Exploration of Molecular Mechanisms of Immunity in the Pacific Oyster (Crassostrea gigas) in Response to Vibrio alginolyticus Invasion
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Crassostrea gigas and Vibrio alginolyticus
2.2. Sample Treatment and RNA Extraction
2.3. Library Construction and RNA Sequencing
2.4. Processing of Data and Differential Expression Analysis
2.5. Functional Enrichment Analysis and PPI Networks
2.6. Gene Expression Validation by RT-PCR
2.7. Histological Analysis
3. Results
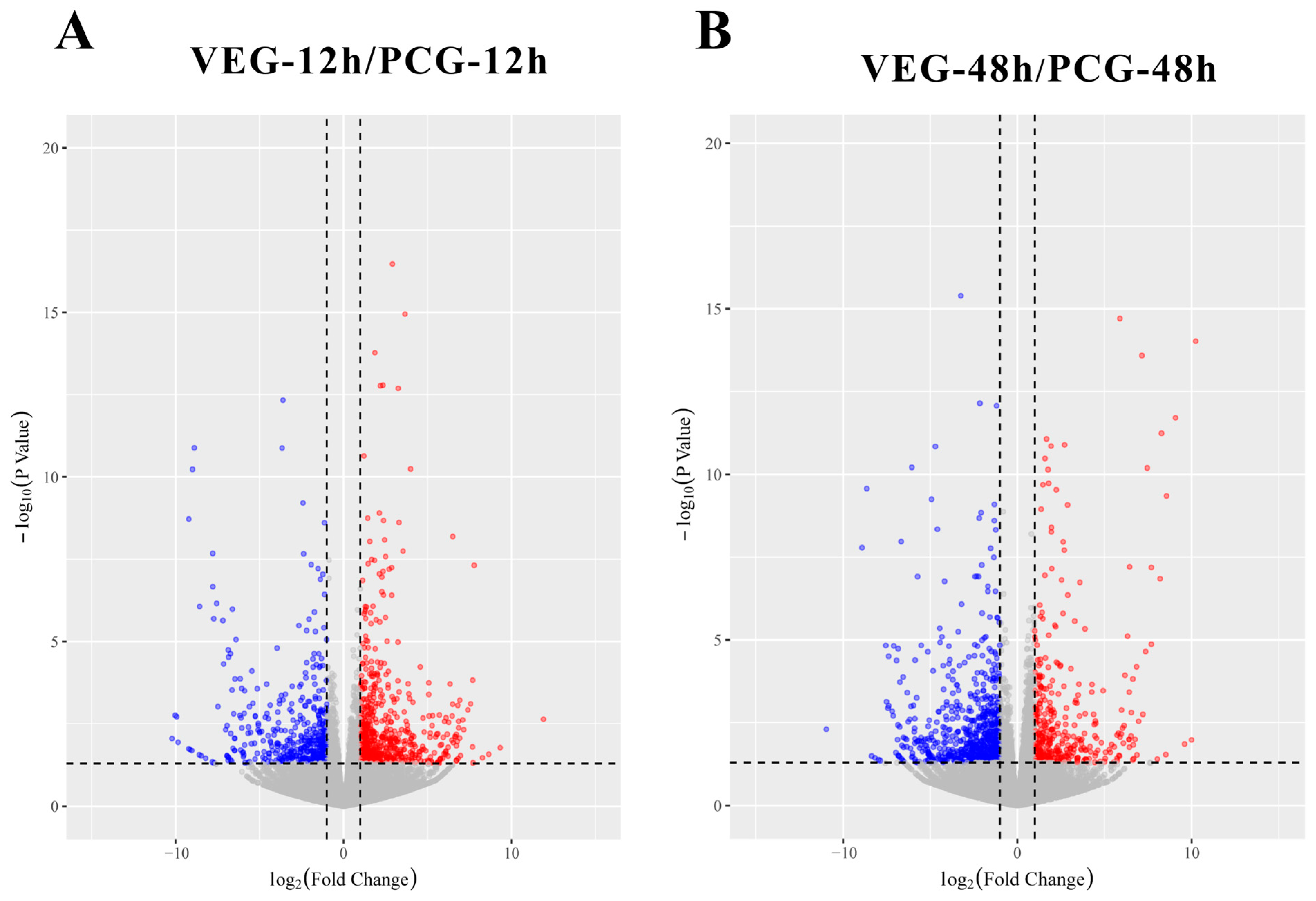
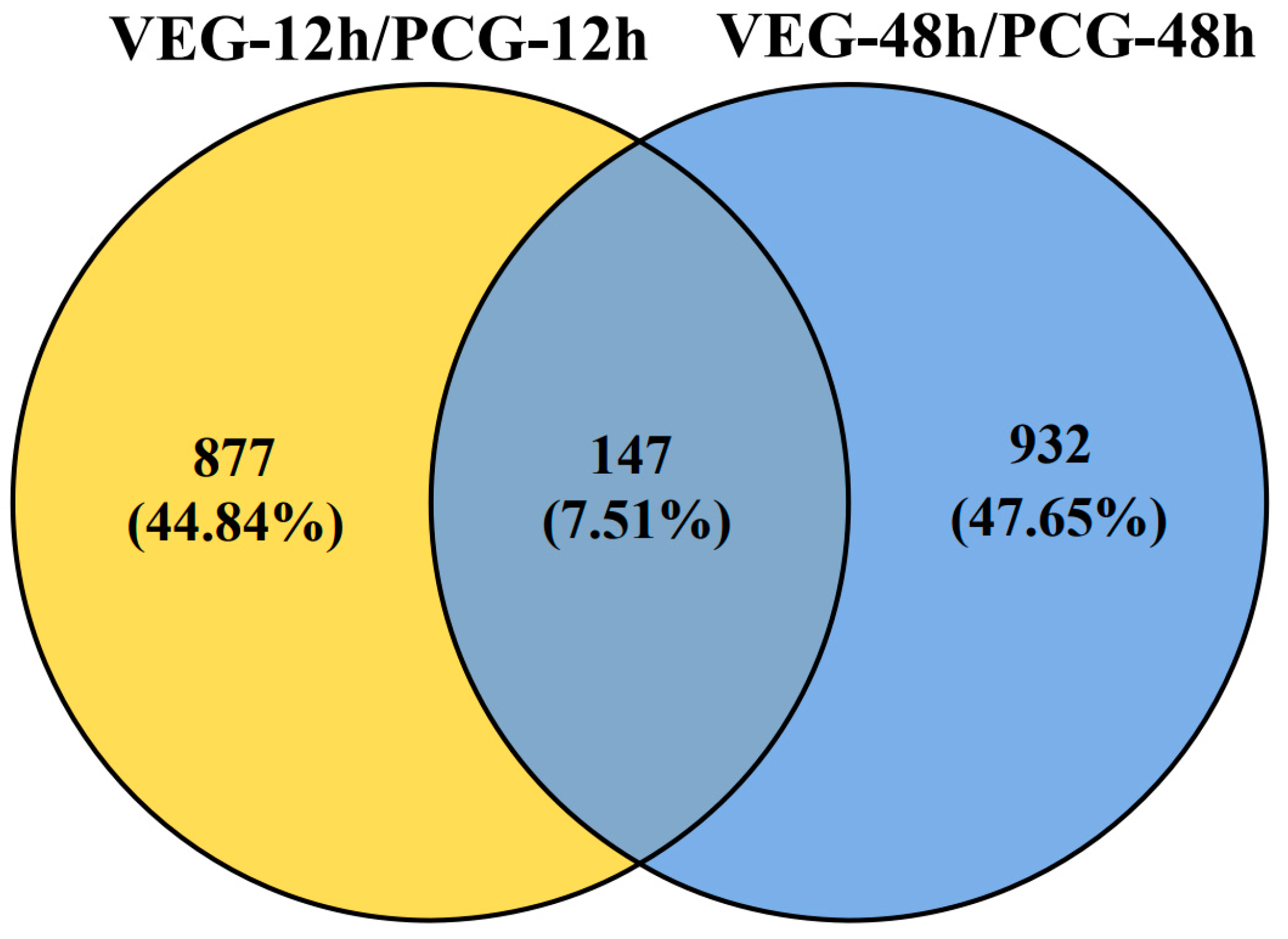
3.1. Identification of DEGs
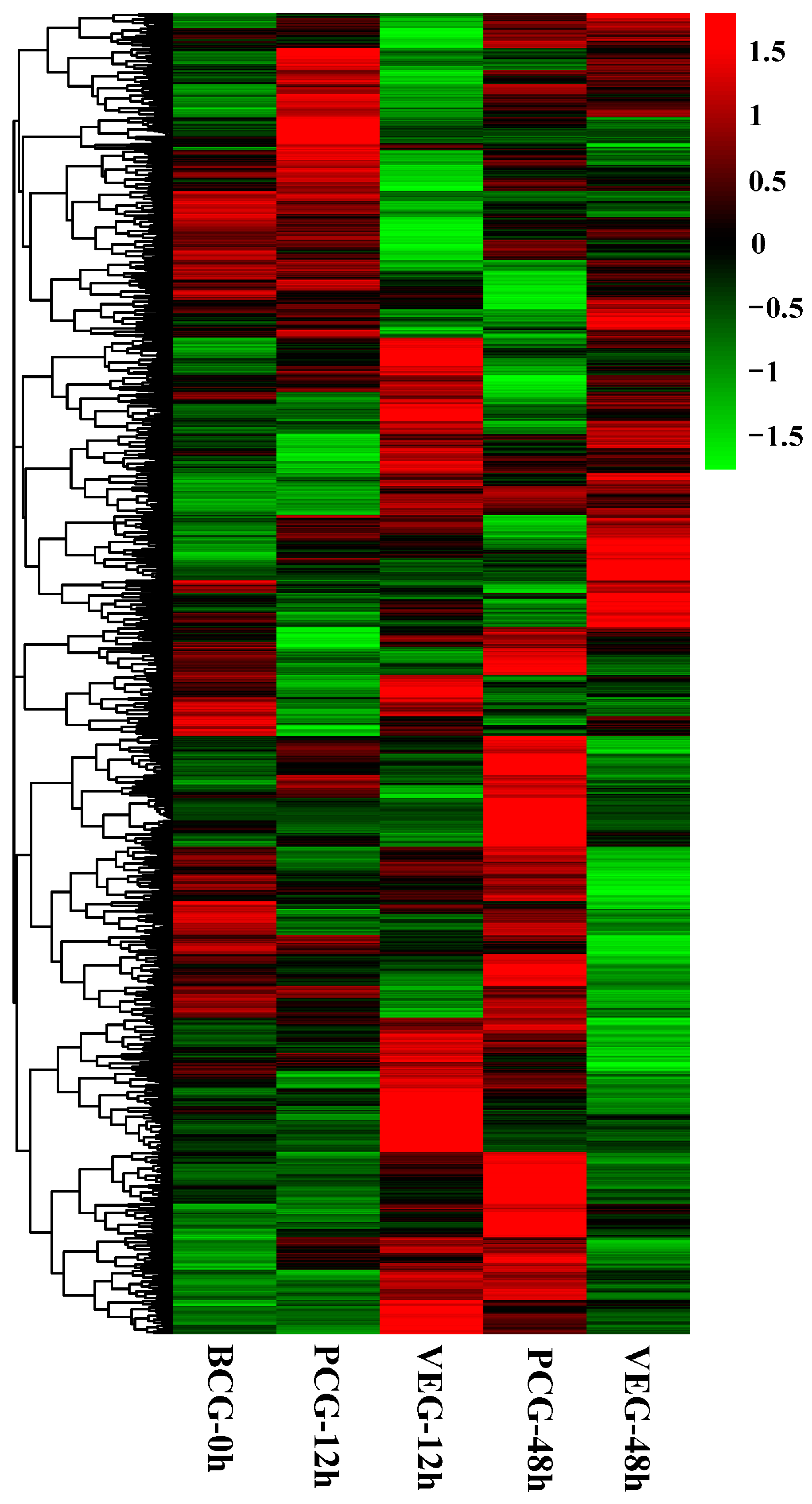
3.2. Analysis of DEGs
3.3. GO and KEGG Functional Enrichment Analysis of DEGs
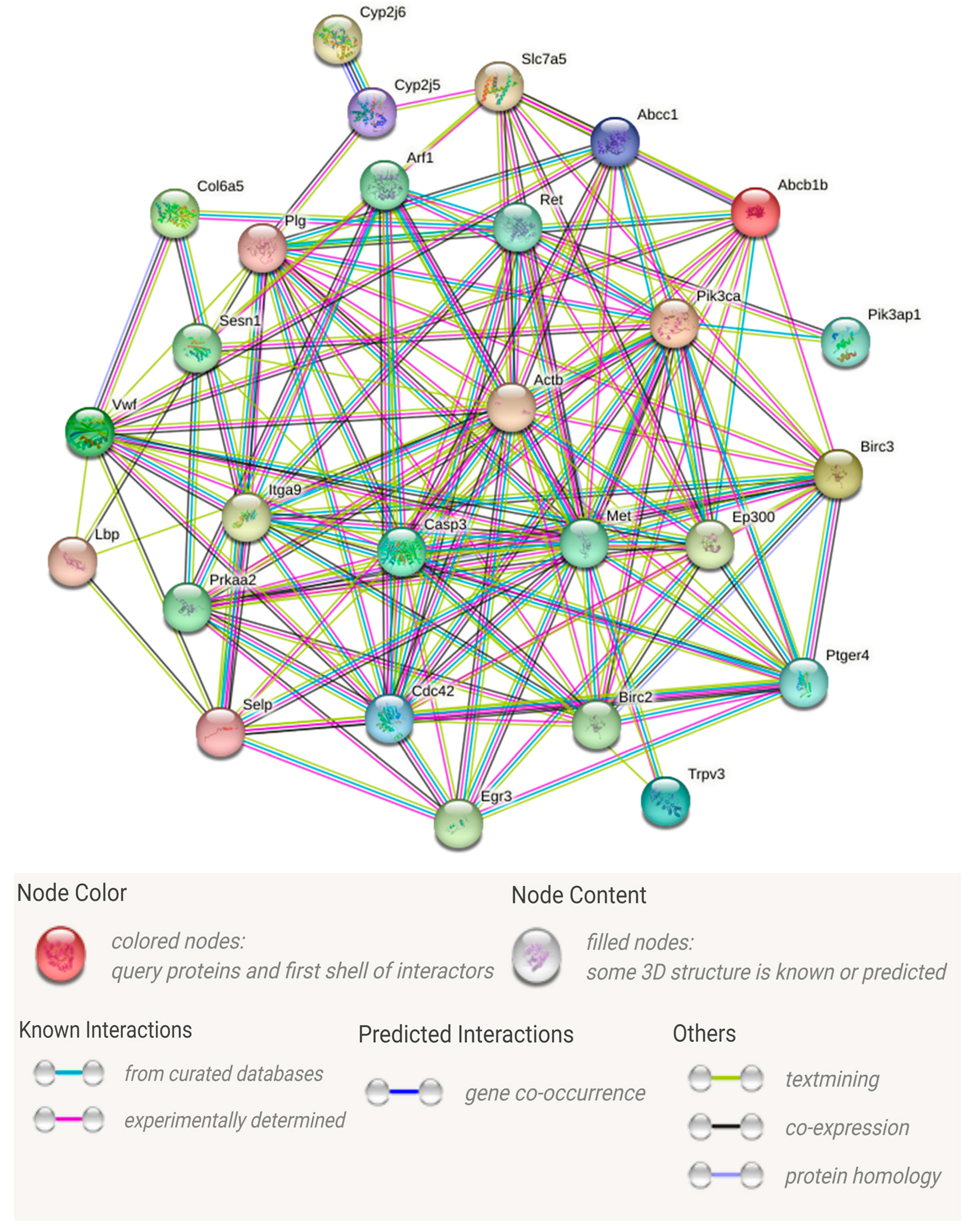
3.4. Analysis of Important DEGs Related to Immune Responses
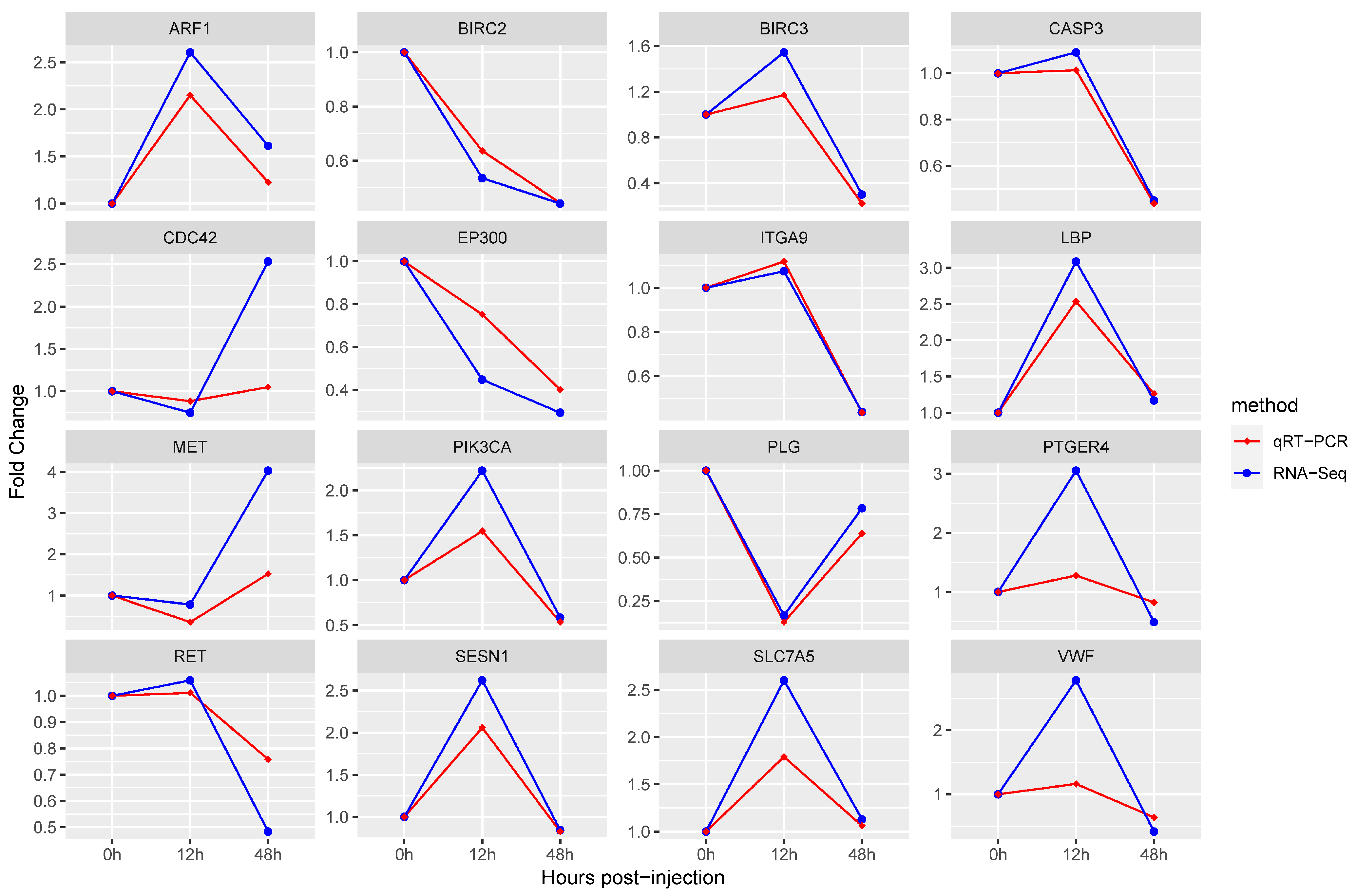
3.5. Quantitative RT-PCR Validation
4. Discussion
4.1. Purposes of Transcriptome Research
4.2. Functional Enrichment Analysis of Immune-Related GO Terms and KEGG Pathways
4.3. Speculation of Hub Genes
4.4. Functional Analysis of KEGG Signaling Pathways and Hub Genes
4.4.1. Inflammatory Mediator Regulation of TRP Channels
4.4.2. NF-κB Signaling Pathway
4.4.3. The Top Three Key Genes in Terms of Number of Interactions
4.4.4. Other Important Hub Genes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, D.; Yang, B.; Li, Q.; Liu, S. Estimation of genetic parameters for female reproduction traits in the Pacific oyster, Crassostrea gigas. Aquaculture 2023, 569, 739387. [Google Scholar] [CrossRef]
- Zhang, F.; Hu, B.; Fu, H.; Jiao, Z.; Li, Q.; Liu, S. Comparative Transcriptome Analysis Reveals Molecular Basis Underlying Fast Growth of the Selectively Bred Pacific Oyster, Crassostrea gigas. Front. Genet. 2019, 10, 610. [Google Scholar] [CrossRef]
- Gestal, C.; Roch, P.; Renault, T.; Pallavicini, A.; Paillard, C.; Novoa, B.; Oubella, R.; Venier, P.; Figueras, A. Study of Diseases and the Immune System of Bivalves Using Molecular Biology and Genomics. Rev. Fish. Sci. 2008, 16 (Suppl. S1), 133–156. [Google Scholar] [CrossRef]
- Wang, L.; Song, X.; Song, L. The oyster immunity. Dev. Comp. Immunol. 2018, 80, 99–118. [Google Scholar] [CrossRef]
- Huang, Q.; Yu, M.; Chen, H.; Zeng, M.; Chen, D. LRFN (leucine-rich repeat and fibronectin type-III domain-containing protein) recognizes bacteria and promotes hemocytic phagocytosis in the Pacific oyster Crassostrea gigas. Fish Shellfish Immunol. 2017, 72, 622–628. [Google Scholar] [CrossRef]
- Xing, D.; Li, Q.; Kong, L.; Yu, H. Heritability estimate for mantle edge pigmentation and correlation with shell pigmentation in the white-shell strain of Pacific oyster, Crassostrea gigas. Aquaculture 2018, 482, 73–77. [Google Scholar] [CrossRef]
