, 154, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Council Regulation (EC)—Official Journal of the European Communities No 1099/2009 of 24 September 2009 on the protection of animals at the time of killing. 2009, pp. 1–30. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex%3A32009R1099 (accessed on 6 May 2024).
- NP 1614/2002; Determination of Moisture Content. Reference Method (ISO 1442:1197). In Portuguese Norm–Meat and Meat Products. Portuguese Institute of Quality, Ministry of Economy and Innovation: Caparica, Portugal, 2002.
- NP-ISO-1615/2002; Determination of Total Ashes. Reference Method. In Portuguese Norm–Meat and Meat Products. Portuguese Institute of Quality, Ministry of Economy and Innovation: Caparica, Portugal, 2002.
- NP 1612/2002; Determination of Total Nitrogen Content. Reference Method (ISO 937:1978). In Portuguese Norm–Meat and Meat Products. Portuguese Institute of Quality, Ministry of Economy and Innovation: Caparica, Portugal, 2002.
- Cunniff, P.; AOAC International. AOAC Official Methods of Analysis of AOAC International, 16th ed.; The Association: Washington, DC, USA, 1995; ISBN 9780935584547. [Google Scholar]
- NP 1987/2002; Carnes e Produtos Cárneos; Determinação do Teor em Hidroxiprolina. (Método de referência). In Portuguese Norm–Meat and Meat Products. Portuguese Institute of Quality, Ministry of Economy and Innovation: Caparica, Portugal, 2002.
- NP 1845/1982; Meat and Meat Products—Determination of Chloride Content—Standard Method. Portuguese Institute of Quality, Ministry of Economy Innovation: Caparica, Portugal, 1982.
- Folch, J.; Lees, M.; Stanley, G.H.S. A simple method for isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Dominguez, R.; Borrajo, P.; Lorenzo, J.M. The effect of cooking methods on nutritional value of foal meat. J. Food Compos. Anal. 2015, 43, 61–67. [Google Scholar] [CrossRef]
- Vieira, C.; Sarmiento-García, A.; García, J.J.; Rubio, B.; Martínez, B. Quality and Shelf Life of Fresh Meat from Iberian Pigs as Affected by a New Form of Presentation of Oleic Acid and an Organic-Acid Mix in the Diet. Foods 2021, 10, 985. [Google Scholar] [CrossRef]
- Ulbricht, T.L.; Southgate, D.A.T. Coronary heart disease: Seven dietary factors. Lancet 1991, 338, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Leite, A.; Vasconcelos, L.; Ferreira, I.; Domínguez, R.; Pereira, E.; Rodrigues, S.; Lorenzo, J.M.; Teixeira, A. Effect of the inclusion of olive cake in the diet on the physicochemical characteristics of dry-cured loin and dry-cured “cachaço” of Bísaro pig. Appl. Sci. 2023, 13, 1439. [Google Scholar] [CrossRef]
- Bermúdez, R.; Franco, D.; Carballo, J.; Lorenzo, J.M. Physicochemical changes during manufacture and final sensory characteristics of dry-cured Celta ham. Effect muscle type. Food Control 2014, 43, 263–269. [Google Scholar] [CrossRef]
- Asensio, M.; Silva, A.; Armenteros, M.; Caballero, D.; Martín, N.; Lorido, L.; Sánchez-Montero, L.; Fernandez, C.; Noguera, J.L.; Ramos, M. Quality evaluation of dry-cured shoulder from different genetic lines of Iberian pigs. Arch. Zootec. 2018, 67, 155–160. [Google Scholar] [CrossRef]
