Milk Odd- and Branched-Chain Fatty Acids as Biomarkers of Rumen Fermentation
Abstract
:Simple Summary
Abstract
1. Introduction
2. The Content of OBCFAs in Milk Fat
| Type of Diet | TMR | TMR + RSO | TMR | TMR + QTE15 | TMR + QTE30 | CS + GS | GS + H | GS-LO | GS-MD | GS-HI | GS100 | GS67 | GS33 | GS0 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Date of Feeding | – | October–February | June–September | April | October–December | |||||||||
| Breed | Holstein | German Holstein | Holstein | Swedish Red | Holstein-Friesian | |||||||||
| C11:0 | 0.080 | 0.061 | – | – | – | – | – | – | – | – | – | – | – | – |
| C15:0 | 1.221 | 1.024 | 1.02 | 0.96 | 0.92 | 0.928 | 0.914 | 0.94 | 1.00 | 1.06 | 1.23 | 1.05 | 0.93 | 0.91 |
| C17:0 | 0.480 | 0.428 | 0.50 | 0.49 | 0.47 | 0.479 | 0.500 | 0.53 | 0.57 | 0.67 | 0.70 | 0.66 | 0.59 | 0.52 |
| isoC13:0 | 0.031 | 0.026 | – | – | – | – | – | – | – | – | – | – | – | – |
| anteisoC13:0 | 0.022 | 0.016 | – | – | – | – | – | – | – | – | – | – | – | – |
| isoC14:0 | 0.136 | 0.147 | 0.064 | 0.058 | 0.054 | 0.059 | 0.074 | 0.07 | 0.07 | 0.08 | 0.09 | 0.08 | 0.08 | 0.09 |
| isoC15:0 | 0.190 | 0.166 | 0.20 | 0.18 | 0.18 | 0.172 | 0.187 | 0.18 | 0.19 | 0.21 | 0.30 | 0.24 | 0.24 | 0.21 |
| anteisoC15:0 | 0.817 | 0.750 | 0.45 | 0.42 | 0.45 | 0.369 | 0.407 | 0.39 | 0.40 | 0.42 | 0.45 | 0.39 | 0.39 | 0.41 |
| isoC16:0 | 0.351 | 0.244 | 0.17 | 0.16 | 0.16 | 0.169 | 0.188 | 0.26 | 0.29 | 0.26 | 0.16 | 0.15 | 0.18 | 0.19 |
| isoC17:0 | 0.112 | 0.127 | 0.30 | 0.29 | 0.29 | 0.300 | 0.331 | 0.33 | 0.29 | 0.47 | 0.38 | 0.37 | 0.37 | 0.37 |
| anteisoC17:0 | 0.334 | 0.285 | 0.43 | 0.42 | 0.44 | – | – | – | – | – | 0.41 | 0.40 | 0.40 | 0.42 |
| OBCFA | 4.116 | 3.683 | 35.5 | 33.8 | 32.7 | – | – | 2.77 | 2.85 | 3.25 | – | – | – | – |
| Reference | [2] | [51] | [21] | [45] | [46] | |||||||||
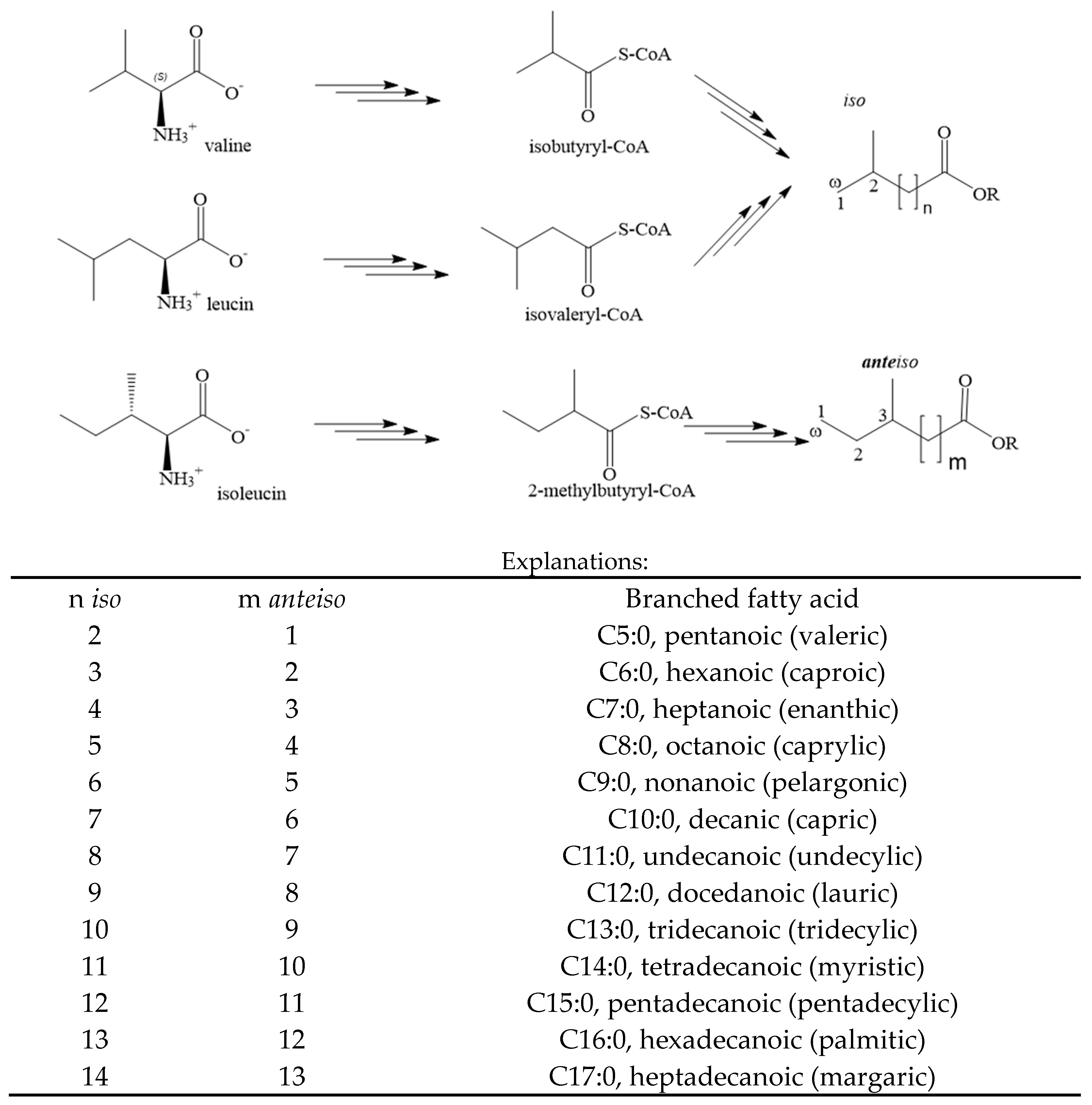
3. The Synthesis of OBCFAs in Cow’s Milk Fat
4. OBCFAs as a Biomarker of Rumen Fermentation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bernbäck, S.; Bläckberg, L.; Hernell, O. The complete digestion of human milk triacylglycerol in vitro requires gastric lipase, pancreatic colipase-dependent lipase, and bile salt-stimulated lipase. J. Clinic. Investig. 1990, 85, 1221–1226. [Google Scholar] [CrossRef] [PubMed]
- Baumann, E.; Chouinard, P.Y.; Lebeuf, Y.; Rico, D.E.; Gervais, R. Effect of lipid supplementation on milk odd- and branched-chain fatty acids in dairy cows. J. Dairy Sci. 2016, 99, 6311–6323. [Google Scholar] [CrossRef] [PubMed]
- Kaneda, T. Iso- and anteiso-fatty acids in bacteria: Biosynthesis, function, and taxonomic significance. Microbiol. Rev. 1991, 55, 288–302. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Hao, X.; Li, Y.; Luo, G.; Zhang, Y.; Xin, H. The relationship between odd- and branched-chain fatty acids and microbial nucleic acid bases in rumen. Asian-Australas. J. Anim. Sci. 2017, 30, 1590–1597. [Google Scholar] [CrossRef] [PubMed]
- Rutkowska, J.; Adamska, A.; Bialek, M. Fatty acid profile of the milk of cows reared in the mountain region of Poland. J. Dairy Res. 2012, 79, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Vlaeminck, B.; Fievez, V.; Cabrita, A.R.J.; Fonseca, A.J.M.; Dewhurst, R.J. Factors affecting odd- and branched-chain fatty acids in milk: A review. Anim. Feed Sci. Technol. 2006, 131, 389–417. [Google Scholar] [CrossRef]
- Vlaeminck, B.; Dufour, C.; van Vuuren, A.M.; Cabrita, A.R.J.; Dewhurst, R.J.; Demeyer, D.; Fievez, V. Use of odd and branched-chain fatty acids in rumen contents and milk as a potential microbial marker. J. Dairy Sci. 2005, 88, 1031–1042. [Google Scholar] [CrossRef]
- Correddu, F.; Gaspa, G.; Pulina, G.; Nudda, A. Grape seed and linseed, alone and in combination, enhance unsaturated fatty acids in the milk of Sarda dairy sheep. J. Dairy Sci. 2016, 99, 1725–1735. [Google Scholar] [CrossRef] [PubMed]
