The Effects of Dietary Saccharomyces cerevisiae Supplementation on Gut Microbiota Composition and Gut Health in Aged Labrador Retrievers
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Feeding Management
2.2. Experimental Design and Sample Collection
2.3. Total Tract Apparent Digestibility of Nutrients
2.4. Fecal Microbiota Analysis
2.5. Fecal Metabolite Concentrations
2.6. Antioxidant Parameters and Inflammation Indicators
2.7. Gut Barrier Function Parameters
2.8. Statistical Analysis
3. Results
3.1. Body Weight and Body Condition Score
3.2. Hematology Indexes
3.3. Coefficients of the Total Tract Apparent Digestibility of Nutrients
3.4. Fecal Quality Score and Microbial Metabolite Concentration
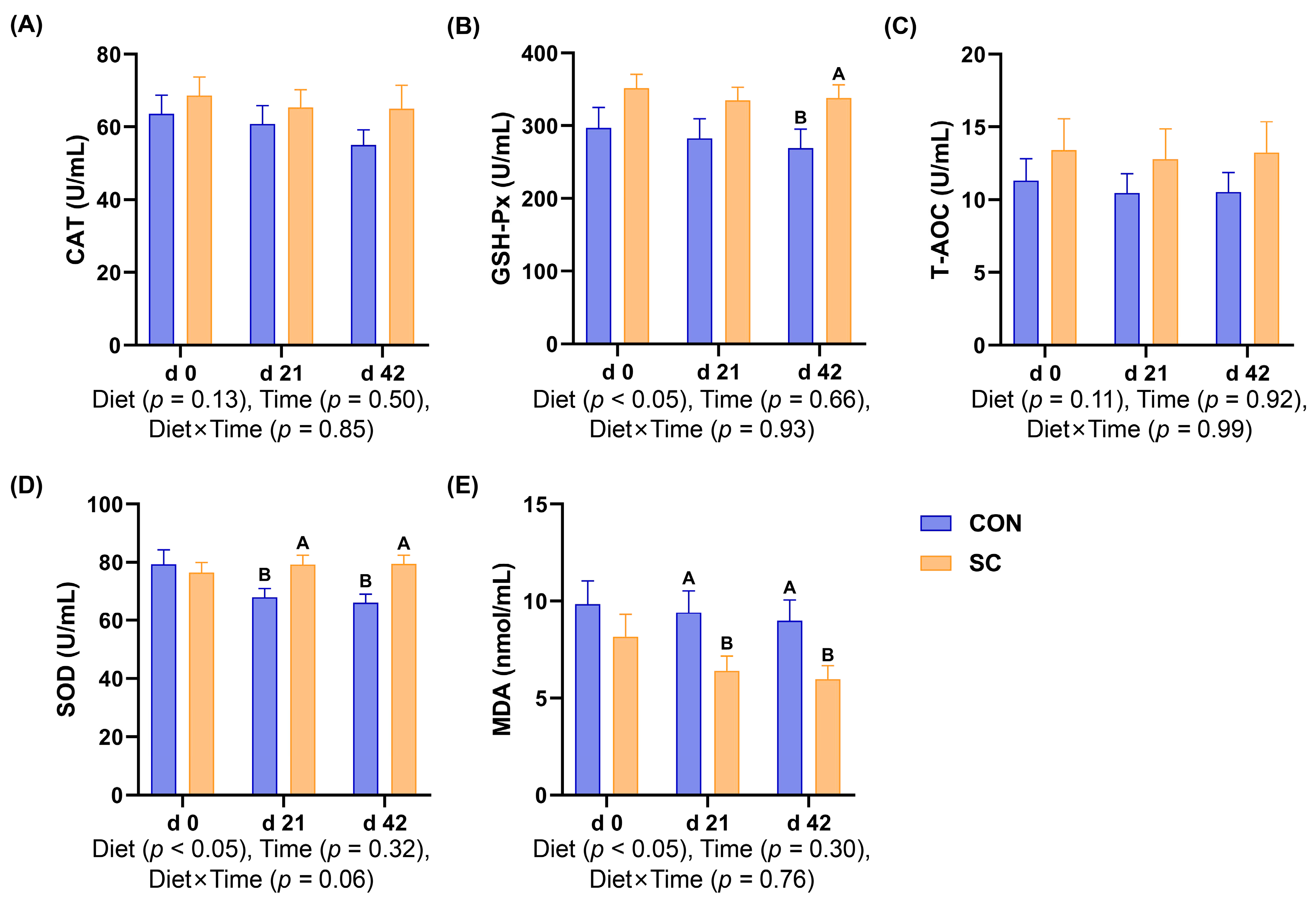
3.5. Antioxidant Indexes
3.6. Inflammatory Factors
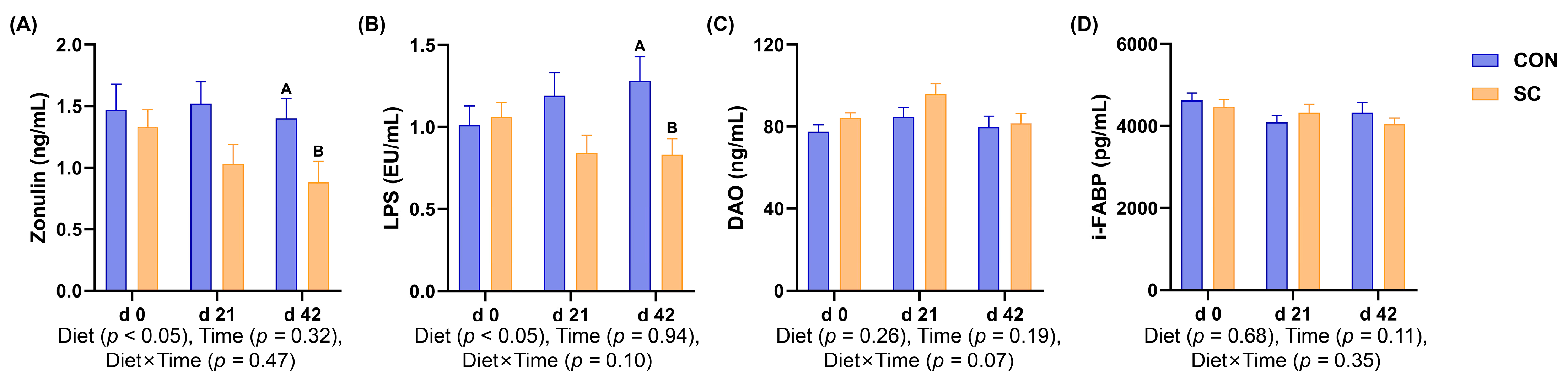
3.7. Intestinal Barrier Function Parameters
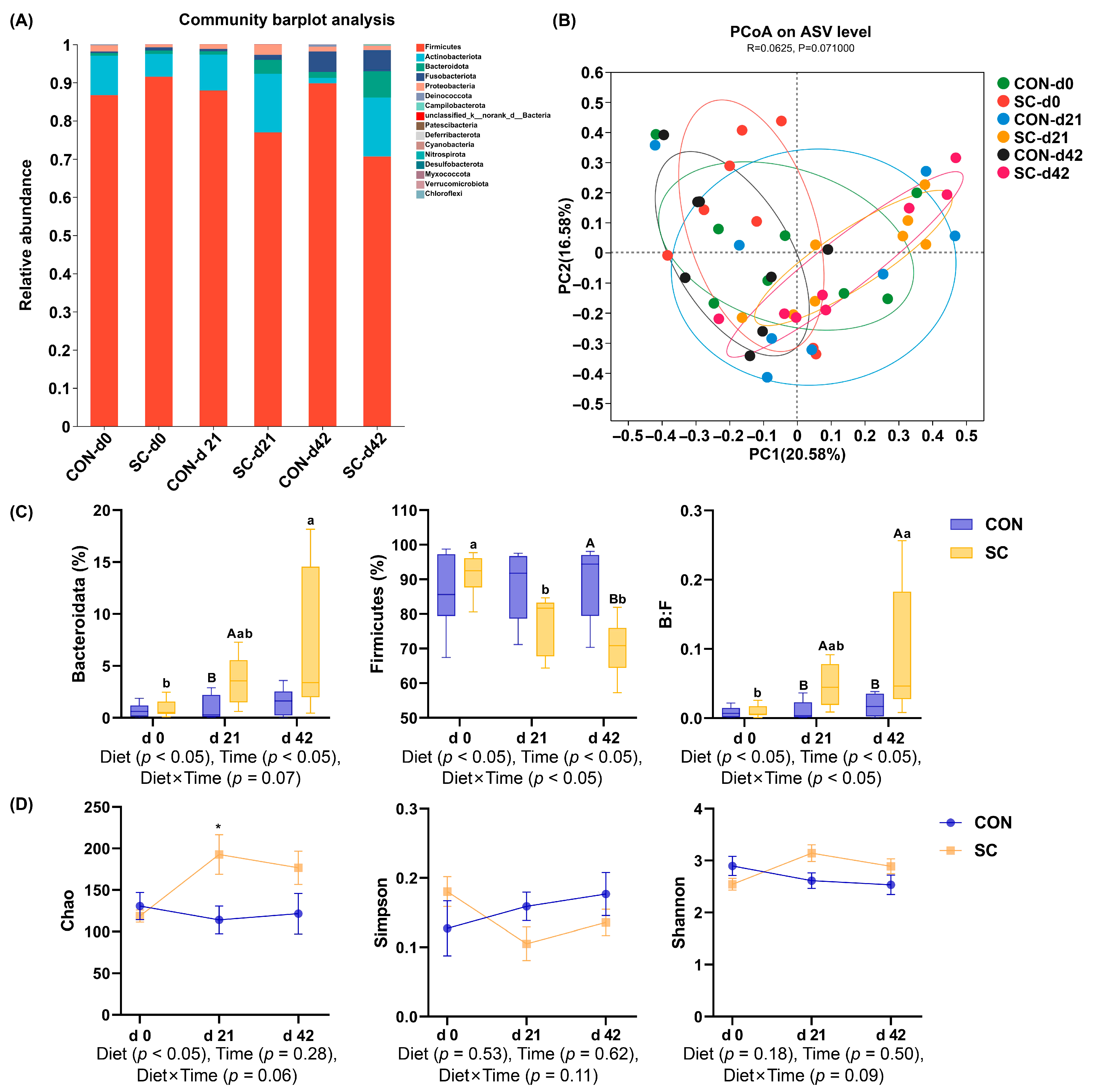
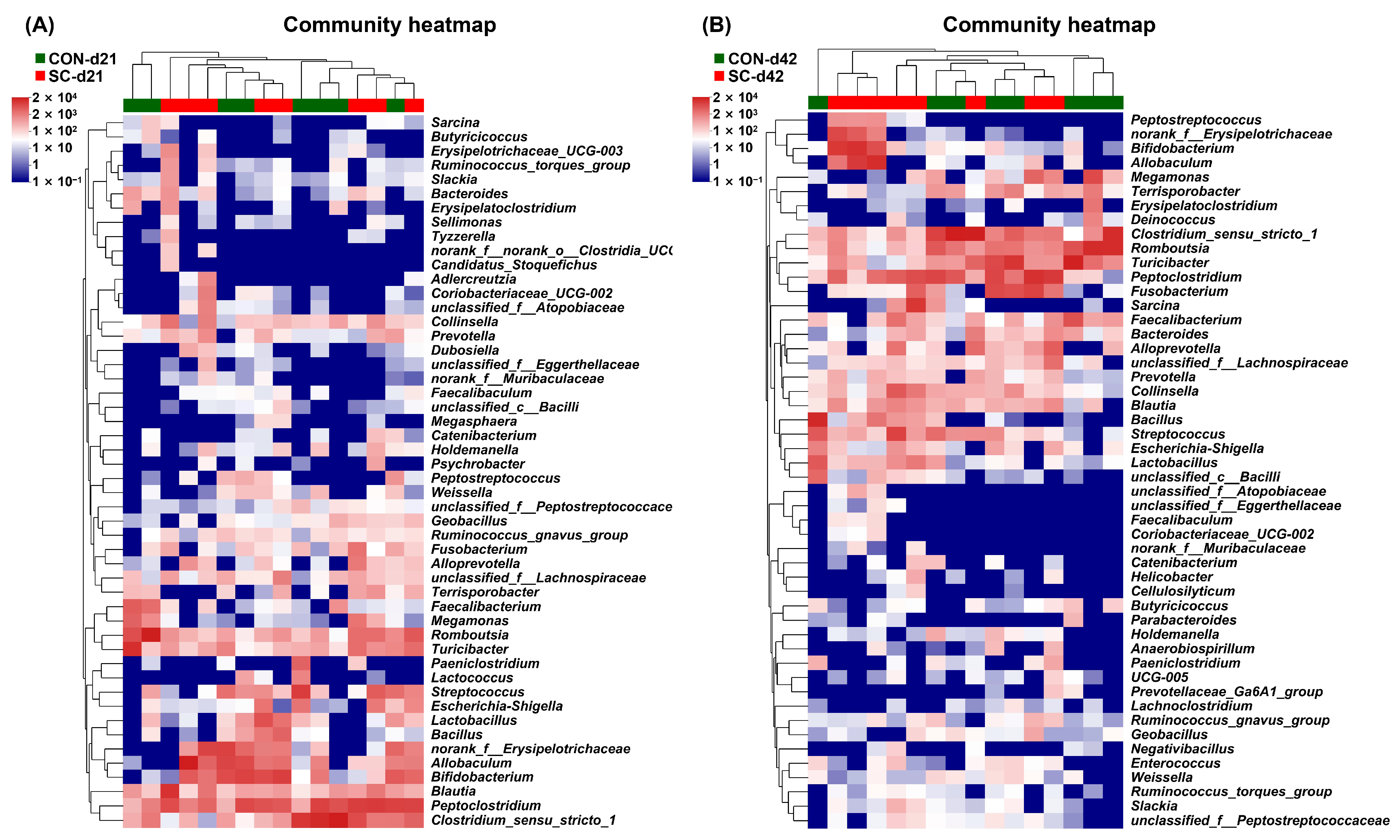
3.8. Fecal Microbiota Composition and Structure
3.9. The Relative Abundance of Fecal Microbiota at the Family Level
3.10. The Relative Abundance of Fecal Microbiota at the Genus Level
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Berg, G.; Rybakova, D.; Fischer, D.; Cernava, T.; Verges, M.C.; Charles, T.; Chen, X.; Cocolin, L.; Eversole, K.; Corral, G.H.; et al. Microbiome definition re-visited: Old concepts and new challenges. Microbiome 2020, 8, 103. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Mo, R.; Li, M.; Qu, Y.; Wang, H.; Liu, T.; Liu, P.; Wu, Y. Comparison of the Effects of Enzymolysis Seaweed Powder and Saccharomyces boulardii on Intestinal Health and Microbiota Composition in Kittens. Metabolites 2023, 13, 637. [Google Scholar] [CrossRef] [PubMed]
- Grześkowiak, Ł.; Endo, A.; Beasley, S.; Salminen, S. Microbiota and probiotics in canine and feline welfare. Anaerobe 2015, 34, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Pilla, R.; Suchodolski, J.S. The gut microbiome of dogs and cats, and the influence of diet. Vet. Clin. North. Am. Small Anim. Pract. 2021, 51, 605–621. [Google Scholar] [CrossRef] [PubMed]
- Ziese, A.L.; Suchodolski, J.S. Impact of changes in gastrointestinal microbiota in canine and feline digestive diseases. Vet. Clin. North. Am. Small Anim. Pract. 2021, 51, 155–169. [Google Scholar] [CrossRef] [PubMed]
- DeJong, E.N.; Surette, M.G.; Bowdish, D.M.E. The Gut Microbiota and Unhealthy Aging: Disentangling Cause from Consequence. Cell Host Microbe. 2020, 28, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Garrigues, Q.; Apper, E.; Chastant, S.; Mila, H. Gut microbiota development in the growing dog: A dynamic process influenced by maternal, environmental and host factors. Front. Vet. Sci. 2022, 9, 964649. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Pinteño, A.; Pilla, R.; Manteca, X.; Suchodolski, J.; Torre, C.; Salas-Mani, A. Age-associated changes in intestinal health biomarkers in dogs. Front. Vet. Sci. 2023, 10, 1213287. [Google Scholar] [CrossRef]
- Cho, H.W.; Choi, S.; Seo, K.; Kim, K.H.; Jeon, J.H.; Kim, C.H.; Lim, S.; Jeong, S.; Chun, J.L. Gut microbiota profiling in aged dogs after feeding pet food contained Hericium erinaceus. J. Anim. Sci. Technol. 2022, 64, 937–949. [Google Scholar] [CrossRef]