- Huang, B.; Tang, X.; Zhang, L.; Li, L.; Zhang, G. IKKε-like plays an important role in the innate immune signaling of the Pacific oyster (Crassostrea gigas). Fish Shellfish Immunol. 2019, 93, 551–558. [Google Scholar] [CrossRef]
- Chen, H.; Cai, X.; Qiu, H.; Fang, J.; Wu, X. A novel C-type lectin from Crassostrea gigas involved in the innate defense against Vibrio alginolyticus. Biochem. Biophys. Res. Commun. 2021, 566, 155–163. [Google Scholar] [CrossRef]
- De Decker, S.; Normand, J.; Saulnier, D.; Pernet, F.; Castagnet, S.; Boudry, P. Responses of diploid and triploid Pacific oysters Crassostrea gigas to Vibrio infection in relation to their reproductive status. J. Invertebr. Pathol. 2011, 106, 179–191. [Google Scholar] [CrossRef]
- Yang, B.; Zhai, S.; Li, X.; Tian, J.; Li, Q.; Shan, H.; Liu, S. Identification of Vibrio alginolyticus as a causative pathogen associated with mass summer mortality of the Pacific Oyster (Crassostrea gigas) in China. Aquaculture 2021, 535, 736363. [Google Scholar] [CrossRef]
- Javier, D.; Barja, J.L.; Romalde, J.L. New Insights into Pathogenic Vibrios Affecting Bivalves in Hatcheries: Present and Future Prospects. Front. Microbiol. 2017, 8, 762. [Google Scholar]
- Gómez-León, J.; Villamil, L.; Lemos, M.L.; Novoa, B.; Figueras, A. Isolation of Vibrio alginolyticus and Vibrio splendidus from aquacultured carpet shell clam (Ruditapes decussatus) larvae associated with mass mortalities. Appl. Environ. Microbiol. 2005, 71, 98–104. [Google Scholar] [CrossRef]
- González-Escalona, N.; Blackstone, G.M.; DePaola, A. Characterization of a Vibrio alginolyticus strain, isolated from Alaskan oysters, carrying a hemolysin gene similar to the thermostable direct hemolysin-related hemolysin gene (trh) of Vibrio parahaemolyticus. Appl. Environ. Microbiol. 2006, 72, 7925–7929. [Google Scholar] [CrossRef]
- Xie, J.; Bu, L.; Jin, S.; Wang, X.; Zhao, Q.; Zhou, S.; Xu, Y. Outbreak of vibriosis caused by Vibrio harveyi and Vibrio alginolyticus in farmed seahorse Hippocampus kuda in China. Aquaculture 2020, 523, 735168. [Google Scholar] [CrossRef]
- Reilly, G.D.; Reilly, C.A.; Smith, E.G.; Baker-Austin, C. Vibrio alginolyticus-associated wound infection acquired in British waters, Guernsey, July 2011. Eurosurveillance 2011, 16, 321–326. [Google Scholar] [CrossRef]
- Zhou, K.; Tian, K.Y.; Liu, X.Q.; Liu, W.; Zhang, X.Y.; Liu, J.Y.; Sun, F. Characteristic and Otopathogenic Analysis of a Vibrio alginolyticus Strain Responsible for Chronic Otitis Externa in China. Front. Microbiol. 2021, 12, 750642. [Google Scholar] [CrossRef]
- Park, M.S.; Kim, Y.D.; Kim, B.M.; Kim, Y.J.; Kim, J.K.; Rhee, J.S. Effects of Antifouling Biocides on Molecular and Biochemical Defense System in the Gill of the Pacific Oyster Crassostrea gigas. PLoS ONE 2016, 11, e0168978. [Google Scholar] [CrossRef]
- Jemaà, M.; Morin, N.; Cavelier, P.; Cau, J.; Strub, J.M.; Delsert, C. Adult somatic progenitor cells and hematopoiesis in oysters. J. Exp. Biol. 2014, 217, 3067–3077. [Google Scholar] [CrossRef]
