In Vivo Imaging of Rabbit Follicles through Combining Ultrasound Bio-Microscopy and Intravital Window
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Equipment
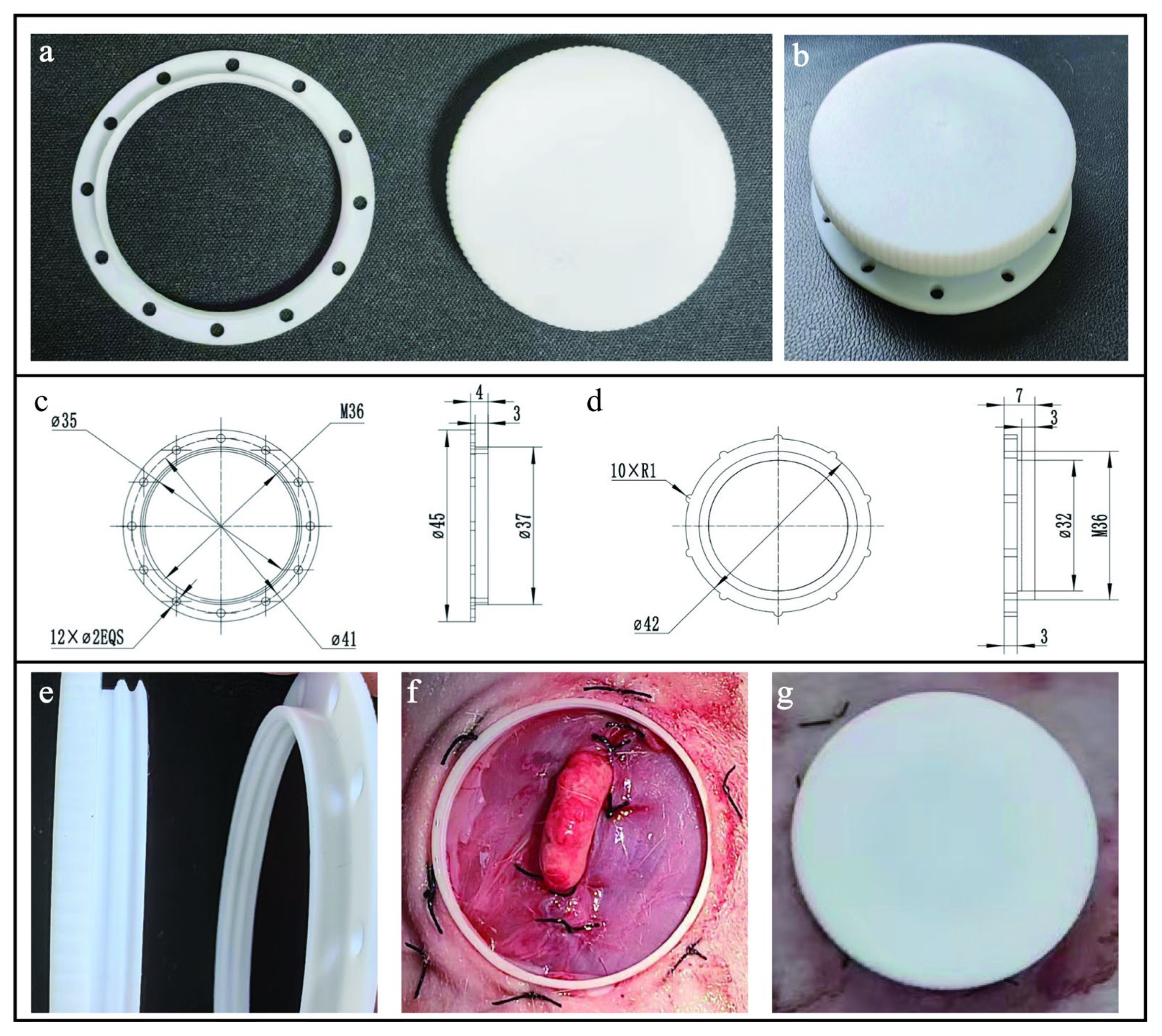
2.2. Design of Intravital Window
2.3. Surgical Procedure
2.4. Statistical Analysis
3. Results
3.1. The Impact of Surgery on Female Rabbit Physiology
3.2. High-Resolution Image of Follicles
3.3. Monitoring of Follicular Development in Female Rabbits
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, S.; Zeng, W.; Li, R.; Hoffman, L.C.; He, Z.; Sun, Q.; Li, H. Rabbit meat production and processing in China. Meat Sci. 2018, 145, 320–328. [Google Scholar] [CrossRef]
- Fan, J.; Kitajima, S.; Watanabe, T.; Xu, J.; Zhang, J.; Liu, E.; Chen, Y.E. Rabbit models for the study of human atherosclerosis: From pathophysiological mechanisms to translational medicine. Pharmacol. Ther. 2015, 146, 104–119. [Google Scholar] [CrossRef]
- Brower, M. Practitioner’s guide to pocket pet and rabbit theriogenology. Theriogenology 2006, 66, 618–623. [Google Scholar] [CrossRef]
- Dal Bosco, A.; Rebollar, P.G.; Boiti, C.; Zerani, M.; Castellini, C. Ovulation induction in rabbit does: Current knowledge and perspectives. Anim. Reprod. Sci. 2011, 129, 106–117. [Google Scholar] [CrossRef]
- Deanesly, R.; Hill, J.P. The development and vascularisation of the corpus luteum in the mouse and rabbit. Proc. R. Soc. Lond. 1930, 107, 60–76. [Google Scholar] [CrossRef]
- Armstrong, R.W.; McCallion, D.J.; YoungLai, E.V. Morphological changes in the rabbit ovary after active immunization to testosterone. Cell Tissue Res. 1978, 193, 533–542. [Google Scholar] [CrossRef]
- Marshall, F.H.; Verney, E.B. The occurrence of ovulation and pseudo-pregnancy in the rabbit as a result of central nervous stimulation. J. Physiol. 1936, 86, 327–336. [Google Scholar] [CrossRef]
- Kauffman, R.D.; Schmidt, P.M.; Rall, W.F.; Hoeg, J.M. Superovulation of rabbits with FSH alters in vivo development of vitrified morulae. Theriogenology 1998, 50, 1081–1092. [Google Scholar] [CrossRef]