- Ali, M.S.; Lee, E.B.; Hsu, W.H.; Suk, K.; Sayem, S.A.J.; Ullah, H.M.A.; Lee, S.J.; Park, S.C. Probiotics and Postbiotics as an Alternative to Antibiotics: An Emphasis on Pigs. Pathogens 2023, 12, 874. [Google Scholar] [CrossRef]
- Kim, S.K.; Guevarra, R.B.; Kim, Y.T.; Kwon, J.; Kim, H.; Cho, J.H.; Kim, H.B.; Lee, J.H. Role of Probiotics in Human Gut Microbiome-Associated Diseases. J. Microbiol. Biotechnol. 2019, 29, 1335–1340. [Google Scholar] [CrossRef] [PubMed]
- Simon, E.; Călinoiu, L.F.; Mitrea, L.; Vodnar, D.C. Probiotics, Prebiotics, and Synbiotics: Implications and Beneficial Effects against Irritable Bowel Syndrome. Nutrients. 2021, 13, 2112. [Google Scholar] [CrossRef] [PubMed]
- Bastos, T.S.; de Lima, D.C.; Souza, C.M.M.; Maiorka, A.; de Oliveira, S.G.; Bittencourt, L.C.; Félix, A.P. Bacillus subtilis and Bacillus licheniformis reduce faecal protein catabolites concentration and odour in dogs. BMC Vet. Res. 2020, 16, 116. [Google Scholar] [CrossRef] [PubMed]
- Bastos, T.S.; Souza, C.M.M.; Legendre, H.; Richard, N.; Pilla, R.; Suchodolski, J.S.; de Oliveira, S.G.; Lesaux, A.A.; Félix, A.P. Effect of Yeast Saccharomyces cerevisiae as a Probiotic on Diet Digestibility, Fermentative Metabolites, and Composition and Functional Potential of the Fecal Microbiota of Dogs Submitted to an Abrupt Dietary Change. Microorganisms 2023, 11, 506. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.; Pengo, G.; Galosi, L.; Berardi, S.; Tambella, A.M.; Attili, A.R.; Gavazza, A.; Cerquetella, M.; Jergens, A.E.; Guard, B.C.; et al. Effects of the Probiotic Mixture Slab51® (SivoMixx®) as Food Supplement in Healthy Dogs: Evaluation of Fecal Microbiota, Clinical Parameters and Immune Function. Front. Vet. Sci. 2020, 7, 613. [Google Scholar] [CrossRef]
- Xu, H.; Huang, W.; Hou, Q.; Kwok, L.Y.; Laga, W.; Wang, Y.; Ma, H.; Sun, Z.; Zhang, H. Oral Administration of Compound Probiotics Improved Canine Feed Intake, Weight Gain, Immunity and Intestinal Microbiota. Front. Immunol. 2019, 10, 666. [Google Scholar] [CrossRef] [PubMed]
- White, R.; Atherly, T.; Guard, B.; Rossi, G.; Wang, C.; Mosher, C.; Webb, C.; Hill, S.; Ackermann, M.; Sciabarra, P.; et al. Randomized, controlled trial evaluating the effect of multi-strain probiotic on the mucosal microbiota in canine idiopathic inflammatory bowel disease. Gut Microbes. 2017, 8, 451–466. [Google Scholar] [CrossRef] [PubMed]
- Ziese, A.L.; Suchodolski, J.S.; Hartmann, K.; Busch, K.; Anderson, A.; Sarwar, F.; Sindern, N.; Unterer, S. Effect of probiotic treatment on the clinical course, intestinal microbiome, and toxigenic Clostridium perfringens in dogs with acute hemorrhagic diarrhea. PLoS ONE. 2018, 13, e0204691. [Google Scholar] [CrossRef]
- Palma, M.L.; Zamith-Miranda, D.; Martins, F.S.; Bozza, F.A.; Nimrichter, L.; Montero-Lomeli, M.; Marques, E.T., Jr.; Douradinha, B. Probiotic Saccharomyces cerevisiae strains as biotherapeutic tools: Is there room for improvement? Appl. Microbiol. Biotechnol. 2015, 99, 6563–6570. [Google Scholar] [CrossRef] [PubMed]