- Bachère, E.; Rosa, R.D.; Schmitt, P.; Poirier, A.C.; Merou, N.; Charrière, G.M.; Destoumieux-Garzón, D. The new insights into the oyster antimicrobial defense: Cellular, molecular and genetic view. Fish Shellfish Immunol. 2015, 46, 50–64. [Google Scholar] [CrossRef]
- Guan, Y.; He, M.; Wu, H. Differential mantle transcriptomics and characterization of growth-related genes in the diploid and triploid pearl oyster Pinctada fucata. Mar. Genom. 2017, 33, 31–38. [Google Scholar] [CrossRef]
- Zeng, Z.; Tan, Q.; Huang, Z.; Shi, B.; Ke, C. Differential Gene expression related to morphological variation in the adductor muscle tissues of diploid and triploid fujian oysters, Crassostrea angulata. Aquac. Res. 2019, 50, 3567–3578. [Google Scholar] [CrossRef]
- Zhang, L.; Li, L.; Zhu, Y.; Zhang, G.; Guo, X. Transcriptome analysis reveals a rich gene set related to innate immunity in the Eastern oyster (Crassostrea virginica). Mar. Biotechnol. 2014, 16, 17–33. [Google Scholar] [CrossRef]
- Liu, X.; Li, Z.; Li, Q.; Bao, X.; Jiang, L.; Yang, J. Acute exposure to polystyrene nanoplastics induced oxidative stress in Sepia esculenta Larvae. Aquac. Rep. 2024, 35, 102004. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, B.W.; Zheng, Y.D.; Xin, L.S.; Chen, W.B.; Yu, T.; Li, C.; Wang, C.M.; Bai, C.M. Identification and characterization of infectious pathogens associated with mass mortalities of Pacific Oyster (Crassostrea gigas) cultured in northern china. Biology 2023, 12, 759. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Wang, W.; Sun, G.; Feng, Y.; Xu, X.; Li, B.; Luo, Q.; Li, Y.; Yang, J.; et al. The investigation on stress mechanisms of Sepia esculenta larvae in the context of global warming and ocean acidification. Aquac. Rep. 2024, 36, 102120. [Google Scholar] [CrossRef]
- Ge, J.; Liu, C.; Tan, J.; Bian, L.; Chen, S. Transcriptome analysis of scyphozoan jellyfish Rhopilema esculentum from polyp to medusa identifies potential genes regulating strobilation. Dev. Genes Evol. 2018, 228, 243–254. [Google Scholar] [CrossRef]
- Zhang, E.; Li, Z.; Li, B.; Fu, J.; Feng, Y.; Sun, G.; Xu, X.; Cui, C.; Wang, W.; Yang, J. Investigating the molecular mechanism of sterility in female triploid Pacific oyster (Crassostrea gigas). Aquac. Rep. 2024, 34, 101885. [Google Scholar] [CrossRef]
- Zhang, E.; Dong, L.; Bao, X.; Yang, X.; Li, Y.; Feng, Y.; Yang, J.; Li, Z.; Wang, W. Transcriptome profiling combined with network analysis deepens the understanding of immune response mechanisms in blood of pacific oyster Crassostrea gigas infected by Vibrio alginolyticus. Front. Mar. Sci. 2022, 9, 1017445. [Google Scholar] [CrossRef]
- Qi, H.; Li, L.; Zhang, G. Construction of a chromosome-level genome and variation map for the Pacific oyster Crassostrea gigas. Mol. Ecol. Resour. 2021, 21, 1670–1685. [Google Scholar] [CrossRef]