- Delgado, G.L.; Gómez, C.S.; Rubio, L.M.S.; Iturbe, C.H.F.; Méndez, M.D. Evolution in the shoulder composition of hairless Mexican pigs throughout the curing and drying processes. Meat Sci. 2002, 61, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Caballero, D.; Asensio, M.; Fernández, C.; Reina, R.; García-Casco, J.; Martín, N.; Silva, A. Chemical-instrumental-sensory traits and data mining for classifying dry-cured Iberian shoulders from pigs with different diets. J. Food Meas. Charact. 2019, 13, 2935–2950. [Google Scholar] [CrossRef]
- Pérez-Ciria, L.; Ripoll, G.; Sanz, M.A.; Blanco, M.; Miana-Mena, F.J.; Latorre, M.A. Impact of gilt immunocastration on weight losses and instrumental and chemical characteristics of Teruel dry-cured ham. Meat Sci. 2015, 104, 52–57. [Google Scholar] [CrossRef]
- Sha, K.; Lang, Y.M.; Sun, B.Z.; Su, H.W.; Li, H.P.; Zhang, L.; Lei, Y.H.; Li, H.B.; Zhang, Y. Changes in Lipid Oxidation, Fatty Acid Profile and Volatile Compounds of Traditional Kazakh Dry-Cured Beef during Processing and Storage. J. Food Process. Preserv. 2017, 41, 13059. [Google Scholar]
- Bermúdez, R.; Pateiro, M.; Domínguez, R.; Lorenzo, J.M.; Franco, D.; Carballo, J. Physicochemical changes of semimembranosus muscle during the processing of dry-cured ham from Celta pig. Effect of crossbreeding with Duroc and Landrace genotypes. Anim. Prod. Sci. 2018, 58, 1958–1965. [Google Scholar] [CrossRef]
- Gomez, M.; Dominguez, R.; Fonseca, S.; Lorenzo, J.M. Effect of Finishing Diet on Physico-Chemical and Lipolytic Parameters and Volatile Compounds Throughout the Manufacture of Dry-Cured Foal Cecina. Austin J. Nutr. Food Sci. 2015, 3, 1056. [Google Scholar]
- Leite, A.; Vasconcelos, L.; Ferreira, I.; Sarmietno-Garcia, A.; Domínguez, R.; Santos, E.M.; Campagnol, P.C.B.; Rodrigues, S.; Lorenzo, J.M.; Teixeira, A. Chemical, physicochemical and sensorial characterization of nitrite-free dry-cured Bísaro shoulder. Foods 2022, 11, 3079. [Google Scholar] [CrossRef] [PubMed]
- Caballero, D.; Asensio, M.; Fernández, C.; Reina, R.; García, M.J.; Noguera, J.L.; Silva, A. Effects of genotypes and crossbreeding on the quality parameters of dry-cured shoulders from different Iberian genetic pig lines. J. Food Meas. Charact. 2020, 14, 818–829. [Google Scholar] [CrossRef]
- Reina, R.; Pulgar, J.S.; Tovar, J.; López-Beusa, P.; García, C. Quality of dry-cured ham compared with quality of dry-cured shoulder. J. Food Sci. 2013, 78, 8. [Google Scholar] [CrossRef]
- Vargas-Ramella, M.; Domínguez, R.; Pateiro, M.; Franco, D.; Barba, F.J.; Lorenzo, J.M. Chemical and physicochemical changes during the dry-cured processing of deer loin. Int. J. Food Sci. Technol. 2020, 55, 1025–1031. [Google Scholar] [CrossRef]
- De Jesús, M.C.; Domínguez, R.; Cantalapiedra, J.; Iglesias, A.; Lorenzo, J.M.; Lorenzo, J.M. Effect of the amount of chestnuts in the diet of Celta pigs on the fatty acid profile of dry-cured lacon. Grasas Aceites 2016, 67, e119. [Google Scholar]