- Zanello, G.; Meurens, F.; Serreau, D.; Chevaleyre, C.; Melo, S.; Berri, M.; D’Inca, R.; Auclair, E.; Salmon, H. Effects of dietary yeast strains on immunoglobulin in colostrum and milk of sows. Vet. Immunol. Immunopathol. 2013, 152, 20–27. [Google Scholar] [CrossRef]
- Lin, C.Y.; Carroll, M.Q.; Miller, M.J.; Rabot, R.; Swanson, K.S. Supplementation of Yeast Cell Wall Fraction Tends to Improve Intestinal Health in Adult Dogs Undergoing an Abrupt Diet Transition. Front. Vet. Sci. 2020, 7, 597939. [Google Scholar] [CrossRef]
- Bampidis, V.; Azimonti, G.; Bastos, M.L.; Christensen, H.; Dusemund, B.; Fašmon Durjava, M.; Kouba, M.; López-Alonso, M.; López Puente, S.; Marcon, F.; et al. Safety and efficacy of a feed additive consisting of Saccharomyces cerevisiae MUCL 39885 (Biosprint®) for cats and dogs (Prosol S.p.A.). EFSA J. 2021, 19, e06699. [Google Scholar] [CrossRef]
- Laflamme, D. Developmental and validation of a body condition score system for dogs. Canine Pract. 1997, 22, 10–15. [Google Scholar]
- Chaney, A.L.; Marbach, E.P.J.C.c. Modified reagents for determination of urea and ammonia. Clin. Chem. 1962, 8, 130–132. [Google Scholar] [CrossRef]
- de Vos, W.M.; Tilg, H.; Van Hul, M.; Cani, P.D. Gut microbiome and health: Mechanistic insights. Gut. 2022, 71, 1020–1032. [Google Scholar] [CrossRef]
- Zhang, B.; Pan, C.; Feng, C.; Yan, C.; Yu, Y.; Chen, Z.; Guo, C.; Wang, X. Role of mitochondrial reactive oxygen species in homeostasis regulation. Redox Rep. 2022, 27, 45–52. [Google Scholar] [CrossRef]
- Blokhina, O.; Virolainen, E.; Fagerstedt, K.V. Antioxidants, oxidative damage and oxygen deprivation stress: A review. Ann. Bot. 2003, 91, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Dreger, H.; Westphal, K.; Weller, A.; Baumann, G.; Stangl, V.; Meiners, S.; Stangl, K. Nrf2-dependent upregulation of antioxidative enzymes: A novel pathway for proteasome inhibitor-mediated cardioprotection. Cardiovasc. Res. 2009, 83, 354–361. [Google Scholar] [CrossRef]
- Cheng, J.; Wang, F.; Yu, D.F.; Wu, P.F.; Chen, J.G. The cytotoxic mechanism of malondialdehyde and protective effect of carnosine via protein cross-linking/mitochondrial dysfunction/reactive oxygen species/MAPK pathway in neurons. Eur. J. Pharmacol. 2011, 650, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Vertuani, S.; Angusti, A.; Manfredini, S. The antioxidants and pro-antioxidants network: An overview. Curr. Pharm. Des. 2004, 10, 1677–1694. [Google Scholar] [CrossRef] [PubMed]
- Brunt, V.E.; Gioscia-Ryan, R.A.; Richey, J.J.; Zigler, M.C.; Cuevas, L.M.; Gonzalez, A.; Vázquez-Baeza, Y.; Battson, M.L.; Smithson, A.T.; Gilley, A.D.; et al. Suppression of the gut microbiome ameliorates age-related arterial dysfunction and oxidative stress in mice. J. Physiol. 2019, 597, 2361–2378. [Google Scholar] [CrossRef]
- Dawood, M.A.; Abd El-Kader, M.F.; Farid, M.A.; Abd-Elghany, M.F.; Alkafafy, M.; Van Doan, H. Enhanced the growth, immune and antioxidative responses of European Seabass (Dicentrarchus labrax). Ann. Anim. Sci. 2021, 21, 1423–1433. [Google Scholar] [CrossRef]