- Wendling, C.C.; Wegner, K.M. Relative contribution of reproductive investment, thermal stress and Vibrio infection to summer mortality phenomena in Pacific oysters. Aquaculture 2013, 412–413, 88–96. [Google Scholar] [CrossRef]
- Alfaro, A.C.; Nguyen, T.V.; Merien, F. The complex interactions of Ostreid herpesvirus 1, Vibrio bacteria, environment and host factors in mass mortality outbreaks of Crassostrea gigas. Rev. Aquac. 2018, 11, 1148–1168. [Google Scholar] [CrossRef]
- Saulnier, D.; De Decker, S.; Haffner, P.; Cobret, L.; Robert, M.; Garcia, C. A large-scale epidemiological study to identify bacteria pathogenic to Pacific oyster Crassostrea gigas and correlation between virulence and metalloprotease-like activity. Microb. Ecol. 2010, 59, 787–798. [Google Scholar] [CrossRef]
- Li, Y.; Song, X.; Wang, W.; Wang, L.; Yi, Q.; Jiang, S.; Jia, Z.; Du, X.; Qiu, L.; Song, L. The hematopoiesis in gill and its role in the immune response of Pacific oyster Crassostrea gigas against secondary challenge with Vibrio splendidus. Dev. Comp. Immunol. 2017, 71, 59–69. [Google Scholar] [CrossRef]
- Laith, A.A.; Ros-Amira, M.K.; Sheikh, H.I.; Effendy, A.W.M.; Najiah, M. Histopathological and immunological changes in green mussel, Perna viridis, challenged with Vibrio alginolyticus. Fish Shellfish Immunol. 2021, 118, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Cannuel, R.; Beninger, P.G.; McCombie, H.; Boudry, P. Gill Development and Its Functional and Evolutionary Implications in the Blue Mussel Mytilus edulis (Bivalvia: Mytilidae). Biol. Bull. 2009, 217, 173–188. [Google Scholar] [CrossRef]
- Liu, Z.-P.; Wu, L.-Y.; Wang, Y.; Zhang, X.-S.; Chen, L. Bridging protein local structures and protein functions. Amino Acids 2008, 35, 627–650. [Google Scholar] [CrossRef]
- Parenti, A.; De Logu, F.; Geppetti, P.; Benemei, S. What is the evidence for the role of TRP channels in inflammatory and immune cells? Br. J. Pharmacol. 2016, 173, 953–969. [Google Scholar] [CrossRef]
- Moran, M.M.; McAlexander, M.A.; Bíró, T.; Szallasi, A. Transient receptor potential channels as therapeutic targets. Nat. Rev. Drug Discov. 2011, 10, 601–620. [Google Scholar] [CrossRef]
- Nilius, B.; Owsianik, G.; Voets, T.; Peters, J.A. Transient receptor potential cation channels in disease. Physiol. Rev. 2007, 87, 165–217. [Google Scholar] [CrossRef]
- Nathan, C. Specificity of a third kind: Reactive oxygen and nitrogen intermediates in cell signaling. J. Clin. Investig. 2003, 111, 769–778. [Google Scholar] [CrossRef]