- Fievez, V.; Colman, E.; Castro-Montoya, J.M.; Stefanov, I.; Vlaeminck, B. Milk odd- and branched-chain fatty acids as biomarkers of rumen function—An update. Anim. Feed Sci. Technol. 2012, 1, 51–65. [Google Scholar] [CrossRef]
- Sun, L.L.; Liu, L.; Brenna, J.T.; Wu, Z.H.; Ma, L.; Bu, D.P. Odd-and branched-chain fatty acids in milk fat from Holstein dairy cows are influenced by physiological factors. Animal 2022, 16, 100545. [Google Scholar] [CrossRef]
- Yan, Y.; Wang, Z.; Wang, X.; Wang, Y.; Xiang, J.; Kothapalli, K.S.D.; Brenna, J.T. Branched chain fatty acids positional distribution in human milk fat and common human food fats and uptake in human intestinal cells. J. Funct. Foods 2017, 29, 172–177. [Google Scholar] [CrossRef]
- Mollica, M.P.; Trinchese, G.; Cimmino, F.; Penna, E.; Cavaliere, G.; Tudisco, R.; Musco, N.; Manca, C.; Catapano, A.; Monda, M.; et al. Milk fatty acid profiles in different animal species: Focus on the potential effect of selected pufas on metabolism and brain functions. Nutrients 2021, 13, 1111. [Google Scholar] [CrossRef] [PubMed]
- Lopez, A.; Bellagamba, F.; Savoini, G.; Moretti, V.M.; Cattaneo, D. Characterization of fat quality in cow milk from alpine farms as influenced by seasonal variations of diets. Animals 2022, 12, 515. [Google Scholar] [CrossRef]
- Fox, P.F. Milk Lipids|Fat Globules in Milk. Encyclopedia of Dairy Sciences, 2nd ed.; Academic Press: Cambridge, MA, USA, 2011; pp. 675–679. [Google Scholar]
- Pacheco-Pappenheim, S.; Yener, S.; Heck, J.M.L.; Dijkstra, J.; van Valenberg, H.J.F. Seasonal variation in fatty acid and triacylglycerol composition of bovine milk fat. J. Dairy Sci. 2021, 104, 8479–8492. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Chen, M.; Luo, R.; Huang, G.; Wu, X.; Zheng, N.; Zhang, Y.; Wang, J. Fatty acid profiles of milk from Holstein cows, Jersey cows, buffalos, yaks, humans, goats, camels, and donkeys based on gas chromatography–mass spectrometry. J. Dairy Sci. 2022, 105, 1687–1700. [Google Scholar] [CrossRef] [PubMed]
- Bauman, D.E.; Griinari, J.M. Nutritional regulation of milk fat synthesis. Annu. Rev. Nutrit. 2003, 23, 203–227. [Google Scholar] [CrossRef]
- Teng, F.; Wang, P.; Yang, L.; Ma, Y.; Day, L. Quantification of fatty acids in human, cow, buffalo, goat, yak, and camel milk using an improved One-Step GC-FID method. Food Anal. Meth. 2017, 10, 2881–2891. [Google Scholar] [CrossRef]
- Carta, S.; Correddu, F.; Battacone, G.; Pulina, G.; Nudda, A. Comparison of milk odd- and branched-chain fatty acids among human, dairy species and artificial substitutes. Foods 2022, 11, 4118. [Google Scholar] [CrossRef]
- Stoop, W.M.; Bovenhuis, H.; Heck, J.M.L.; Van Arendonk, J.A.M. Effect of lactation stage and energy status on milk fat composition of Holstein-Friesian cows. J. Dairy Sci. 2009, 92, 1469–1478. [Google Scholar] [CrossRef]
- Craninx, M.; Steen, A.; Van Laar, H.; Van Nespen, T.; Martin-Tereso, J.; De Baets, B.; Fievez, V. Effect of lactation stage on the odd-and branched-chain milk fatty acids of dairy cattle under grazing and indoor conditions. J. Dairy Sci. 2008, 91, 2662–2677. [Google Scholar] [CrossRef]
- Dudi, K.; Devi, I.; Vinay, V.V.; Dhaigude, V. Economic Importance and Management Strategies for Alleviation of MilkFat Depression in DairyAnimals: A Review. Agric. Rev. 2022, 43, 62–69. [Google Scholar]
- Zhang, Y.; Liu, K.; Hao, X.; Xin, H. The relationships between odd- and branched-chain fatty acids to ruminal fermentation parameters and bacterial populations with different dietary ratios of forage and concentrate. J. Anim. Physiol. Anim. Nutr. 2017, 101, 1103–1114. [Google Scholar] [CrossRef] [PubMed]
- Toral, P.G.; Hervás, G.; Della Badia, A.; Gervais, R.; Frutos, P. Effect of dietary lipids and other nutrients on milk odd-and branched-chain fatty acid composition in dairy ewes. J. Dairy Sci. 2020, 103, 11413–11423. [Google Scholar] [CrossRef]
- Xin, H.; Khan, N.A.; Liu, X.; Jiang, X.; Sun, F.; Zhang, S.; Sun, Y.; Zhang, Y.; Li, X. Profiles of odd- and branched-chain fatty acids and their correlations with rumen fermentation parameters, microbial protein synthesis, and bacterial populations based on pure carbohydrate incubation in vitro. Front. Nutr. 2021, 8, 733352. [Google Scholar] [CrossRef] [PubMed]