- Al-Badrany, M.S. Laparoscopic ovariectomy in rabbits. Iraqi J. Vet. Sci. 2009, 23, 51–55. [Google Scholar] [CrossRef]
- Miller, J.B.; Keyes, P.L. Transition of the rabbit corpus luteum to estrogen dependence during early luteal development. Endocrinology 1978, 102, 31–38. [Google Scholar] [CrossRef]
- Garcia-Garcia, R.M.; Arias-Alvarez, M.; Sanchez-Rodriguez, A.; Lorenzo, P.L.; Rebollar, P.G. Role of nerve growth factor in the reproductive physiology of female rabbits: A review. Theriogenology 2020, 150, 321–328. [Google Scholar] [CrossRef]
- Robertson, L.; Cattoni, J.C.; Shand, R.I.; Jeffcoate, I.A. A critical evaluation of ultrasonic monitoring of superovulation in cattle. Br. Vet. J. 1993, 149, 477–484. [Google Scholar] [CrossRef]
- Adams, G.P.; Griffin, P.G.; Ginther, O.J. In situ morphologic dynamics of ovaries, uterus, and cervix in llamas. Biol. Reprod. 1989, 41, 551–558. [Google Scholar] [CrossRef]
- Ravindra, J.P.; Rawlings, N.C.; Evans, A.C.; Adams, G.P. Ultrasonographic study of ovarian follicular dynamics in ewes during the oestrous cycle. J. Reprod. Fertil. 1994, 101, 501–509. [Google Scholar] [CrossRef]
- Kaulfuss, K.H.; Brabant, S.; Blume, K.; May, J. Optimization of embryo transfer programs in ewes by transrectal ultrasonographic ovary diagnosis (B-mode) in superovulated donors. Dtsch. Tierärztliche Wochenschr. 1995, 102, 208–212. [Google Scholar]
- Robertson, R.D.; Picker, R.H.; Wilson, P.C.; Saunders, D.M. Assessment of ovulation by ultrasound and plasma estradiol determinations. Obs. Gynecol. 1979, 54, 686–691. [Google Scholar]
- Ylostalo, P.; Lingren, P.G.; Nillius, S.J. Ultrasonic measurement of ovarian follicles, ovarian and uterine size during induction of ovulation with human gonadotrophins. Acta Endocrinol. 1981, 98, 592–598. [Google Scholar] [CrossRef]
- Pallares, P.; Gonzalez-Bulnes, A. Use of ultrasound imaging for early diagnosis of pregnancy and determination of litter size in the mouse. Lab. Anim. 2009, 43, 91–95. [Google Scholar] [CrossRef]