- Martín-Mateos, M.J.; Amaro-Blanco, G.; Manzano, R.; Andrés, A.I.; Ramírez, R. Efficacy of modified active packaging with oxygen scavengers for the preservation of sliced Iberian dry-cured shoulder. Food Sci. Technol. Int. 2022, 29, 318–330. [Google Scholar] [CrossRef] [PubMed]
- Fernández, M.; Ordóñez, J.A.; Cambero, I.; Santos, C.; Pin, C.; de la Hoz, L. Fatty acid compositions of selected varieties of Spanish dry ham related to their nutritional implications. Food Chem. 2007, 101, 107–112. [Google Scholar]
- Baum, S.J.; Kris-Etherton, P.M.; Willett, W.C.; Lichtenstein, A.H.; Rudel, L.L.; Maki, K.C.; Whelan, J.; Ramsden, C.E.; Block, R.C. Fatty acids in cardiovascular health and disease: A comprehensive update. J. Clin. Lipidol. 2012, 6, 216–234. [Google Scholar] [CrossRef]
- Jiménez-Colmenero, F.; Ventanas, J.; Toldrá, F. Nutritional composition of dry-cured ham and its role in a healthy diet. Meat Sci. 2010, 84, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M. A comprehensive review on lipid oxidation in meat and meat products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P.; Cleland, L.G.; Christiansen, E.N. Omega-6/omega-3 essential fatty acid ratio: The scientific evidence. Scand. J. Nutr. 2004, 48, 49–50. [Google Scholar] [CrossRef]
- British Nutrition Foundation. Unsaturated Fatty Acids: Nutritional and Physiological Significance: The Report from the British Nutrition Foundation’s Task Force; Chapman and Hall Ltd.: London, UK, 1992. [Google Scholar]
- Simopoulos, A. An Increase in the Omega-6/Omega-3 Fatty Acid Ratio Increases the Risk for Obesity. Nutrients 2016, 8, 128. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. Evolutionary aspects of the dietary Omega-6:Omega-3 fatty acid ratio: Medical implications. World Rev. Nutr. Diet. 2009, 100, 1–21. [Google Scholar] [PubMed]
- Simopoulos, A.P. Omega-6/omega-3 essential fatty acids: Biological effects. World Rev. Nutr. Diet. 2009, 99, 1–16. [Google Scholar] [PubMed]
- Chen, J.; Liu, H. Nutritional Indices for Assessing Fatty acids: A Mini-Review. Int. J. Mol. Sci. 2020, 21, 5695. [Google Scholar] [CrossRef] [PubMed]
- Cava, R.; Estévez, M.; Ruiz, J.; Morcuende, D. Physicochemical characteristics of three muscles from free-range reared Iberian pigs slaughtered at 90 kg live weight. Meat Sci. 2003, 63, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.T.; Wicker, L.R. 8—Discriminant Analysis. In Handbook of Applied Multivariate Statistics and Mathematical Modeling; Tinsley, H.E.A., Brown, S.D., Eds.; Academic Press: Cambridge, MA, USA, 2000; pp. 209–235. ISBN 9780126913606. [Google Scholar]
- Greenacre, M.; Groenen, P.J.F.; Hastie, T.; D′Enza, A.I.; Markos, A. Principal component analysis. Nat. Rev. Methods Primers 2022, 2, 100. [Google Scholar] [CrossRef]
| Diets | |||||
|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | T5 | |
| Olive cake | 0 | 10 | 10 | 10 | 10 |