- Musco, N.; Tudisco, R.; Esposito, G.; Iommelli, P.; Totakul, P.; D’Aniello, B.; Lombardi, P.; Amato, R.; Wanapat, M.; Infascelli, F. Effects of linseed supplementation on milk production, composition, odd- and branched-chain fatty acids, and on serum biochemistry in cilentana grazing goats. Animals 2022, 12, 783. [Google Scholar] [CrossRef] [PubMed]
- Schwendel, B.H.; Morel, P.C.H.; Wester, T.J.; Tavendale, M.H.; Deadman, C.; Fong, B.; Shadbolt, N.M.; Thatcher, A.; Otter, D.E. Fatty acid profile differs between organic and conventionally produced cow milk independent of season or milking time. J. Dairy Sci. 2015, 98, 1411–1425. [Google Scholar] [CrossRef] [PubMed]
- Bainbridge, M.L.; Saldinger, L.K.; Barlow, J.W.; Alvez, J.P.; Roman, J.; Kraft, J. Alteration of rumen bacteria and protozoa through grazing regime as a tool to enhance the bioactive fatty acid content of bovine milk. Front. Microbiol. 2018, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Colman, E.; Fokkink, W.B.; Craninx, M.; Newbold, J.R.; De Baets, B.; Fievez, V. Effect of induction of subacute ruminal acidosis on milk fat profile and rumen parameters. J. Dairy Sci. 2010, 93, 4759–4773. [Google Scholar] [CrossRef] [PubMed]
- Roman-Garcia, Y.; Denton, B.L.; Mitchell, K.E.; Lee, C.; Socha, M.T.; Firkins, J.L. Conditions stimulating neutral detergent fiber degradation by dosing branched-chain volatile fatty acids. I: Comparison with branched-chain amino acids and forage source in ruminal batch cultures. J. Dairy Sci. 2021, 104, 6739–6755. [Google Scholar] [CrossRef]
- Luo, Z.; Evans, A.C.O.; Bu, D. The relation and variation of OBCFA content in rumen fluid, blood and milk from lactating dairy cows. Livest. Sci. 2024, 281, 105417. [Google Scholar] [CrossRef]
- Matamoros, C.; Cai, J.; Patterson, A.D.; Harvatine, K.J. Comparison of the effects of short-term feeding of sodium acetate and sodium bicarbonate on milk fat production. J. Dairy Sci. 2021, 104, 7572–7582. [Google Scholar] [CrossRef] [PubMed]
- Prado, L.A.; Schmidely, P.; Nozière, P.; Ferlay, A. Milk saturated fatty acids, odd- and branched-chain fatty acids, and isomers of C18:1, C18:2, and C18: 3n -3 according to their duodenal flows in dairy cows: A meta-analysis approach. J. Dairy Sci. 2019, 102, 3053–3070. [Google Scholar] [CrossRef] [PubMed]
- Purba, R.A.P.; Yuangklang, C.; Paengkoum, S.; Paengkoum, P. Milk fatty acid composition. rumen microbial population and animal performance in response to diets rich in linoleic acid supplemented with Piper betle leaves in Saanen goats. Anim. Prod. Sci. 2020, 62, 1391–1401. [Google Scholar] [CrossRef]
- Li, Y.; Shi, C.; Deng, J.; Qiu, X.; Zhang, S.; Wang, H.; Qin, X.; He, Y.; Cao, B.; Su, H. Effects of Grape Pomace on Growth Performance. Nitrogen Metabolism. Antioxidants. and Microbial Diversity in Angus Bulls. Antioxidants 2024, 13, 412. [Google Scholar] [CrossRef] [PubMed]
- Pegolo, S.; Stocco, G.; Mele, M.; Schiavon, S.; Bittante, G.; Cecchinato, A. Factors affecting variations in the detailed fatty acid profile of Mediterranean buffalo milk determined by 2-dimensional gas chromatography. J. Dairy Sci. 2017, 100, 2564–2576. [Google Scholar] [CrossRef] [PubMed]
- Pegolo, S.; Cecchinato, A.; Casellas, J.; Conte, G.; Mele, M.; Schiavon, S.; Bittante, G. Genetic and environmental relationships of detailed milk fatty acids profile determined by gas chromatography in Brown Swiss cows. J. Dairy Sci. 2016, 99, 1315–1330. [Google Scholar] [CrossRef] [PubMed]
- Conte, G.; Serra, A.; Cremonesi, P.; Chessa, S.; Castiglioni, B.; Cappucci, A.; Bulleri, E.; Mele, M. Investigating mutual relationship among milk fatty acids by multivariate factor analysis in dairy cows. Livest. Sci. 2016, 188, 124–132. [Google Scholar] [CrossRef]