- Holmström, K.M.; Finkel, T. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat. Rev. Mol. Cell Biol. 2014, 15, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Jiao, Z.; Li, Y.; Tian, J.; Ren, L.; Zhang, F.; Li, Q.; Liu, S. Transient Receptor Potential (TRP) Channels in the Pacific Oyster (Crassostrea gigas): Genome-Wide Identification and Expression Profiling after Heat Stress between C. gigas and C. angulata. Int. J. Mol. Sci. 2021, 22, 3222. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Zhang, T.; Zhang, F.; Liao, T.; Li, Z. Transcriptome analysis of digestive diverticula of Hong Kong oyster (Crassostrea hongkongesis) infected with Vibrio harveyi. Fish Shellfish Immunol. 2023, 142, 109120. [Google Scholar] [CrossRef] [PubMed]
- Qu, F.; Xiang, Z.; Wang, F.; Qi, L.; Xu, F.; Xiao, S.; Yu, Z. Prostaglandin E receptor 4 (PTGER4) involved in host protection against immune challenge in oyster, Crassostrea hongkongensis. Fish Shellfish Immunol. 2015, 42, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Takayama, K.; García-Cardeña, G.; Sukhova, G.K.; Comander, J.; Gimbrone, M.A.; Libby, P. Prostaglandin E2 Suppresses Chemokine Production in Human Macrophages through the EP4 Receptor*. J. Biol. Chem. 2002, 277, 44147–44154. [Google Scholar] [CrossRef] [PubMed]
- Nataraj, C.; Thomas, D.W.; Tilley, S.L.; Nguyen, M.T.; Mannon, R.; Koller, B.H.; Coffman, T.M. Receptors for prostaglandin E (2) that regulate cellular immune responses in the mouse. J. Clin. Investig. 2001, 108, 1229–1235. [Google Scholar] [CrossRef] [PubMed]
- Guijarro, C.; Egido, J. Transcription factor-kappa B (NF-kappa B) and renal disease. Kidney Int. 2001, 59, 415–424. [Google Scholar] [CrossRef]
- Oeckinghaus, A.; Ghosh, S. The NF-kappaB family of transcription factors and its regulation. Cold Spring Harb. Perspect. Biol. 2009, 1, a000034. [Google Scholar] [CrossRef] [PubMed]
- Pahl, H.L.; Baeuerle, P.A. A novel signal transduction pathway from the endoplasmic reticulum to the nucleus is mediated by transcription factor NF-kappa B. EMBO J. 1995, 14, 2580–2588. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-kappaB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Li, M.; Ding, Y.; Mu, Y.; Ao, J.; Chen, X. Molecular cloning and characterization of caspase-3 in large yellow croaker (Pseudosciaena crocea). Fish Shellfish Immunol. 2011, 30, 910–916. [Google Scholar] [CrossRef] [PubMed]
- Yamato, A.; Soda, M.; Ueno, T.; Kojima, S.; Sonehara, K.; Kawazu, M.; Sai, E.; Yamashita, Y.; Nagase, T.; Mano, H. Oncogenic activity of BIRC2 and BIRC3 mutants independent of nuclear factor-κB-activating potential. Cancer Sci. 2015, 106, 1137–1142. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Yan, D.; Xiao, J.; Feng, H.; Chang, M.X. The Zebrafish Antiapoptotic Protein BIRC2 Promotes Edwardsiella piscicida Infection by Inhibiting Caspases and Accumulating p53 in a p53 Transcription-Dependent and -Independent Manner. Front. Immunol. 2021, 12, 781680. [Google Scholar] [CrossRef]
- Estornes, Y.; Bertrand, M.J. IAPs, regulators of innate immunity and inflammation. Semin. Cell Dev. Biol. 2015, 39, 106–114. [Google Scholar] [CrossRef]