| Olive oil | 0 | 0 | 0 | 0 | 1 |
| Barley grain | 45.80 | 41.20 | 41.20 | 41.20 | 41.20 |
| Wheat grain | 22.60 | 20.40 | 20.40 | 20.40 | 20.40 |
| Soybean meal 47 | 12.90 | 11.60 | 11.60 | 11.60 | 11.60 |
| Rice bran | 5.00 | 4.50 | 4.50 | 4.50 | 4.50 |
| Corn grain | 2.50 | 2.20 | 2.20 | 2.20 | 2.20 |
| DDG’s corn | 5.00 | 4.50 | 4.50 | 4.50 | 4.50 |
| Beet molasses | 4.00 | 3.60 | 3.60 | 3.60 | 3.60 |
| Minerals and vitamins | 1.70 | 1.70 | 1.70 | 1.70 | 1.70 |
| Supplement min + vit + fitase | 0.50 | 0.30 | 0.30 | 0.30 | 0.30 |
| Chemical composition of the diet | |||||
| DM | 98.05 | 98.49 | 98.19 | 98.15 | 98.46 |
| OM | 93.90 | 94.20 | 93.75 | 94.16 | 93.98 |
| NDF | 18.01 | 23.39 | 22.97 | 24.04 | 22.88 |
| ADF | 6.40 | 10.62 | 10.48 | 10.50 | 10.06 |
| ADL | 0.89 | 3.06 | 2.81 | 3.09 | 2.86 |
| Cellulose | 5.51 | 7.56 | 7.68 | 7.41 | 7.20 |
| PB | 16.00 | 13.38 | 13.45 | 14.39 | 13.98 |
| GB | 5.41 | 5.53 | 4.96 | 4.30 | 5.20 |
| Fatty acids (g/100 g) | |||||
| ΣSFA | 21.54 | 20.03 | 20.09 | 21.31 | 20.57 |
| ΣMUFA | 28.00 | 42.36 | 38.31 | 29.45 | 36.10 |
| ΣPUFA | 50.46 | 37.61 | 41.60 | 49.24 | 43.34 |
| PUFA/SFA | 2.34 | 2.07 | 1.88 | 2.31 | 2.10 |
| n-6/n-3 | 17.58 | 16.66 | 16.69 | 17.10 | 16.90 |
| Chemical Composition (g/100g) | |||||||
|---|---|---|---|---|---|---|---|
| aw | Moisture | NaCl | Ash | Protein | Total Fat | Collagen | |
| T1 | 0.899 a | 55.76 a | 4.91 ab | 4.88 a | 27.56 a | 11.74 ab | 1.99 ab |
| T2 | 0.897 a | 54.81 a | 4.99 ab | 4.57 a | 26.93 a | 11.08 b | 1.65 b |
| T3 | 0.899 a | 55.38 a | 4.53 ab | 4.35 a | 26.69 a | 11.62 ab | 1.96 ab |
| T4 | 0.899 a | 53.98 a | 5.32 a | 4.78 a | 27.48 a | 14.00 a | 2.48 a |
| T5 | 0.896 a | 56.19 a | 4.46 b | 4.36 a | 26.54 a | 11.00 b | 1.82 b |
| SE | 0.003 | 0.73 | 0.20 | 0.14 | 0.40 | 0.73 | 0.17 |
| p value | ns | ns | * | ns | ns | * | ** |
| Muscle | Time Curing | Chemical Composition (g/100g) | ||||||
|---|---|---|---|---|---|---|---|---|
| aw | Moisture | NaCl | Ash | Protein | Total Fat | Collagen | ||
| SB | RM | 0.960 a | 72.19 a | 1.73 e | 19.12 f | 6.34 f | 1.62 bcd | |
| 6MC | 0.896 b | 42.27 d | 3.75 c | 4.82 d | 34.29 b | 18.71 ab | 0.67 d | |
| 12MC | 0.805 d | 33.24 e | 3.81 c | 5.38 cd | 39.99 a | 23.02 a | 3.62 a | |
| IF | RM | 0.954 a | 71.10 a | 1.49 e | 18.74 f | 7.99 def | 1.64 bc | |
| 6MC | 0.908 b | 52.75 c | 4.32 bc | 5.83 c | 23.64 e | 11.28 cde | 1.64 bcd | |
| 12MC | 0.841 c | 43.99 d | 6.09 a | 7.08 ab | 30.00 c | 13.97 bc | 4.36 a | |
| SP | RM | 0.963 a | 71.88 a | 1.65 e | 19.20 f | 7.29 ef | 0.94 cd | |
| 6MC | 0.915 b | 59.28 b | 4.96 b | 6.16 bc | 26.58 d | 6.32 f | 0.84 cd | |
| 12MC | 0.845 c | 50.32 c | 6.13 a | 7.15 a | 31.81 bc | 12.06 cd | 2.48 b | |
| SEM | 0.005 | 1.072 | 0.221 | 0.210 | 0.587 | 1.115 | 0.246 | |
| significance | *** | *** | *** | *** | *** | *** | ** | |
| Treatment | |||||||
|---|---|---|---|---|---|---|---|
| Fatty Acids (g/100g) | T1 | T2 | T3 | T4 | T5 | SE | p |
| C16:0 | 24.30 | 24.46 | 24.13 | 24.58 | 24.23 | 0.14 | ns |