- Adamska, A.; Rasińska, E.; Rutkowska, J.; Antoniewska, A. Fatty acid profile of commercial Camembert- and Brie-type cheeses available on the Polish market. CyTA. J. Food 2017, 15, 639–645. [Google Scholar]
- Segato, S.; Galaverna, G.; Contiero, B.; Berzaghi, P.; Caligiani, A.; Marseglia, A.; Cozzi, G. Identification of lipid biomarkers to discriminate between the different production systems for Asiago PDO Cheese. J. Agric. Food Chem. 2017, 15, 9887–9892. [Google Scholar] [CrossRef]
- Teter, A.; Domaradzki, P.; Kędzierska-Matysek, M.; Sawicka-Zugaj, W.; Florek, M. Comprehensive investigation of humic-mineral substances from oxyhumolite: Effects on fatty acid composition and health lipid indices in milk and cheese from Holstein-Friesian cows. Appl. Sci. 2023, 13, 9624. [Google Scholar] [CrossRef]
- Nudda, A.; Correddu, F.; Cesarani, A.; Pulina, G.; Battacone, G. Functional odd- and branched-chain fatty acid in sheep and goat milk and cheeses. Dairy 2021, 2, 79–89. [Google Scholar] [CrossRef]
- Ran-Ressler, R.R.; Bae, S.; Lawrence, P.; Wang, D.H.; Brenna, J.T. Branched-chain fatty acid content of foods and estimated intake in the USA. Br. J. Nutr. 2014, 112, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Bainbridge, M.L.; Egolf, E.; Barlow, J.W.; Alvez, J.P.; Roman, J.; Kraft, J. Milk from cows grazing on cool-season pastures provides an enhanced profile of bioactive fatty acids compared to those grazed on a monoculture of pearl millet. Food Chem. 2017, 217, 750–755. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Wredle, E.; Bertilsson, J. Effect of dietary proportion of grass silage on milk fat with emphasis on odd- and branched-chain fatty acids in dairy cows. J. Dairy Sci. 2013, 96, 390–397. [Google Scholar] [CrossRef] [PubMed]
- van Gastelen, S.; Antunes-Fernandes, E.C.; Hettinga, K.A.; Klop, G.; Alferink, S.J.J.; Hendriks, W.H.; Dijkstra, J. Enteric methane production, rumen volatile fatty acid concentrations, and milk fatty acid composition in lactating Holstein-Friesian cows fed grass silage- or corn silage-based diets. J. Dairy Sci. 2015, 98, 1915–1927. [Google Scholar] [CrossRef] [PubMed]
- Rego, O.A.; Rosa, H.J.D.; Regalo, S.M.; Alves, S.P.; Alfaia, C.M.M.; Prates, J.A.M.; Vouzela, C.M.; Bessa, R.J.B. Seasonal changes of CLA isomers and other fatty acids of milk fat from grazing dairy herds in the Azores. J. Sci. Food Agric. 2008, 88, 1855–1859. [Google Scholar] [CrossRef]
- Westreicher-Kristen, E.; Castro-Montoya, J.; Hasler, M.; Susenbeth, A. Relationship of milk odd-and branched-chain fatty acids with urine parameters and ruminal microbial protein synthesis in dairy cows fed different proportions of maize silage and red clover silage. Animals 2020, 10, 316. [Google Scholar] [CrossRef]
- Moate, P.J.; Williams, S.R.O.; Torok, V.A.; Hannah, M.C.; Ribaux, B.E.; Tavendale, M.H.; Eckard, R.J.; Jacobs, J.L.; Auldist, M.J.; Wales, W.J. Grape marcreduces methane emissions when fed to dairy cows. J. Dairy Sci. 2014, 97, 5073–5087. [Google Scholar] [CrossRef]
- Jones, G.A.; McAllister, T.A.; Muir, A.D.; Cheng, K.-J. Effects of sainfoin (Onobrychis viciifolia Scop:) condensed tannins on growth and proteolysis by four strains of ruminal bacteria. Appl. Environ. Microbiol. 1994, 60, 1374–1378. [Google Scholar] [CrossRef]
- Castro-Montoya, J.; Henke, A.; Molkentin, J.; Knappstein, K.; Susenbeth, A.; Dickhoefer, U. Relationship between milk odd and branched-chain fatty acids and urinary purine derivatives in dairy cows supplemented with quebracho tannins—A study to test milk fatty acids as predictors of rumen microbial protein synthesis. Anim. Feed Sci. Technol. 2016, 214, 22–33. [Google Scholar] [CrossRef]
- Nantapo, C.T.W.; Muchenje, V.; Hugo, A. Atherogenicity index and health-related fatty acids in different stages of lactation from Friesian, Jersey and Friesian × Jersey cross cow milk under a pasture-based dairy system. Food Chem. 2014, 146, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Bainbridge, M.L.; Cersosimo, L.M.; Wright, A.D.G.; Kraft, J. Content and composition of branched-chain fatty acids in bovine milk are affected by lactation stage and breed of dairy cow. PLoS ONE 2016, 11, e0150386. [Google Scholar] [CrossRef] [PubMed]