- Hayashi, A.; Giacalone, G.; Yamamoto, T.; Belva, F.; Visconti, G.; Hayashi, N.; Handa, M.; Yoshimatsu, H.; Salgarello, M. Ultra High-frequency Ultrasonographic Imaging with 70 MHz Scanner for Visualization of the Lymphatic Vessels. Plast. Reconstr. Surg. Glob. Open 2019, 7, e2086. [Google Scholar] [CrossRef]
- Dada, T.; Gadia, R.; Sharma, A.; Ichhpujani, P.; Bali, S.J.; Bhartiya, S.; Panda, A. Ultrasound biomicroscopy in glaucoma. Surv. Ophthalmol. 2011, 56, 433–450. [Google Scholar] [CrossRef]
- Pavlin, C.J.; Harasiewicz, K.; Sherar, M.D.; Foster, F.S. Clinical use of ultrasound biomicroscopy. Ophthalmology 1991, 98, 287–295. [Google Scholar] [CrossRef]
- Kapoor, R.; Parameswarappa, D.C.; Dhurandhar, D.; Peguda, H.K.; Rani, P.K. Peering into the eye: A comprehensive look at ultrasound biomicroscopy (UBM) and its diagnostic value in anterior segment disorders. Indian J. Ophthalmol. 2023, 71, 3118–3119. [Google Scholar] [CrossRef]
- Silverman, R.H. High-resolution ultrasound imaging of the eye—A review. Clin. Exp. Ophthalmol. 2009, 37, 54–67. [Google Scholar] [CrossRef]
- Chang, Y.J.; Huang, H.C.; Hsueh, Y.Y.; Wang, S.W.; Su, F.C.; Chang, C.H.; Tang, M.J.; Li, Y.S.; Wang, S.H.; Shung, K.K.; et al. Role of Excessive Autophagy Induced by Mechanical Overload in Vein Graft Neointima Formation: Prediction and Prevention. Sci. Rep. 2016, 6, 22147. [Google Scholar] [CrossRef]
- Pittet, M.J.; Weissleder, R. Intravital imaging. Cell 2011, 147, 983–991. [Google Scholar] [CrossRef]
- Holtmaat, A.; Bonhoeffer, T.; Chow, D.K.; Chuckowree, J.; De Paola, V.; Hofer, S.B.; Hubener, M.; Keck, T.; Knott, G.; Lee, W.C.; et al. Long-term, high-resolution imaging in the mouse neocortex through a chronic cranial window. Nat. Protoc. 2009, 4, 1128–1144. [Google Scholar] [CrossRef]
- Feng, W.; Liu, C.J.; Wang, L.; Zhang, C. An optical clearing imaging window: Realization of mouse brain imaging and manipulation through scalp and skull. J. Cereb. Blood Flow Metab. 2023, 43, 2105–2119. [Google Scholar] [CrossRef]
- Farrar, M.J.; Bernstein, I.M.; Schlafer, D.H.; Cleland, T.A.; Fetcho, J.R.; Schaffer, C.B. Chronic in vivo imaging in the mouse spinal cord using an implanted chamber. Nat. Methods 2012, 9, 297–302. [Google Scholar] [CrossRef]