- Yang, Y.; Li, S.; Zhu, Y.; Che, L.; Wu, Q.; Bai, S.; Shu, G.; Zhao, X.; Guo, P.; Soaud, S.A.; et al. Saccharomyces cerevisiae additions normalized hemocyte differential genes expression and regulated crayfish (Procambarus clarkii) oxidative damage under cadmium stress. Sci. Rep. 2023, 13, 20939. [Google Scholar] [CrossRef]
- Christodoulou, C.; Skourtis, A.; Kyriakaki, P.; Satolias, F.F.; Karabinas, D.; Briche, M.; Salah, N.; Zervas, G.; Mavrommatis, A.; Tsiplakou, E. The Effect of Dietary Supplementation with Probiotic and Postbiotic Yeast Products on Ewes Milk Performance and Immune Oxidative Status. J. Fungi 2023, 9, 1139. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, J.J.; Murray, P.J. Cytokine signaling modules in inflammatory responses. Immunity. 2008, 28, 477–487. [Google Scholar] [CrossRef]
- Liu, C.; Chu, D.; Kalantar-Zadeh, K.; George, J.; Young, H.A.; Liu, G. Cytokines: From Clinical Significance to Quantification. Adv Sci (Weinh). 2021, 8, e2004433. [Google Scholar] [CrossRef]
- Sun, X.; Wang, Y.; Wang, E.; Zhang, S.; Wang, Q.; Zhang, Y.; Wang, Y.; Cao, Z.; Yang, H.; Wang, W.; et al. Effects of Saccharomyces cerevisiae Culture on Ruminal Fermentation, Blood Metabolism, and Performance of High-Yield Dairy Cows. Animals 2021, 11, 2401. [Google Scholar] [CrossRef]
- Kim, E.; Kyoung, H.; Hyung Koh, N.; Lee, H.; Lee, S.; Kim, Y.; Il Park, K.; Min Heo, J.; Song, M. Supplementation of live yeast culture modulates intestinal health, immune responses, and microbiota diversity in broiler chickens. J. Anim. Sci. 2022, 100, skac122. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Kim, Y.H.; Min, J. Anti-inflammatory effects of yeast-derived vacuoles on LPS-induced murine macrophage activation. Microbiol. Spectr. 2023, 11, e0146623. [Google Scholar] [CrossRef]
- Bzducha-Wróbel, A.; Błażejak, S. Antitoxic and antimicrobial properties of the yeast cell wall components. Med. Weter. 2011, 67, 244–249. [Google Scholar]
- Che, T.M.; Song, M.; Liu, Y.; Johnson, R.W.; Kelley, K.W.; Van Alstine, W.G.; Dawson, K.A.; Pettigrew, J.E. Mannan oligosaccharide increases serum concentrations of antibodies and inflammatory mediators in weanling pigs experimentally infected with porcine reproductive and respiratory syndrome virus. J. Anim. Sci. 2012, 90, 2784–2793. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Liu, J.; Yan, Q.; Gu, Z.; August, A.; Huang, W.; Jiang, Z. Konjac Glucomannan Oligosaccharides Prevent Intestinal Inflammation Through SIGNR1-Mediated Regulation of Alternatively Activated Macrophages. Mol. Nutr. Food Res. 2021, 65, e2001010. [Google Scholar] [CrossRef] [PubMed]
- Walachowski, S.; Tabouret, G.; Fabre, M.; Foucras, G. Molecular Analysis of a Short-term Model of β-Glucans-Trained Immunity Highlights the Accessory Contribution of GM-CSF in Priming Mouse Macrophages Response. Front. Immunol. 2017, 8, 1089. [Google Scholar] [CrossRef] [PubMed]
- Carloto, A.C.M.; Bortoleti, B.; Rodrigues, A.C.J.; Silva, T.F.; Tomiotto-Pellissier, F.; Bidóia, D.L.; Gonçalves, M.D.; Assolini, J.P.; Dekker, R.F.H.; Barbosa-Dekker, A.M.; et al. Botryosphaeran, [(1 → 3)(1 → 6)-β-D-glucan], induces apoptosis-like death in promastigotes of Leishmania amazonensis, and exerts a leishmanicidal effect on infected macrophages by activating NF-kB and producing pro-inflammatory molecules. Chem. Biol. Interact. 2022, 351, 109713. [Google Scholar] [CrossRef] [PubMed]