- Bhagwat, A.M.; De Baets, B.; Steen, A.; Vlaeminck, B.; Fievez, V. Prediction of ruminal volatile fatty acid proportions of lactating dairy cows based on milk odd-and branched-chain fatty acid profiles: New models, better predictions. J. Dairy Sci. 2012, 95, 3926–3937. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Li, Y.; Luo, G.; Xin, H.; Zhang, Y.; Li, G. Relations of ruminal fermentation parameters and microbial matters to odd- and branched-chain fatty acids in rumen fluid of dairy cows at different milk stages. Animals 2019, 22, 1019. [Google Scholar] [CrossRef] [PubMed]
- de Souza, J.; Leskinen, H.; Lock, A.L.; Shingfield, K.J.; Huhtanen, P. Between-cow variation in milk fatty acids associated with methane production. PLoS ONE 2020, 15, e0235357. [Google Scholar] [CrossRef] [PubMed]
- Fievez, V.; Vlaeminck, B.; Dhanoa, M.S.; Dewhurst, R.J. Use of principal component analysis to investigate the origin of heptadecenoic and conjugated linoleic acids in milk. J. Dairy Sci. 2003, 86, 4047–4053. [Google Scholar] [CrossRef] [PubMed]
- Dewhurst, R.J.; Moorby, J.M.; Vlaeminck, B.; Fievez, V. Apparent recovery of duodenal odd- and branched-chain fatty acids in milk of dairy cows. J. Dairy Sci. 2007, 90, 1775–1780. [Google Scholar] [CrossRef] [PubMed]
- Vlaeminck, B.; Gervais, R.; Rahman, M.M.; Gadeyne, F.; Gorniak, M.; Doreau, M.; Fievez, V. Postruminal synthesis modifies the odd- and branched-chain fatty acid profile from the duodenum to milk. J. Dairy Sci. 2015, 98, 4829–4840. [Google Scholar] [CrossRef]
- Vazirigohar, M.; Dehghan-Banadaky, M.; Rezayazdi, K.; Nejati-Javaremi, A.; Mirzaei-Alamouti, H.; Patra, A.K. Effects of diets containing supplemental fats on ruminal fermentation and milk odd-and branched-chain fatty acids in dairy cows. J. Dairy Sci. 2018, 101, 6133–6141. [Google Scholar] [CrossRef] [PubMed]
- AbuGhazaleh, A.A.; Potu, R.B.; Ibrahim, S. Short Communication: The Effect of Substituting Fish Oil in Dairy Cow Diets with Docosahexaenoic Acid-Micro Algae on Milk Composition and Fatty Acids Profile’. J. Dairy Sci. 2009, 92, 6156–6159. [Google Scholar] [CrossRef]
- Angulo, J.; Hiller, B.; Olivera, M.; Mahecha, L.; Dannenberger, D.; Nuernberg, G.; Losand, B.; Nuernberg, K. Dietary fatty acid intervention of lactating cows simultaneously affects lipid profiles of meat and milk. J. Sci. Food Agri. 2012, 92, 2968–2974. [Google Scholar] [CrossRef]
- Sterk, A.; Vlaeminck, A.B.; van Vuuren, A.M.; Hendriks, W.H.; Dijkstra, J. Effects of feeding different linseed sources on omasal fatty acid flows and fatty acid profiles of plasma and milk fat in lactating dairy cows. J. Dairy Sci. 2012, 95, 3149–3165. [Google Scholar] [CrossRef]
- Thanh, L.P.; and Suksombat, W. Milk yield, composition, and fatty acid profile in dairy cows fed a high-concentrate diet blended with oil mixtures rich in polyunsaturated fatty acids. Asian-Australas. J. Anim. Sci. 2015, 28, 796–806. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.P.; Avramis, C.A.; Kramer, J.K.G.; Marangoni, A.G. Algal meal supplementation of the cows’ diet alters the physical properties of milk fat. J. Dairy Res. 2004, 71, 66–73. [Google Scholar] [CrossRef]
- Mondello, L. FAMEs Fatty Acid Methyl esters: Mass Spectral Database; Wiley: Oxford, UK, 2011. [Google Scholar]
- Fu, X.; Hafza, N.; Götz, F.; Lämmerhofer, M. Profiling of branched chain and straight chain saturated fatty acids by ultra-high performance liquid chromatography-mass spectrometry. J. Chromatogr. A 2023, 1703, 464111. [Google Scholar] [CrossRef] [PubMed]
- Abdela, N. Sub-acute ruminal acidosis (SARA) and its consequence in dairy cattle: A review of past and recent research at global prospective. Achiev. Life Sci. 2016, 10, 187–196. [Google Scholar] [CrossRef]