- Wu, Y.; Du, H.; Zhu, L.; Zhao, N.; Zhang, S.; Cao, Z.; Zhou, Y.; Sun, Y. Bactericidal permeability-increasing protein/LPS-binding protein (BPI/LBP) enhances resistance of golden pompano Trachinotus ovatus against bacterial infection. Fish Shellfish Immunol. 2022, 131, 872–880. [Google Scholar] [CrossRef] [PubMed]
- Hisatsune, K.; Kiuye, A.; Kondo, S. A comparative study of the sugar composition of O-antigenic lipopolysaccharides isolated from Vibrio alginolyticus and Vibrio parahaemolyticus. Microbio. Immunol. 1981, 25, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zha, H.; Han, X.; Yu, S.; Chai, Y.; Zhong, J.; Zhu, Q. Molecular characterization and functional analysis of the bactericidal permeability-increasing protein/LPS-binding protein (BPI/LBP) from roughskin sculpin (Trachidermus fasciatus). Dev. Comp. Immunol. 2021, 123, 104133. [Google Scholar] [CrossRef] [PubMed]
- Voll, R.E.; Herrmann, M.; Roth, E.A.; Stach, C.; Kalden, J.R.; Girkontaite, I. Immunosuppressive effects of apoptotic cells. Nature 1997, 390, 350–351. [Google Scholar] [CrossRef] [PubMed]
- Hughes, F.M.; Foster, B.; Grewal, S.; Sokolova, I.M. Apoptosis as a host defense mechanism in Crassostrea virginica and its modulation by Perkinsus marinus. Fish Shellfish Immunol. 2010, 29, 247–257. [Google Scholar] [CrossRef]
- Kiss, T. Apoptosis and its functional significance in molluscs. Apoptosis 2010, 15, 313–321. [Google Scholar] [CrossRef]
- Zhou, Z.; Xu, S.; Jiang, L.; Tan, Z.; Wang, J. A Systematic Pan-Cancer Analysis of CASP3 as a Potential Target for Immunotherapy. Front. Mol. Biosci. 2022, 9, 776808. [Google Scholar] [CrossRef] [PubMed]
- Grabert, K.; Engskog-Vlachos, P.; Škandík, M.; Vazquez-Cabrera, G.; Murgoci, A.N.; Keane, L.; Gaetani, M.; Joseph, B.; Cheray, M. Proteome integral solubility alteration high-throughput proteomics assay identifies Collectin-12 as a non-apoptotic microglial caspase-3 substrate. Cell Death Dis. 2023, 14, 192. [Google Scholar] [CrossRef]
- Martenot, C.; Gervais, O.; Chollet, B.; Houssin, M.; Renault, T. Haemocytes collected from experimentally infected Pacific oysters, Crassostrea gigas: Detection of ostreid herpesvirus 1 DNA, RNA, and proteins in relation with inhibition of apoptosis. PLoS ONE 2017, 12, e0177448. [Google Scholar] [CrossRef] [PubMed]
- Sagi, Z.; Hieronymus, T. The Impact of the Epithelial-Mesenchymal Transition Regulator Hepatocyte Growth Factor Receptor/Met on Skin Immunity by Modulating Langerhans Cell Migration. Front. Immunol. 2018, 9, 517. [Google Scholar] [CrossRef] [PubMed]
- Shirasaki, T.; Yamagoe, S.; Shimakami, T.; Murai, K.; Imamura, R.; Ishii, K.A.; Takayama, H.; Matsumoto, Y.; Tajima-Shirasaki, N.; Nagata, N.; et al. Leukocyte cell-derived chemotaxin 2 is an antiviral regulator acting through the proto-oncogene MET. Nat. Commun. 2022, 13, 3176. [Google Scholar] [CrossRef] [PubMed]