- No, H.; Kim, J.; Seo, C.R.; Lee, D.E.; Kim, J.H.; Kuge, T.; Mori, T.; Kimoto, H.; Kim, J.K. Anti-inflammatory effects of β-1,3-1,6-glucan derived from black yeast Aureobasidium pullulans in RAW264.7 cells. Int. J. Biol. Macromol. 2021, 193, 592–600. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, A.; Chanez-Paredes, S.D.; Haest, X.; Turner, J.R. Paracellular permeability and tight junction regulation in gut health and disease. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 417–432. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; An, K.; Li, P.; Li, L.; Xia, Z. Dietary Saccharomyces cerevisiae improves intestinal flora structure and barrier function of Pekin ducks. Poult. Sci. 2023, 102, 101940. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Zhang, L.; Li, P.; Yu, J.; Mu, G.; Li, X.; Tuo, Y. Saccharomyces cerevisiae I4 Showed Alleviating Effects on Dextran Sulfate Sodium-Induced Colitis of Balb/c Mice. Foods. 2022, 11, 1436. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Li, X.; Yue, Y.; Wang, X.; Chen, H.; Gu, Y.; Jia, H.; He, Y.; Yuan, Y.; Yue, T. Ameliorative Effect of Saccharomyces cerevisiae JKSP39 on Fusobacterium nucleatum and Dextran Sulfate Sodium-Induced Colitis Mouse Model. J. Agric. Food Chem. 2022, 70, 14179–14192. [Google Scholar] [CrossRef]
- Shen, J.; Liu, Y.; Ren, X.; Gao, K.; Li, Y.; Li, S.; Yao, J.; Yang, X. Changes in DNA Methylation and Chromatin Structure of Pro-inflammatory Cytokines Stimulated by LPS in Broiler Peripheral Blood Mononuclear Cells. Poult. Sci. 2016, 95, 1636–1645. [Google Scholar] [CrossRef]
- Snow, D.R.; Ward, R.E.; Olsen, A.; Jimenez-Flores, R.; Hintze, K.J. Membrane-rich milk fat diet provides protection against gastrointestinal leakiness in mice treated with lipopolysaccharide. J. Dairy. Sci. 2011, 94, 2201–2212. [Google Scholar] [CrossRef]
- Luk, G.D.; Bayless, T.M.; Baylin, S.B. Plasma postheparin diamine oxidase. Sensitive provocative test for quantitating length of acute intestinal mucosal injury in the rat. J. Clin. Invest. 1983, 71, 1308–1315. [Google Scholar] [CrossRef]
- Stevens, B.R.; Goel, R.; Seungbum, K.; Richards, E.M.; Holbert, R.C.; Pepine, C.J.; Raizada, M.K. Increased human intestinal barrier permeability plasma biomarkers zonulin and FABP2 correlated with plasma LPS and altered gut microbiome in anxiety or depression. Gut. 2018, 67, 1555–1557. [Google Scholar] [CrossRef]
- Vollrath, J.T.; Klingebiel, F.; Bläsius, F.; Greven, J.; Bolierakis, E.; Nowak, A.J.; Simic, M.; Hildebrand, F.; Marzi, I.; Relja, B. I-FABP as a potential marker for intestinal barrier loss in porcine polytrauma. J. Clin. Med. 2022, 11, 4599. [Google Scholar] [CrossRef]
- Chun, J.L.; Ji, S.Y.; Lee, S.D.; Lee, Y.K.; Kim, B.; Kim, K.H. Difference of gut microbiota composition based on the body condition scores in dogs. J. Anim. Sci. Technol. 2020, 62, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Kalenyak, K.; Isaiah, A.; Heilmann, R.M.; Suchodolski, J.S.; Burgener, I.A. Comparison of the intestinal mucosal microbiota in dogs diagnosed with idiopathic inflammatory bowel disease and dogs with food-responsive diarrhea before and after treatment. FEMS Microbiol. Ecol. 2018, 94, fix173. [Google Scholar] [CrossRef]