| C16:1n-7 | 2.74 | 2.83 | 2.73 | 2.85 | 2.78 | 0.051 | ns |
| C18:0 | 11.42 | 11.40 | 11.47 | 11.29 | 11.27 | 0.146 | ns |
| C18:1n-9 | 47.99 ab | 47.76 b | 48.78 a | 47.85 ab | 48.31 ab | 0.294 | * |
| C18:2n-6 | 8.32 | 8.56 | 8.22 | 8.58 | 8.58 | 0.162 | ns |
| SFA | 37.70 | 37.79 | 37.53 | 37.86 | 37.50 | 0.25 | ns |
| MUFA | 52.16 | 52.42 | 52.81 | 52.07 | 52.43 | 0.23 | ns |
| PUFA | 10.14 | 9.79 | 9.66 | 10.07 | 10.07 | 0.20 | ns |
| n-6/n-3 | 24.42 | 24.26 | 24.53 | 24.17 | 24.16 | 0.68 | ns |
| IA | 0.46 | 0.46 | 0.46 | 0.47 | 0.46 | 0.004 | ns |
| IT | 1.14 | 1.15 | 1.14 | 1.15 | 1.13 | 0.012 | ns |
| h/H | 2.27 | 2.24 | 2.31 | 2.24 | 2.29 | 0.022 | ns |
| Fatty Acids | Muscle × Time of Curing | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SB | IF | SP | SEM | SIG. | |||||||
| RM | 6MC | 12MC | RM | 6MC | 12MC | RM | 6MC | 12MC | |||
| C10:0 | 0.014 c | 0.024 bc | 0.031 abc | 0.046 a | 0.031 abc | 0.026 bc | 0.040 ab | 0.026 bc | 0.025 bc | 0.005 | *** |
| C12:0 | 0.026 d | 0.029 bcd | 0.047 abc | 0.054 a | 0.032 bcd | 0.041 abcd | 0.045 ab | 0.024 cd | 0.035 abcd | 0.005 | *** |
| C14:0 | 1.06 bc | 1.19 a | 1.11 abc | 1.06 bc | 1.14 ab | 1.05 bc | 1.08 bc | 1.13 ab | 1.04 cd | 0.020 | * |
| C14:1 | 0.008 cd | 0.004 d | 0.020 abc | 0.031 a | 0.004 d | 0.022 ab | 0.016 bcd | 0.005 d | 0.02 abc | 0.003 | *** |
| C15:0 | 0.32 d | 0.12 bcd | 0.05 d | 0.07 a | 0.14 cd | 0.11 | 0.22 bc | 0.25 ab | 0.12 bcd | 0.029 | *** |
| C16:0 | 23.73 d | 24.71 bc | 26.01 a | 24.72 b | 23.86 cd | 24.73 bc | 23.65 d | 23.86 cd | 24.69 bc | 0.206 | *** |
| C16:1n-7 | 2.72 b | 2.95 ab | 2.66 b | 2.68 b | 2.92 ab | 2.66 b | 2.80 b | 3.11 a | 2.72 b | 0.074 | ns |
| C17:0 | 0.23 a | 0.22 a | 0.14 bc | 0.22 a | 0.21 a | 0.11 c | 0.21 a | 0.19 ab | 0.12 c | 0.013 | ns |
| C17:1n-7 | 0.23 a | 0.22 a | 0.22 a | 0.25 a | 0.26 a | 0.23 a | 0.25 a | 0.24 a | 0.22 a | 0.013 | ns |
| C18:0 | 11.33 bc | 11.20 bc | 12.77 a | 11.88 b | 10.58 cd | 11.78 b | 10.89 cd | 10.16 d | 11.77 b | 0.215 | *** |
| 9t-C18:1 | 0.14 a | 0.17 a | 1.38 a | 0.17 a | 0.18 a | 0.20 a | 0.16 a | 0.16 a | 0.19 a | 0.331 | ns |
| C18:1n-9 | 47.75 bcd | 49.02 ab | 46.57 d | 47.28 cd | 49.88 a | 48.54 abc | 48.50 abc | 48.74 abc | 47.85 bcd | 0.432 | ** |
| 9t,12t-C18:2 | 0.009 b | 0.011 ab | 0.011 ab | 0.020 a | 0.014 ab | 0.011 ab | 0.009 b | 0.017 ab | 0.010 ab | 0.003 | ns |
| C18:2n-6 | 9.33 a | 7.68 de | 6.89 e | 8.54 bcd | 7.94 cde | 7.89 cde | 8.97 ab | 8.83 abc | 8.47 abcd | 0.238 | *** |
| C20:0 | 0.13 ab | 0.14 ab | 0.16 a | 0.16 a | 0.15 a | 0.16 a | 0.15 a | 0.12 b | 0.14 ab | 0.008 | ** |
| C18:3n-6 | 0.010 a | 0.004 a | 0.028 a | 0.027 a | 0.010 a | 0.015 a | 0.023 a | 0.022 a | 0.017 a | 0.008 | ns |
| C20:1n-9 | 0.80 ab | 0.80 ab | 0.68 c | 0.79 ab | 0.86 a | 0.77 abc | 0.80 ab | 0.73 bc | 0.73 bc | 0.024 | * |
| C18:3n-3 | 0.34 ab | 0.30 bcd | 0.21 e | 0.32 ab | 0.37 a | 0.27 d | 0.31 bc | 0.35 ab | 0.28 cd | 0.012 | *** |