- Trevisan, R.; Mello, D.F.; Delapedra, G.; Silva, D.G.H.; Arl, M.; Danielli, N.M.; Metian, M.; Almeida, E.A.; Dafre, A.L. Gills as a glutathione-dependent metabolic barrier in Pacific oysters Crassostrea gigas: Absorption, metabolism and excretion of a model electrophile. Aquat. Toxicol. 2016, 173, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Deane, J.A.; Fruman, D.A. Phosphoinositide 3-Kinase: Diverse Roles in Immune Cell Activation. Annu. Rev. Immunol. 2004, 22, 563–598. [Google Scholar] [CrossRef] [PubMed]
- Canesi, L.; Betti, M.; Ciacci, C.; Scarpato, A.; Citterio, B.; Pruzzo, C.; Gallo, G. Signaling pathways involved in the physiological response of mussel hemocytes to bacterial challenge: The role of stress-activated p38 MAP kinases. Dev. Comp. Immunol. 2002, 26, 325–334. [Google Scholar] [CrossRef]
- Wang, W.; Liu, Q.; Zhang, T.; Chen, L.; Li, S.; Xu, S. Glyphosate induces lymphocyte cell dysfunction and apoptosis via regulation of miR-203 targeting of PIK3R1 in common carp (Cyprinus carpio L.). Fish Shellfish Immunol. 2020, 101, 51–57. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, X.; Han, M.; Liu, Y.; Wang, X.; Yu, H.; Liu, J.; Zhang, Q. Functional differentiation of three phosphatidylinositol 3-kinase catalytic subunit alpha (PIK3CA) in response to Vibrio anguillarum infection in turbot (Scophthalmus maximus). Fish Shellfish Immunol. 2019, 92, 450–459. [Google Scholar] [CrossRef]
- Kirchhausen, T. Three ways to make a vesicle. Nat. Rev. Mol. Cell Biol. 2000, 1, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, X.; Xu, X.; Yang, J.; Liu, X.; Sun, G.; Li, Z. Weighted Gene Co-Expression Network Analysis Based on Stimulation by Lipopolysaccharides and Polyinosinic:polycytidylic Acid Provides a Core Set of Genes for Understanding Hemolymph Immune Response Mechanisms of Amphioctopus fangsiao. Animals 2023, 14, 80. [Google Scholar] [CrossRef]
- Mao, F.; Wong, N.K.; Lin, Y.; Zhang, X.; Liu, K.; Huang, M.; Xu, D.; Xiang, Z.; Li, J.; Zhang, Y.; et al. Transcriptomic Evidence Reveals the Molecular Basis for Functional Differentiation of Hemocytes in a Marine Invertebrate, Crassostrea gigas. Front. Immunol. 2020, 11, 911. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Chen, J.; Tan, F.; Wang, B.; Xu, W.; Yuan, C. ITGA9: Potential Biomarkers and Therapeutic Targets in Different Tumors. Curr. Pharm. Des. 2022, 28, 1412–1418. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, M.A.; Ribeiro, A.L.C.; Costa, B.R.C.; Vago, J.P.; Lima, K.M.; Carneiro, F.S.; Ortiz, M.M.O.; Lima, G.L.N.; Carmo, A.A.F.; Rocha, R.M.; et al. Plasmin and plasminogen induce macrophage reprogramming and regulate key steps of inflammation resolution via annexin A1. Blood 2017, 129, 2896–2907. [Google Scholar] [CrossRef]
- Yang, F.; Chen, R. Sestrin1 exerts a cytoprotective role against oxygen-glucose deprivation/reoxygenation-induced neuronal injury by potentiating Nrf2 activation via the modulation of Keap1. Brain Res. 2021, 1750, 147165. [Google Scholar] [CrossRef]