- Shinkai, T.; Ikeyama, N.; Kumagai, M.; Ohmori, H.; Sakamoto, M.; Ohkuma, M.; Mitsumori, M. Prevotella lacticifex sp. nov., isolated from the rumen of cows. Int. J. Syst. Evol. Microbiol. 2022, 72, 005278. [Google Scholar] [CrossRef] [PubMed]
- Or-Rashid, M.M.; Odongo, N.E.; McBride, B.W. Fatty acid composition of ruminal bacteria and protozoa, with emphasis on conjugated linoleic acid, vaccenic acid, and odd-chain and branched-chain fatty acids. J. Anim. Sci. 2007, 85, 1228–1234. [Google Scholar] [CrossRef]
- Vlaeminck, B.; Fievez, V.; Tamminga, S.; Dewhurst, R.J.; van Vuuren, A.; de Brabander, D.; Demeyer, D. Milk odd- and branched-chain fatty acids in relation to the rumen fermentation pattern. J. Dairy Sci. 2006, 89, 3954–3964. [Google Scholar] [CrossRef]
- Dewanckele, L.; Jeyanathan, J.; Vlaeminck, B.; Fievez, V. Identifying and exploring biohydrogenating rumen bacteria with emphasis on pathways including trans-10 intermediates. BMC Microbiol. 2020, 20, 198. [Google Scholar] [CrossRef]
- Diez-Gonzalez, F.; Bond, D.R.; Jennings, E.; Russell, J.B. Alternative schemes of butyrate production in butyrivibrio fibrisolvens and their relationship to acetate utilization, lactate production, and phylogeny. Arch. Microbiol. 1999, 171, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Snyder, E.; Credille, B. Diagnosis and treatment of clinical rumen acidosis. Vet. Clin. Food Anim. Pract. 2017, 33, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Heirbaut, S.; Jeyanathan, J.; Jing, X.P.; de Neve, N.; Vandaele, L.; Fievez, V. Subacute ruminal acidosis phenotypes in periparturient dairy cows differ in ruminal and salivary bacteria and in the in vitro fermentative activity of their ruminal microbiota. J. Dairy Sci. 2022, 105, 3969–3987. [Google Scholar] [CrossRef]
- Mao, S.Y.; Zhang, R.Y.; Wang, D.S.; Zhu, W.Y. Impact of subacute ruminal acidosis (SARA) adaptation on rumen microbiota in dairy cattle using pyrosequencing. Anaerobe 2013, 24, 12–19. [Google Scholar] [CrossRef]
- McCann, J.C.; Luan, S.; Cardoso, F.C.; Derakhshani, H.; Khafipour, E.; Loor, J.J. Induction of subacute ruminal acidosis affects the ruminal microbiome and epithelium. Front. Microbiol. 2016, 7, 701. [Google Scholar] [CrossRef] [PubMed]
- Kell, D.B.; Pretorius, E. On the translocation of bacteria and their lipopolysaccharides between blood and peripheral locations in chronic, inflammatory diseases: The central roles of LPS and LPS-induced cell death. Integrat. Biol. 2015, 7, 1339–1377. [Google Scholar] [CrossRef] [PubMed]
- Matamoros, C.; Hao, F.; Tian, Y.; Patterson, A.D.; Harvatine, K.J. Interaction of sodium acetate supplementation and dietary fiber level on feeding behavior, digestibility, milk synthesis, and plasma metabolites. J. Dairy Sci. 2022, 105, 8824–8838. [Google Scholar] [CrossRef]
- Sandri, E.C.; Lévesque, J.; Marco, A.; Couture, Y.; Gervais, R.; Rico, D.E. Transient reductions in milk fat synthesis and their association with the ruminal and metabolic profile in dairy cows fed high-starch, low-fat diets. Animal 2020, 14, 2523–2534. [Google Scholar] [CrossRef]
- Jing, L.; Dewanckele, L.; Vlaeminck, B.; van Straalen, W.M.; Koopmans, A.; Fievez, V. Susceptibility of dairy cows to subacute ruminal acidosis is reflected in milk fatty acid proportions, with C18: 1 trans-10 as primary and C15: 0 and C18: 1 trans-11 as secondary indicators. J. Dairy Sci. 2018, 101, 9827–9840. [Google Scholar] [CrossRef]
- Yang, H.; Heirbaut, S.; Jing, X.; De Neve, N.; Vandaele, L.; Jeyanathan, J.; Fievez, V. Susceptibility of dairy cows to subacute ruminal acidosis is reflected in both prepartum and postpartum bacteria as well as odd-and branched-chain fatty acids in feces. J. Anim. Sci. Biotechnol. 2022, 13, 87. [Google Scholar] [CrossRef]
- Gómez-Cortés, P.; de la Fuente, M.A.; Toral, P.G.; Frutos, P.; Juárez, M.; Hervás, G. Effects of different forage: Concentrate ratios in dairy ewe diets supplemented with sunflower oil on animal performance and milk fatty acid profile. J. Dairy Sci. 2011, 94, 4578–4588. [Google Scholar] [CrossRef] [PubMed]