- Petersen, J.; Du, W.; Adkisson, C.; Gravekamp, C.; Oktay, M.H.; Condeelis, J.; Panarelli, N.C.; McAuliffe, J.C.; Entenberg, D. Stabilized Window for Intravital Imaging of the Murine Pancreas. Jove-J. Vis. Exp. 2023, 6, e65498. [Google Scholar] [CrossRef]
- Du, W.; Adkisson, C.; Ye, X.; Duran, C.L.; Chellakkan Selvanesan, B.; Gravekamp, C.; Oktay, M.H.; McAuliffe, J.C.; Condeelis, J.S.; Panarelli, N.C.; et al. SWIP-a stabilized window for intravital imaging of the murine pancreas. Open Biol. 2022, 12, 210273. [Google Scholar] [CrossRef]
- Ritsma, L.; Steller, E.J.; Ellenbroek, S.I.; Kranenburg, O.; Borel Rinkes, I.H.; van Rheenen, J. Surgical implantation of an abdominal imaging window for intravital microscopy. Nat. Protoc. 2013, 8, 583–594. [Google Scholar] [CrossRef]
- Entenberg, D.; Voiculescu, S.; Guo, P.; Borriello, L.; Wang, Y.; Karagiannis, G.S.; Jones, J.; Baccay, F.; Oktay, M.; Condeelis, J. A permanent window for the murine lung enables high-resolution imaging of cancer metastasis. Nat. Methods 2018, 15, 73–80. [Google Scholar] [CrossRef]
- Rakhilin, N.; Garrett, A.; Eom, C.Y.; Chavez, K.R.; Small, D.M.; Daniel, A.R.; Kaelberer, M.M.; Mejooli, M.A.; Huang, Q.; Ding, S.; et al. An intravital window to image the colon in real time. Nat. Commun. 2019, 10, 5647. [Google Scholar] [CrossRef]
- Wang, S.; Larina, I.V. In vivo dynamic 3D imaging of oocytes and embryos in the mouse oviduct. Cell Rep. 2021, 36, 109382. [Google Scholar] [CrossRef]
- Huang, Q.; Cohen, M.A.; Alsina, F.C.; Devlin, G.; Garrett, A.; McKey, J.; Havlik, P.; Rakhilin, N.; Wang, E.; Xiang, K.; et al. Intravital imaging of mouse embryos. Science 2020, 368, 181–186. [Google Scholar] [CrossRef]
- Meissner, S.; Knels, L.; Schnabel, C.; Koch, T.; Koch, E.J.J.o.B.O. Three-dimensional Fourier domain optical coherence tomography in vivo imaging of alveolar tissue in the intact thorax using the parietal pleura as a window. J. Biomed. Opt. 2010, 15, 016030. [Google Scholar] [CrossRef]