| C21:0 | 0.031 abc | 0.015 bc | 0.008 bc | 0.044 a | 0.033 abc | 0.012 bc | 0.034 ab | 0.015 bc | 0.005 c | 0.007 | ns |
| C20:2n-6 | 0.34 a | 0.29 b | 0.31 ab | 0.32 ab | 0.31 ab | 0.33 ab | 0.34 ab | 0.30 ab | 0.33 ab | 0.013 | ns |
| C22:0 | 0.02 c | 0.02 c | 0.03 bc | 0.06 a | 0.03 bc | 0.05 ab | 0.05 ab | 0.04 abc | 0.05 ab | 0.005 | ns |
| C20:3n-6 | 0.13 ab | 0.08 cd | 0.06 d | 0.12 ab | 0.11 abc | 0.10 bcd | 0.14 a | 0.14 a | 0.10 bc | 0.008 | *** |
| C20:3n-3 | 0.03 b | 0.01 bc | 0.01 bc | 0.08 a | 0.02 bc | 0.001 c | 0.08 a | 0.02 bc | 0.004 bc | 0.007 | *** |
| C20:4n-6 | 1.02 a | 0.63 bc | 0.001 d | 0.42 c | 0.69 b | 0.003 d | 0.98 a | 1.22 a | 0.001 d | 0.062 | *** |
| C24:1n-9 | 0.18 a | 0.09 de | 0.07 e | 0.16 ab | 0.12 bcd | 0.12 cde | 0.18 a | 0.16 abc | 0.11 bcde | 0.010 | *** |
| C22:6n-3 | 0.06 ab | 0.02 d | 0.03 cd | 0.06 ab | 0.05 bc | 0.05 abc | 0.08 a | 0.06 ab | 0.05 bc | 0.007 | ns |
| SFA | 36.90 cd | 37.69 bc | 40.78 a | 38.71 b | 36.23 cd | 38.74 b | 36.37 cd | 35.88 d | 38.84 b | 0.361 | *** |
| MUFA | 51.84 cd | 53.26 ab | 51.64 cd | 51.36 d | 54.24 a | 52.58 bcd | 52.69 bc | 53.14 ab | 51.88 bcd | 0.335 | *** |
| PUFA | 11.26 a | 9.05 cd | 7.58 e | 9.93 bc | 9.52 cd | 8.68 de | 10.94 a | 10.98 ab | 9.28 cd | 0.291 | *** |
| n-6/n-3 | 26.40 a | 26.62 a | 28.06 a | 20.24 d | 20.83 cd | 26.13 ab | 22.32 bcd | 25.24 abc | 26.89 ab | 1.005 | ns |
| IA | 0.44 e | 0.47 bc | 0.52 a | 0.47 b | 0.45 cde | 0.48 bc | 0.44 e | 0.44 de | 0.47 bcd | 0.007 | *** |
| IT | 1.11 cd | 1.16 bc | 1.32 a | 1.18 b | 1.08 d | 1.19 b | 1.08 d | 1.06 d | 1.19 b | 0.018 | *** |
| h/H | 2.36 a | 2.23 bc | 1.98 d | 2.20 c | 2.36 ab | 2.21 c | 2.38 a | 2.37 ab | 2.20 c | 0.033 | *** |
| Variable | F Ratio | Prob > F |
|---|---|---|
| aw | 20.508 | 0.0000000 |
| Moisture | 14.631 | 0.0000000 |
| Ash | 38.971 | 0.0000000 |
| Total Fat | 5.979 | 0.0000000 |
| Protein | 20.587 | 0.0000000 |
| Collagen | 12.769 | 0.0000000 |
| SFA | 9.448 | 0.0000000 |
| n-6/n-3 | 6.384 | 0.0000000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leite, A.; Vasconcelos, L.; Rodrigues, S.; Pereira, E.; Domínguez-Valencia, R.; Lorenzo, J.M.; Teixeira, A. Effect of Olive Cake in Bísaro Pig Feed on Physicochemical Composition and Fatty Acid Profile of Three Different Muscles of Dry-Cured Shoulder. Animals 2024, 14, 1697. https://doi.org/10.3390/ani14111697
Leite A, Vasconcelos L, Rodrigues S, Pereira E, Domínguez-Valencia R, Lorenzo JM, Teixeira A. Effect of Olive Cake in Bísaro Pig Feed on Physicochemical Composition and Fatty Acid Profile of Three Different Muscles of Dry-Cured Shoulder. Animals. 2024; 14(11):1697. https://doi.org/10.3390/ani14111697
Chicago/Turabian StyleLeite, Ana, Lia Vasconcelos, Sandra Rodrigues, Etelvina Pereira, Rubén Domínguez-Valencia, José Manuel Lorenzo, and Alfredo Teixeira. 2024. "Effect of Olive Cake in Bísaro Pig Feed on Physicochemical Composition and Fatty Acid Profile of Three Different Muscles of Dry-Cured Shoulder" Animals 14, no. 11: 1697. https://doi.org/10.3390/ani14111697