- Harper, M.T.; Oh, J.; Melgar, A.; Nedelkov, K.; Räisänen, S.; Chen, X.; Hristov, A.N. Production effects of feeding extruded soybean meal to early-lactation dairy cows. J. Dairy Sci. 2019, 102, 8999–9016. [Google Scholar] [CrossRef] [PubMed]
- Chapinal, N.; Leblanc, S.J.; Carson, M.E.; Leslie, K.E.; Godden, S.; Capel, M.; Santos, J.E.P.; Overton, M.W.; Duffield, T.F. Herd-level association of serum metabolites in the transition period with disease, milk production, and early lactation reproductive performance. J. Dairy Sci. 2012, 95, 5676–5682. [Google Scholar] [CrossRef] [PubMed]
- Jorjong, S.; van Knegsel, A.T.M.; Verwaeren, J.; Bruckmaier, R.M.; De Baets, B.; Kemp, B.; Fievez, V. Milk fatty acids as possible biomarkers to diagnose hyperketonemia in early lactation. J. Dairy Sci. 2015, 98, 5211–5221. [Google Scholar] [CrossRef] [PubMed]
- Mann, S.; Nydam, D.V.; Lock, A.L.; Overton, T.R.; McArt, J.A.A. Short communication: Association of milk fatty acids with early lactation hyperketonemia and elevated concentration of nonesterified fatty acids. J. Dairy Sci. 2016, 99, 5851–5857. [Google Scholar] [CrossRef] [PubMed]
- Pires, J.A.A.; Larsen, T.; Leroux, C. Milk metabolites and fatty acids as noninvasive biomarkers of metabolic status and energy balance in early-lactation cows. J. Dairy Sci. 2022, 105, 201–220. [Google Scholar] [CrossRef]
- Rico, D.E.; Chouinard, P.Y.; Hassanat, F.; Benchaar, C.; Gervais, R. Prediction of enteric methane emissions from holstein dairy cows fed various forage sources. Animal 2016, 10, 203–211. [Google Scholar] [CrossRef]
- Chilliard, Y.; Martin, C.; Rouel, J.; Doreau, M. Milk fatty acids in dairy cows fed whole crude linseed, extruded linseed, or linseed oil, and their relationship with methane output. J. Dairy Sci. 2009, 92, 5199–5211. [Google Scholar] [CrossRef]
- van Gastelen, S.; Dijkstra, J.; Binnendijk, G.; Duval, S.M.; Heck, J.M.; Kindermann, M.; Bannink, A. 3-Nitrooxypropanol decreases methane emissions and increases hydrogen emissions of early lactation dairy cows, with associated changes in nutrient digestibility and energy metabolism. J. Dairy Sci. 2020, 103, 8074–8093. [Google Scholar] [CrossRef]
- van Gastelen, S.; Dijkstra, J.; Heck, J.M.; Kindermann, M.; Klop, A.; de Mol, R.; Bannink, A. Methane mitigation potential of 3-nitrooxypropanol in lactating cows is influenced by basal diet composition. J. Dairy Sci. 2022, 105, 4064–4082. [Google Scholar] [CrossRef]
- Yanibada, B.; Hohenester, U.; Pétéra, M.; Canlet, C.; Durand, S.; Jourdan, F.; Boudra, H. Milk metabolome reveals variations on enteric methane emissions from dairy cows fed a specific inhibitor of the methanogenesis pathway. J. Dairy Sci. 2021, 104, 12553–12566. [Google Scholar] [CrossRef] [PubMed]
| FA | Milk | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Buffalo | Cow | Goat | Sheep | ||||||
| [19] | [36] | [19] | [37] | [38] | [19] | [11] | [19] | [8] | |
| C11:0 | 0.02 | 0.06 | 0.09 | 0.06 | 0.08 | 0.19 | – | 0.29 | 0.1 |
| C15:0 | 1.15 | 1.34 | 1.06 | 1.18 | 1.05 | 0.75 | – | 1.18 | 1.26 |
| C17:0 | 0.51 | 0.54 | 0.46 | 0.54 | 0.46 | 0.79 | – | 0.76 | 0.65 |
| iso-C13:0 | 0.02 | 0.07 | 0.09 | 0.06 | 0.08 | 0.03 | 0.01 | 0.03 | 0.06 |
| anteiso-C13:0 | 0.04 | – | – | – | – | 0.02 | – | 0.04 | 0.01 |
| iso-C14:0 | 0.19 | 0.26 | 0.12 | 0.17 | 0.11 | 0.1 | 0.06 | 0.12 | 0.1 |
| iso-C15:0 | 0.32 | 0.4 | 0.22 | 0.28 | 0.21 | 0.19 | 0.16 | 0.29 | 0.2 |
| anteiso-C15:0 | 0.54 | 0.64 | 0.44 | 0.53 | 45 | 0.33 | 0.32 | 0.56 | 0.49 |
| iso-C16:0 | 0.39 | 0.46 | 0.233 | 0.32 | 0.22 | 0.25 | 0.19 | 0.33 | 0.29 |
| iso-C17:0 | 0.24 | 0.3 | 0.25 | 0.32 | 0.26 | 0.31 | 0.42 | 0.42 | 0.36 |
| anteiso-C17:0 | 0.37 | 0.41 | 0.42 | 0.42 | 0.4 | 0.39 | 0.44 | 0.5 | 0.5 |
| BCFA | 2.1 | 2.65 | 1.78 | 2.08 | 1.74 | 1.6 | – | 2.29 | 2.01 |
| OBCFA | 3.78 | 5.02 | 3.38 | 3.97 | 3.64 | 3.33 | – | 4.51 | 3.88 |
| Σ iso-FA/Σ BCFA, % | 55.19 | 56.22 | 51.66 | 55.29 | 50.57 | 53.93 | 53.88 | 51.75 | 50.25 |
| Σ anteiso-FA/Σ BCFA, % | 44.81 | 39.62 | 48.34 | 45.67 | 48.85 | 46.07 | 46.12 | 48.25 | 49.75 |