- Cervantes, M.P.; Singh, J.; Palomino, J.M.; Adams, G.P. Surgical translocation and ultrasound bio-microscopy of the ovaries in rabbits. Anim. Reprod. Sci. 2013, 138, 133–141. [Google Scholar] [CrossRef]
- Jaiswal, R.S.; Singh, J.; Adams, G.P. High-resolution ultrasound biomicroscopy for monitoring ovarian structures in mice. Reprod. Biol. Endocrin. 2009, 7, 69. [Google Scholar] [CrossRef]
- Chang, M.C. Fertilization and Normal Development of Follicular Oocytes in the Rabbit. Science 1955, 121, 867–869. [Google Scholar] [CrossRef]
- Naseer, Z.; Ahmad, E.; Epikmen, E.T.R.; Uçan, U.U.; Boyacioğlu, M.; İpek, E.; Akosy, M.J.T. Quercetin supplemented diet improves follicular development, oocyte quality, and reduces ovarian apoptosis in rabbits during summer heat stress. Theriogenology 2017, 96, 136–141. [Google Scholar] [CrossRef]



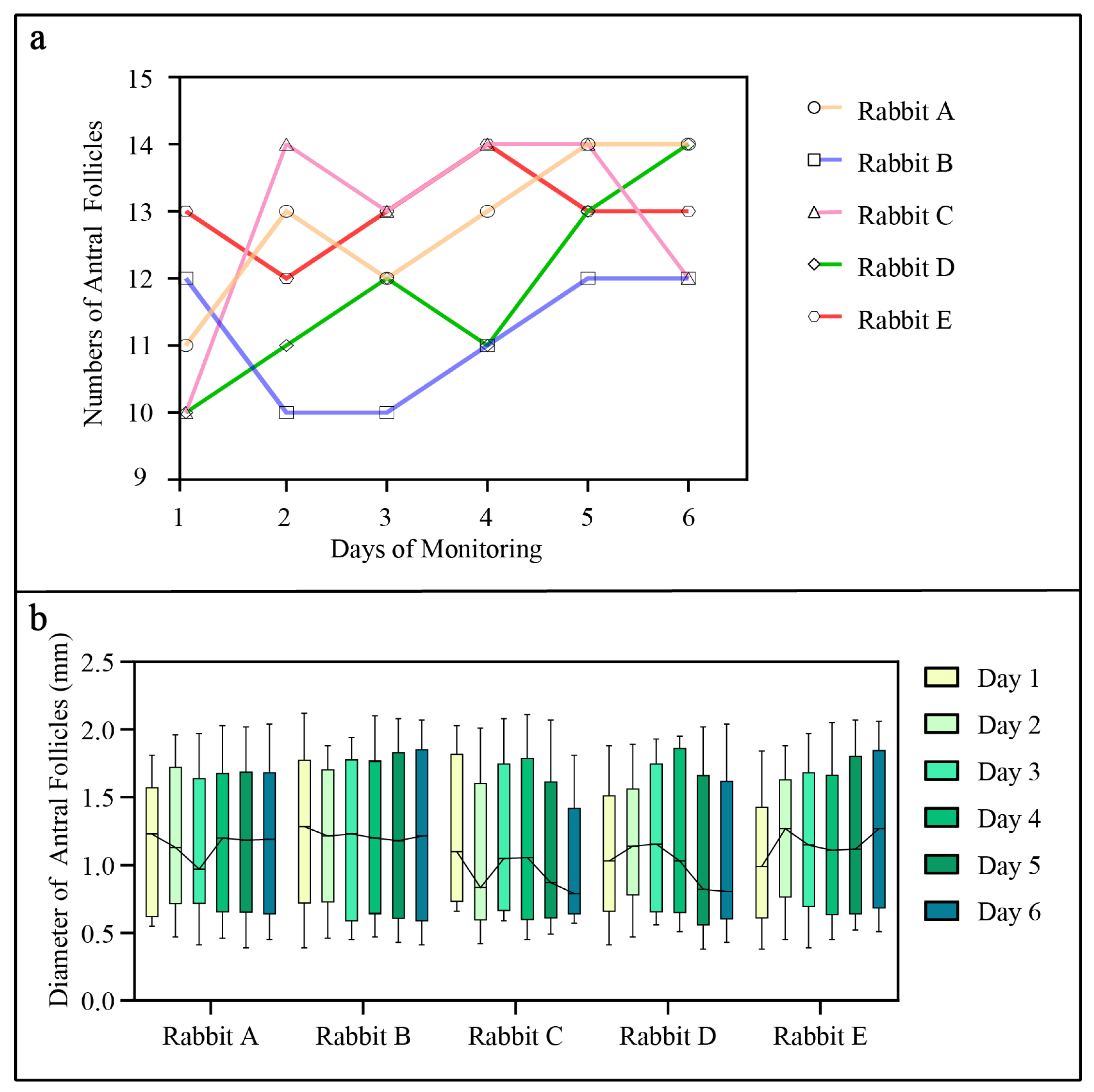
| Rabbit | Ovarian Antral Follicle | |
|---|---|---|
| Numbers | Mean ± SEM (Range) | |
| a | 14 | 1.18 ± 0.14 (0.55–2.01) |
| b | 14 | 1.19 ± 0.15 (0.43–1.94) |
| c | 15 | 1.09 ± 0.13 (0.35–1.80) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Yan, C.; Tao, S.; He, Y.; Zhao, J.; Wang, Y.; Wu, Y.; Liu, N.; Qin, Y. In Vivo Imaging of Rabbit Follicles through Combining Ultrasound Bio-Microscopy and Intravital Window. Animals 2024, 14, 1727. https://doi.org/10.3390/ani14121727
Yang L, Yan C, Tao S, He Y, Zhao J, Wang Y, Wu Y, Liu N, Qin Y. In Vivo Imaging of Rabbit Follicles through Combining Ultrasound Bio-Microscopy and Intravital Window. Animals. 2024; 14(12):1727. https://doi.org/10.3390/ani14121727
Chicago/Turabian StyleYang, Lihan, Chang Yan, Siming Tao, Yifeilong He, Jing Zhao, Yanya Wang, Yingjie Wu, Ning Liu, and Yinghe Qin. 2024. "In Vivo Imaging of Rabbit Follicles through Combining Ultrasound Bio-Microscopy and Intravital Window" Animals 14, no. 12: 1727. https://doi.org/10.3390/ani14121727
APA StyleYang, L., Yan, C., Tao, S., He, Y., Zhao, J., Wang, Y., Wu, Y., Liu, N., & Qin, Y. (2024). In Vivo Imaging of Rabbit Follicles through Combining Ultrasound Bio-Microscopy and Intravital Window. Animals, 14(12), 1727. https://doi.org/10.3390/ani14121727