- Suchodolski, J.S. Analysis of the gut microbiome in dogs and cats. Vet. Clin. Pathol. 2022, 50 (Suppl. S1), 6–17. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Xie, Y.; Wu, Z.; Qian, Q.; Yang, H.; Li, S.; Li, X. Preventive Effects of Apple Polyphenol Extract on High-Fat-Diet-Induced Hepatic Steatosis Are Related to the Regulation of Hepatic Lipid Metabolism, Autophagy, and Gut Microbiota in Aged Mice. J. Agric. Food Chem. 2023, 71, 20011–20033. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Dong, H.; Zhou, J.; Luo, Y.; Yuan, M.M.; Zhan, J.F.; Liu, Y.L.; Xia, J.Y.; Zhang, L. Aged gut microbiota contribute to different changes in antioxidant defense in the heart and liver after transfer to germ-free mice. PLoS ONE. 2023, 18, e0289892. [Google Scholar] [CrossRef]
- Vacca, M.; Celano, G.; Calabrese, F.M.; Portincasa, P.; Gobbetti, M.; De Angelis, M. The Controversial Role of Human Gut Lachnospiraceae. Microorganisms. 2020, 8, 573. [Google Scholar] [CrossRef]
- Biddle, A.; Stewart, L.; Blanchard, J.; Leschine, S. Untangling the genetic basis of fibrolytic specialization by Lachnospiraceae and Ruminococcaceae in diverse gut communities. Diversity 2013, 5, 627–640. [Google Scholar] [CrossRef]
- Edwards, M.; Edwards, A.; Millard, P.; Kocher, A. Mannose rich fraction of Saccharomyces cerevisiae promotes growth and enhances carcass yield in commercially housed grower–finisher pigs. Anim. Feed. Sci. Technol. 2014, 197, 227–232. [Google Scholar] [CrossRef]
- Zou, J.; Shen, Y.; Chen, M.; Zhang, Z.; Xiao, S.; Liu, C.; Wan, Y.; Yang, L.; Jiang, S.; Shang, E.; et al. Lizhong decoction ameliorates ulcerative colitis in mice via modulating gut microbiota and its metabolites. Appl. Microbiol. Biotechnol. 2020, 104, 5999–6012. [Google Scholar] [CrossRef]
- Biagi, E.; Nylund, L.; Candela, M.; Ostan, R.; Bucci, L.; Pini, E.; Nikkïla, J.; Monti, D.; Satokari, R.; Franceschi, C.; et al. Through ageing, and beyond: Gut microbiota and inflammatory status in seniors and centenarians. PLoS ONE 2010, 5, e10667. [Google Scholar] [CrossRef]
- Khaledi, M.; Poureslamfar, B.; Alsaab, H.O.; Tafaghodi, S.; Hjazi, A.; Singh, R.; Alawadi, A.H.; Alsaalamy, A.; Qasim, Q.A.; Sameni, F. The role of gut microbiota in human metabolism and inflammatory diseases: A focus on elderly individuals. Ann. Microbiol. 2024, 74, 1. [Google Scholar] [CrossRef]
- Shi, X.; Ma, T.; Sakandar, H.A.; Menghe, B.; Sun, Z. Gut microbiome and aging nexus and underlying mechanism. Appl. Microbiol. Biotechnol. 2022, 106, 5349–5358. [Google Scholar] [CrossRef]
- Kimoto-Nira, H.; Mizumachi, K.; Okamoto, T.; Sasaki, K.; Kurisaki, J. Influence of long-term consumption of a Lactococcus lactis strain on the intestinal immunity and intestinal flora of the senescence-accelerated mouse. Br. J. Nutr. 2009, 102, 181–185. [Google Scholar] [CrossRef]