| FA | Cheese | Yoghurt | Butter | Sour Cream | Ice Cream | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cow | Goat | Sheep | ||||||||||
| [39] 1 | [40] 2 | [41] 3 | [42] | [42] | [43] | [43] | [43] | [43] | ||||
| P-UL | H-UL | MS-LL | 0 | 60 | ||||||||
| C11:0 | 0.32–0.39 | – | – | – | 0.02 | 0.02 | – | – | – | – | – | – |
| C15:0 | 1.12–1.20 | 1.23 | 1.24 | 1.28 | 0.23 | 0.27 | 0.89 | 1.21 | – | – | – | – |
| C17:0 | 0.27–0.62 | 0.62 | 0.57 | 0.61 | 0.16 | 0.18 | 0.75 | 0.66 | – | – | – | – |
| iso-C13:0 | 0.11–0.13 | – | – | – | 0.02 | 0.02 | 0.02 | 0.02 | – | – | – | – |
| anteiso-C13:0 | 0.10–0.16 | – | – | – | 0.02 | 0.03 | 0.07 | 0.05 | – | – | – | – |
| iso-C14:0 | 0.25–0.29 | – | – | – | 0.03 | 0.03 | 0.11 | 0.1 | 0.12 | 0.17 | 0.05 | 0.14 |
| iso-C15:0 | 1.07–1.28 | 0.38 | 0.31 | 0.31 | 0.05 | 0.06 | 0.24 | 0.28 | 0.15 | 0.01 | 0.11 | 0.33 |
| anteiso-C15:0 | 0.48–0.53 | 0.66 | 0.62 | 0.59 | 0.11 | 0.12 | 0.39 | 0.55 | 0.62 | 0.63 | 0.46 | 0.42 |
| iso-C16:0 | 0.15–0.16 | 0.46 | 0.34 | 0.34 | 0.07 | 0.07 | 0.25 | 0.29 | 0.29 | 0.34 | 0.24 | 0.46 |
| iso-C17:0 | – | 0.46 | 0.42 | 0.38 | 0.07 | 0.08 | 0.17 | 0.41 | 0.25 | 0.31 | 0.3 | 0.17 |
| anteiso-C17:0 | 0.37–0.45 | 0.42 | 0.39 | 0.38 | 0.12 | 0.14 | 0.4 | 0.5 | 0.59 | 0.38 | 0.36 | 0.57 |
| OBCFA | 4.74–5.39 | 4.53 | 4.13 | 4.12 | 0.98 | 1.13 | 3.12 | 4.2 | – | – | – | – |
| FA | Early | Middle | Late | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HO | HO * | HO | JE | CB | HO | HO * | HO | JE | CB | HO | HO * | HO | JE | CB | |
| C11:0 | 0.03 | 0.08 | 0.21 | 0.24 | 0.25 | 0.03 | 0.07 | 0.26 | 0.28 | 0.28 | 0.04 | 0.05 | 0.26 | 0.28 | 0.30 |
| C15:0 | 0.84 | 1.12 | 1.21 | 1.19 | 1.30 | 0.84 | 1.01 | 1.24 | 1.20 | 1.16 | 0.78 | 0.94 | 1.13 | 1.21 | 1.20 |
| C17:0 | 0.57 | 0.51 | 0.68 | 0.65 | 0.72 | 0.43 | 0.50 | 0.67 | 0.65 | 0.65 | 0.47 | 0.51 | 0.62 | 0.65 | 0.70 |
| isoC13:0 | – | 0.05 | 0.02 | 0.02 | 0.02 | – | 0.06 | 0.02 | 0.02 | 0.02 | – | 0.06 | 0.04 | 0.03 | 0.05 |
| anteisoC13:0 | – | 0.04 | 0.05 | 0.08 | 0.07 | – | 0.05 | 0.10 | 0.09 | 0.09 | – | 0.05 | 0.09 | 0.10 | 0.11 |
| isoC14:0 | – | 0.15 | 0.07 | 0.12 | 0.12 | – | 0.15 | 0.10 | 0.12 | 0.12 | – | 0.16 | 0.13 | 0.13 | 0.16 |
| isoC15:0 | 0.14 | 0.19 | 0.19 | 0.18 | 0.20 | 0.15 | 0.20 | 0.19 | 0.18 | 0.19 | 0.15 | 0.27 | 0.21 | 0.22 | 0.23 |
| anteisoC15:0 | 0.38 | – | 0.42 | 0.36 | 0.42 | 0.39 | – | 0.43 | 0.37 | 0.40 | 0.38 | – | 0.43 | 0.42 | 0.48 |
| isoC16:0 | 0.25 | 0.36 | 0.19 | 0.26 | 0.25 | 0.19 | 0.32 | 0.26 | 0.32 | 0.26 | 0.16 | 0.32 | 0.30 | 0.35 | 0.39 |
| isoC17:0 | 0.44 | 0.54 | 0.30 | 0.24 | 0.30 | 0.52 | 0.55 | 0.26 | 0.23 | 0.26 | 0.55 | 0.60 | 0.26 | 0.23 | 0.30 |
| anteisoC17:0 | 0.83 | 0.46 | 0.09 | 0.10 | 0.10 | 1.13 | 0.43 | 0.14 | 0.11 | 0.09 | 1.01 | 0.44 | 0.06 | 0.07 | 0.09 |
| OBCFA | 3.54 | 3.99 | 5.10 | 5.05 | 5.59 | 3.57 | 3.82 | 5.51 | 5.23 | 5.16 | 3.69 | 3.89 | 5.16 | 5.38 | 5.80 |
| Reference | [55] | [10] | [53] | [55] | [10] | [53] | [55] | [10] | [53] | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kupczyński, R.; Pacyga, K.; Lewandowska, K.; Bednarski, M.; Szumny, A. Milk Odd- and Branched-Chain Fatty Acids as Biomarkers of Rumen Fermentation. Animals 2024, 14, 1706. https://doi.org/10.3390/ani14111706
Kupczyński R, Pacyga K, Lewandowska K, Bednarski M, Szumny A. Milk Odd- and Branched-Chain Fatty Acids as Biomarkers of Rumen Fermentation. Animals. 2024; 14(11):1706. https://doi.org/10.3390/ani14111706
Chicago/Turabian StyleKupczyński, Robert, Katarzyna Pacyga, Kamila Lewandowska, Michał Bednarski, and Antoni Szumny. 2024. "Milk Odd- and Branched-Chain Fatty Acids as Biomarkers of Rumen Fermentation" Animals 14, no. 11: 1706. https://doi.org/10.3390/ani14111706
APA StyleKupczyński, R., Pacyga, K., Lewandowska, K., Bednarski, M., & Szumny, A. (2024). Milk Odd- and Branched-Chain Fatty Acids as Biomarkers of Rumen Fermentation. Animals, 14(11), 1706. https://doi.org/10.3390/ani14111706