| Pathways | Number of DEGs |
|---|---|
| Inflammatory mediator regulation of TRP channels | 7 |
| Central carbon metabolism in cancer | 5 |
| PI3K-Akt signaling pathway | 5 |
| Salmonella infection | 3 |
| NF-kappa B signaling pathway | 3 |
| MicroRNAs in cancer | 3 |
| Pathways in cancer | 3 |
| Endocytosis | 2 |
| Staphylococcus aureus infection | 2 |
| p53 signaling pathway | 2 |
| Apoptosis | 2 |
| C-type lectin receptor signaling pathway | 2 |
| Network Stats | |
|---|---|
| Number of nodes | 27 |
| Number of edges | 145 |
| Average node degree | 10.7 |
| Clustering coefficient | 0.709 |
| Expected number of edges | 99 |
| PPI enrichment p-value | 8.29 × 10−6 |
| Gene Name (Abbreviation) | Gene Name (Official Full Name) | Number of Protein–Protein Interactions | Number of KEGG Signaling Pathway |
|---|---|---|---|
| CASP3 | Caspase 3 | 20 | 3 |
| MET | MET proto-oncogene, receptor tyrosine kinase | 19 | 1 |
| PIK3CA | Phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha | 17 | 4 |
| CDC42 | Cell division cycle 42 | 16 | 1 |
| PLG | Plasminogen | 16 | 1 |
| EP300 | E1A binding protein p300 | 15 | 1 |
| ITGA9 | Integrin subunit alpha 9 | 14 | 1 |
| BIRC3 | Baculoviral IAP repeat containing 3 | 13 | 1 |
| PTGER4 | Prostaglandin E receptor 4 | 13 | 2 |
| BIRC2 | Baculoviral IAP repeat containing 2 | 12 | 1 |
| VWF | Von Willebrand factor | 12 | 1 |
| RET | Ret proto-oncogene | 11 | 2 |
| ARF1 | ADP ribosylation factor 1 | 10 | 1 |
| SLC7A5 | Solute carrier family 7 member 5 | 9 | 2 |
| SESN1 | Sestrin 1 | 6 | 1 |
| LBP | lipopolysaccharide binding protein | 4 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, E.; Li, Z.; Dong, L.; Feng, Y.; Sun, G.; Xu, X.; Wang, Z.; Cui, C.; Wang, W.; Yang, J. Exploration of Molecular Mechanisms of Immunity in the Pacific Oyster (Crassostrea gigas) in Response to Vibrio alginolyticus Invasion. Animals 2024, 14, 1707. https://doi.org/10.3390/ani14111707
Zhang E, Li Z, Dong L, Feng Y, Sun G, Xu X, Wang Z, Cui C, Wang W, Yang J. Exploration of Molecular Mechanisms of Immunity in the Pacific Oyster (Crassostrea gigas) in Response to Vibrio alginolyticus Invasion. Animals. 2024; 14(11):1707. https://doi.org/10.3390/ani14111707
Chicago/Turabian StyleZhang, Enshuo, Zan Li, Luyao Dong, Yanwei Feng, Guohua Sun, Xiaohui Xu, Zhongping Wang, Cuiju Cui, Weijun Wang, and Jianmin Yang. 2024. "Exploration of Molecular Mechanisms of Immunity in the Pacific Oyster (Crassostrea gigas) in Response to Vibrio alginolyticus Invasion" Animals 14, no. 11: 1707. https://doi.org/10.3390/ani14111707
APA StyleZhang, E., Li, Z., Dong, L., Feng, Y., Sun, G., Xu, X., Wang, Z., Cui, C., Wang, W., & Yang, J. (2024). Exploration of Molecular Mechanisms of Immunity in the Pacific Oyster (Crassostrea gigas) in Response to Vibrio alginolyticus Invasion. Animals, 14(11), 1707. https://doi.org/10.3390/ani14111707