- Li, P.; Ji, B.; Luo, H.; Sundh, D.; Lorentzon, M.; Nielsen, J. One-year supplementation with Lactobacillus reuteri ATCC PTA 6475 counteracts a degradation of gut microbiota in older women with low bone mineral density. NPJ Biofilms Microbiomes 2022, 8, 84. [Google Scholar] [CrossRef]
- Ni, Y.; Yang, X.; Zheng, L.; Wang, Z.; Wu, L.; Jiang, J.; Yang, T.; Ma, L.; Fu, Z. Lactobacillus and Bifidobacterium Improves Physiological Function and Cognitive Ability in Aged Mice by the Regulation of Gut Microbiota. Mol. Nutr. Food Res. 2019, 63, e1900603. [Google Scholar] [CrossRef]


| Items | Content |
|---|---|
| Ingredient, % | |
| Chicken meal | 30.00 |
| Pea | 7.00 |
| Peeled barley | 13.50 |
| Chicken liver meal | 5.00 |
| Tapioca starch | 15.00 |
| Chicken oil | 8.50 |
| Sweet potato granules | 8.00 |
| Fish meal | 6.00 |
| Carrot powder | 1.00 |
| Pumpkin powder | 2.00 |
| Fish oil | 2.50 |
| Premix 1 | 1.50 |
| Total | 100.00 |
| Nutrient levels | |
| Metabolic energy, kcal/kg | 4.235 |
| Crude protein, % | 32.85 |
| Ether extract, % | 18.32 |
| Calcium, % | 1.23 |
| Phosphorus, % | 0.97 |
| Items | CON | SC | SEM | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Day 0 | Day 21 | Day 42 | Day 0 | Day 21 | Day 42 | Diet | Time | Diet × Time | ||
| Body weight, kg | 28.39 | 28.44 | 28.59 | 29.09 | 29.42 | 29.73 | 0.50 | 0.37 | 0.95 | 0.99 |
| Daily intake, g/d | 417.25 | 427.67 | 420.03 | 418.00 | 406.56 | 426.34 | 4.86 | 0.64 | 0.86 | 0.50 |
| Body condition score | 4.80 | 4.90 | 5.10 | 4.80 | 4.80 | 4.90 | 0.09 | 0.56 | 0.63 | 0.90 |
| Items | CON | SC | SEM | p-Value | Reference Interval | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Day 0 | Day 21 | Day 42 | Day 0 | Day 21 | Day 42 | Diet | Time | |||
| Hematological indexes | ||||||||||
| WBCs, 109/L | 13.83 | 14.94 | 14.48 | 15.23 | 12.51 | 10.76 | 0.52 | 0.16 | 0.24 | 6.00~16.9 |
| Lymphocytes, 109/L | 3.58 | 3.40 | 3.67 | 3.76 | 3.58 | 2.82 | 0.22 | 0.81 | 0.65 | 1.10~6.30 |
| Lymphocytes, % | 18.24 | 19.01 | 18.88 | 18.24 | 18.44 | 15.91 | 0.47 | 0.17 | 0.49 | 12.0~30.0 |
| Monocytes, 109/L | 0.92 | 0.89 | 0.94 | 0.92 | 0.86 | 0.86 | 0.05 | 0.63 | 0.84 | 0.15~1.35 |
| Gran, 109/L | 9.09 | 9.05 | 8.81 | 10.39 | 9.43 | 9.52 | 0.40 | 0.55 | 0.62 | 6.20~14.8 |
| Monocytes, % | 6.77 | 5.97 | 7.35 | 6.38 | 5.29 | 5.57 | 0.29 | 0.07 | 0.35 | 3.00~10.0 |
| Gran, % | 70.81 | 73.98 | 73.51 | 70.55 | 69.75 | 69.16 | 0.97 | 0.12 | 0.9 | 63.0~87.0 |
| RBCs, 1012/L | 6.48 | 6.08 | 6.33 | 6.14 | 6.25 | 5.76 | 0.17 | 0.67 | 0.65 | 5.50~8.50 |
| HGB, g/L | 151.16 | 165.49 | 157.47 | 139.30 | 137.90 | 149.14 | 5.33 | 0.2 | 0.73 | 120~180 |
| PLTs, 109/L | 294.10 | 291.10 | 303.38 | 328.15 | 324.82 | 306.31 | 13.15 | 0.85 | 0.97 | 175~500 |
| RDW, % | 12.83 | 12.57 | 12.85 | 13.61 | 13.04 | 13.00 | 0.19 | 0.09 | 0.57 | 12.0~21.0 |
| MCHC, g/L | 344.08 | 333.66 | 343.93 | 354.92 | 350.34 | 351.23 | 3.56 | 0.16 | 0.57 | 300~369 |
| MPV, fL | 9.46 | 9.23 | 9.55 | 9.59 | 9.34 | 8.89 | 0.16 | 0.67 | 0.62 | 6.70~11.1 |
| Biochemical indexes | ||||||||||
| ALT, U/L | 38.05 | 36.01 | 40.73 | 40.80 | 38.64 | 43.68 | 1.94 | 0.27 | 0.51 | 10.0~100 |
| AST, U/L | 26.65 | 25.18 | 28.49 | 34.46 | 32.63 | 36.88 | 2.06 | 0.09 | 0.58 | 0~50.0 |
| GLU, mmol/L | 5.30 | 5.00 | 5.97 | 6.26 | 5.61 | 5.81 | 0.15 | 0.05 | 0.12 | 3.89~7.94 |
| BUN, mmol/L | 3.44 | 3.13 | 3.56 | 3.66 | 3.35 | 3.80 | 0.12 | 0.31 | 0.32 | 3.20~7.00 |
| ALP, U/L | 152.40 | 144.66 | 163.49 | 179.26 | 170.18 | 186.32 | 7.52 | 0.05 | 0.49 | 23.0~212 |
| Cr, μmol/L | 63.78 | 61.41 | 57.39 | 51.24 | 48.55 | 54.89 | 2.76 | 0.08 | 0.9 | 44.0~159 |
| TCHO, mmol/L | 4.31 | 3.96 | 4.51 | 4.69 | 4.33 | 4.93 | 0.17 | 0.31 | 0.31 | 2.84~8.27 |
| TP, g/L | 65.62 | 61.41 | 69.40 | 73.82 | 67.01 | 74.11 | 1.95 | 0.13 | 0.22 | 52.0~82.0 |
| ALB, g/L | 32.26 | 30.53 | 34.53 | 31.57 | 29.85 | 33.76 | 0.87 | 0.72 | 0.18 | 22.0~39.0 |
| Digestibility (%) | CON | SC | SEM | p-Value |
|---|---|---|---|---|
| Dry matter | 84.53 | 83.87 | 0.66 | 0.52 |
| Organic matter | 85.61 | 86.97 | 0.75 | 0.21 |
| Crude protein | 86.90 | 85.14 | 0.86 | 0.15 |
| Ether extract | 90.69 | 90.52 | 0.85 | 0.88 |
| Gross energy | 85.34 | 87.13 | 1.04 | 0.22 |
| Items | CON | SC | SEM | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Day 0 | Day 21 | Day 42 | Day 0 | Day 21 | Day 42 | Diet | Time | Diet × Time | ||
| Fecal score | 2.90 | 2.60 | 2.70 | 2.70 | 2.50 | 2.70 | 0.11 | 0.67 | 0.68 | 0.94 |
| Short-chain fatty acids (µmol/g dry matter basis) | ||||||||||
| Acetate | 351.16 | 336.52 | 332.97 | 335.39 | 348.55 | 344.87 | 6.20 | 0.83 | 0.96 | 0.60 |
| Propionate | 149.01 | 144.68 | 151.41 | 157.06 | 155.03 | 158.43 | 4.69 | 0.39 | 0.91 | 0.99 |
| Butyrate | 82.53 | 79.23 | 80.98 | 80.26 | 89.93 | 83.63 | 1.39 | 0.19 | 0.63 | 0.16 |
| Valerate | 3.48 | 3.65 | 4.01 | 3.08 | 3.42 | 3.74 | 0.20 | 0.47 | 0.51 | 0.99 |
| Branched-chain fatty acids (µmol/g dry matter basis) | ||||||||||
| Isovalerate | 9.98 | 9.51 | 9.77 | 9.34 | 9.85 | 9.81 | 0.16 | 0.80 | 0.94 | 0.47 |
| Isobutyrate | 7.25 | 6.47 | 7.79 | 7.38 | 7.12 | 6.50 | 0.23 | 0.71 | 0.65 | 0.22 |
| Ammonia, μmol/g | 179.04 | 173.67 | 169.53 A | 172.89 | 152.83 | 132.46 B | 4.95 | <0.05 | 0.10 | 0.41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, Y.; Li, D.; Zhang, M.; Liu, P.; Wang, H.; Li, Y.; Wu, Y. The Effects of Dietary Saccharomyces cerevisiae Supplementation on Gut Microbiota Composition and Gut Health in Aged Labrador Retrievers. Animals 2024, 14, 1713. https://doi.org/10.3390/ani14121713
Cui Y, Li D, Zhang M, Liu P, Wang H, Li Y, Wu Y. The Effects of Dietary Saccharomyces cerevisiae Supplementation on Gut Microbiota Composition and Gut Health in Aged Labrador Retrievers. Animals. 2024; 14(12):1713. https://doi.org/10.3390/ani14121713
Chicago/Turabian StyleCui, Yingyue, Deping Li, Mingrui Zhang, Pan Liu, Haotian Wang, Yingying Li, and Yi Wu. 2024. "The Effects of Dietary Saccharomyces cerevisiae Supplementation on Gut Microbiota Composition and Gut Health in Aged Labrador Retrievers" Animals 14, no. 12: 1713. https://doi.org/10.3390/ani14121713
APA StyleCui, Y., Li, D., Zhang, M., Liu, P., Wang, H., Li, Y., & Wu, Y. (2024). The Effects of Dietary Saccharomyces cerevisiae Supplementation on Gut Microbiota Composition and Gut Health in Aged Labrador Retrievers. Animals, 14(12), 1713. https://doi.org/10.3390/ani14121713







